Combined Intra-Articular PN HPT™ and Hyaluronic Acid: Regeneration Medicine in Knee Osteoarthritis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Primary Endpoints
2.2. Secondary Endpoint
2.3. Sample Size and Statistical Analysis
3. Results
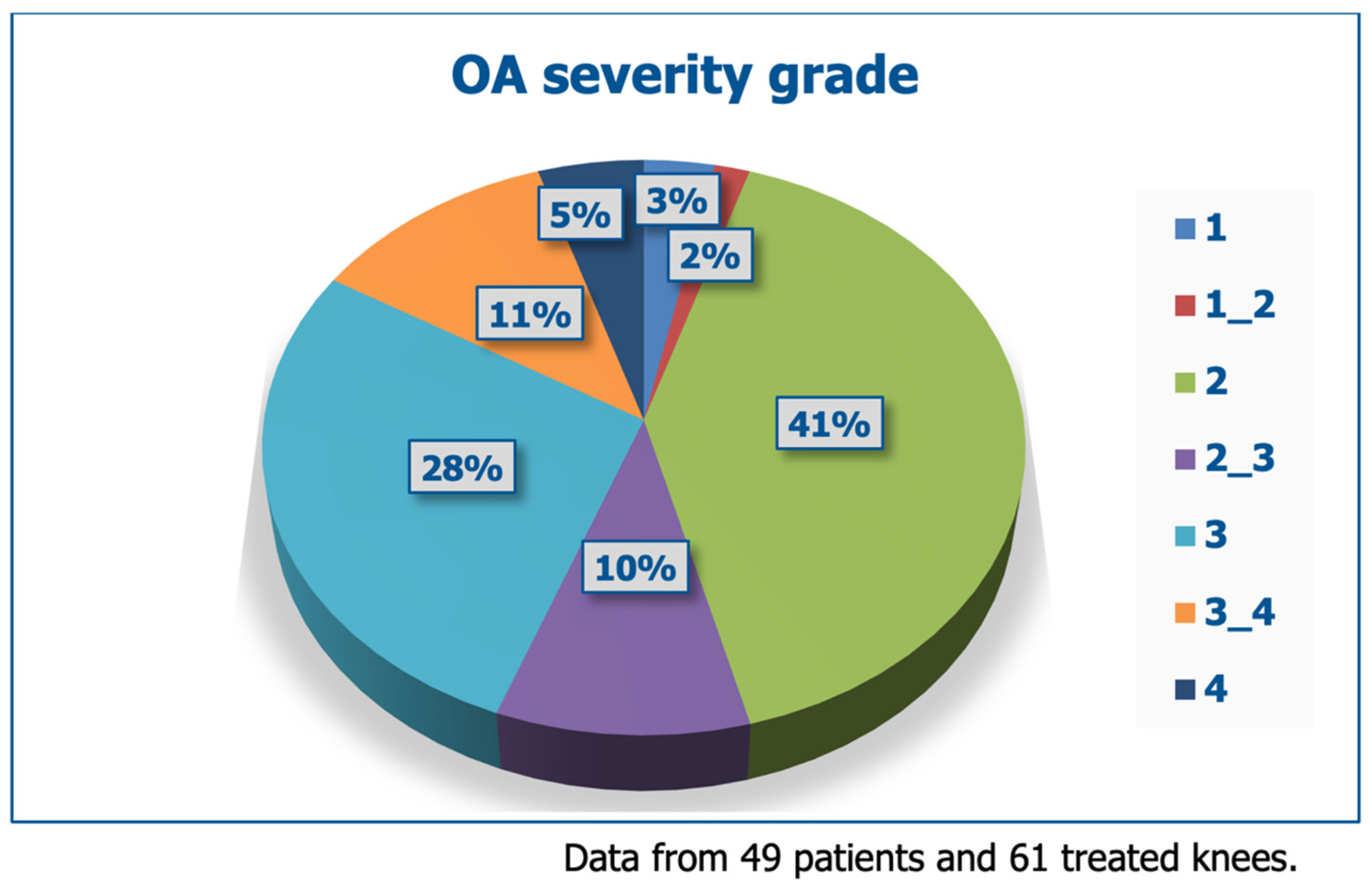
3.1. Baseline Demographics and Dropouts
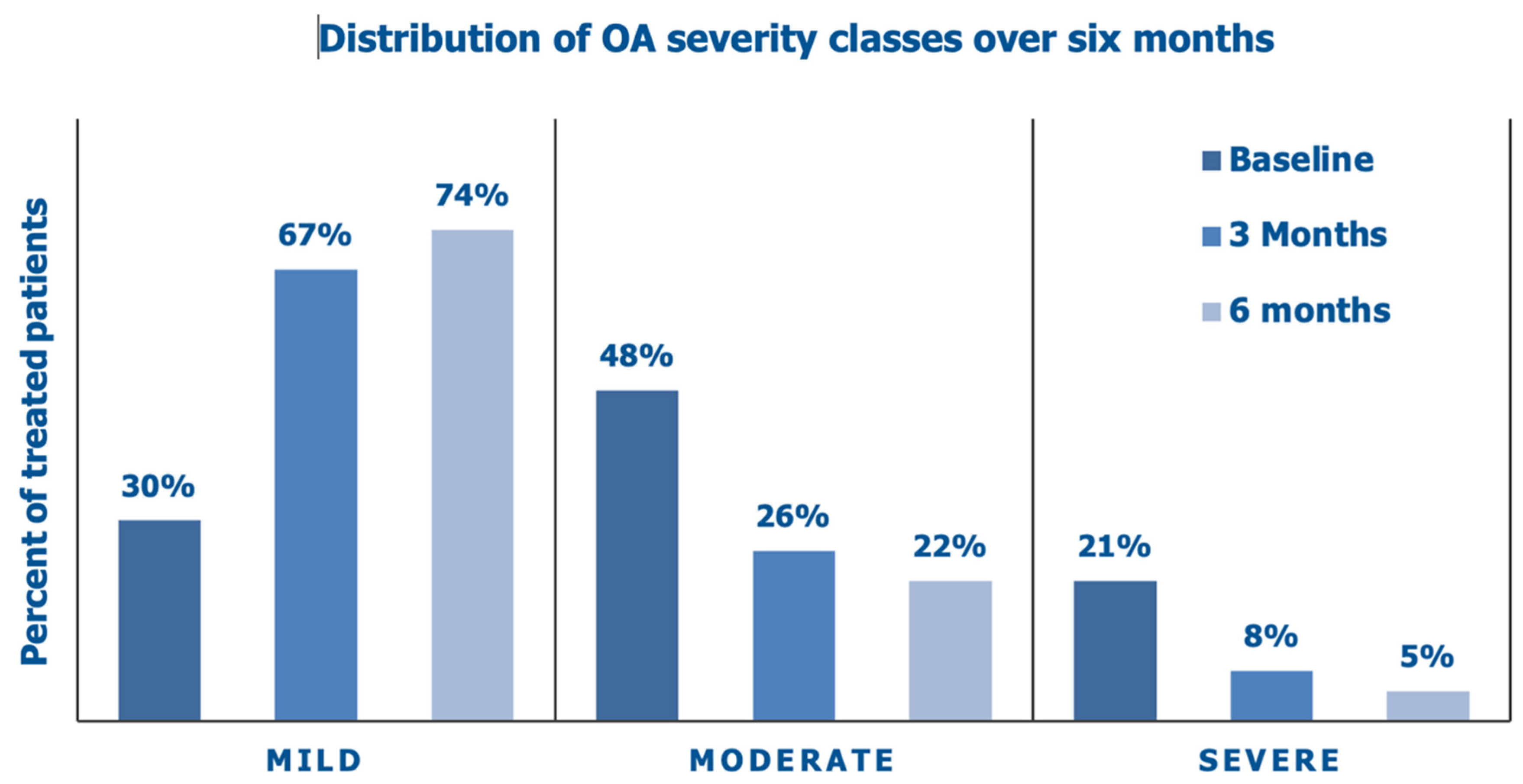
3.2. Primary Endpoints
3.3. Secondary Endpoint (WOMAC Score)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Butler, J.; Rydell, N.W.; Balazs, E.A. Hyaluronic acid in synovial fluid. VI. Effect of intra-articular injection of hyaluronic acid on the clinical symptoms of arthritis in track horses. Acta Vet. Scand. 1970, 11, 139–155. [Google Scholar] [PubMed]
- Gupta, R.C.; Lall, R.; Srivastava, A.; Sinha, A. Hyaluronic Acid: Molecular mechanisms and therapeutic mechanisms and therapeutic trajectory. Front. Vet. Sci. 2019, 6, 192. [Google Scholar] [CrossRef] [PubMed]
- Walvekar, P.; Lulinski, P.; Kumar, P.; Aminabhavi, T.M.; Choonara, Y.E. A review of hyaluronic acid-based therapeutics for the treatment and management of arthritis. Int. J. Biol. Macromol. 2024, 264 Pt 2, 130645. [Google Scholar] [CrossRef] [PubMed]
- Balazs, E.A.; Denlinger, J.L. Viscosupplementation: A new concept in the treatment of osteoarthritis. J. Rheumatol. 1993, 39, 3–9. [Google Scholar]
- Martin, J.A.; Buckwalter, J.A. Aging, articular cartilage chondrocyte senescence and osteoarthritis. Biogerontology 2002, 3, 257–264. [Google Scholar] [CrossRef]
- Peng, H.; Zhou, J.-l.; Liu, S.-q.; Hu, Q.-j.; Ming, J.-h.; Qiu, B. Hyaluronic acid inhibits nitric oxide-induced apoptosis and dedifferentiation of articular chondrocytes In Vitro. Inflamm. Res. 2010, 59, 519–530. [Google Scholar] [CrossRef]
- Vanelli, R.; Costa, P.; Rossi, S.M.P.; Benazzo, F. Efficacy of intra-articular polynucleotides in the treatment of knee osteoarthritis: A randomized, double-blind clinical trial. Knee Surg. Sports Traumatol. Arthrosc. 2010, 18, 901–907. [Google Scholar] [CrossRef]
- Giarratana, L.S.; Marelli, B.M.; Crapanzano, C.; De Martinis, S.E.; Gala, L.; Ferraro, M.; Marelli, N.; Albisetti, W. A randomized, double-blind clinical trial on the treatment of knee osteoarthritis: The efficacy of polynucleotides compared to standard hyaluronan viscosupplementation. Knee 2014, 21, 661–668. [Google Scholar] [CrossRef]
- Stagni, C.; Rocchi, M.; Mazzotta, A.; Del Piccolo, N.; Rani, N.; Govo, M.ni; Vivarelli, L.; Veronesi, F.; Fini, M.; Dallari, D. Randomised, double-blind comparison of a fixed co-formulation of intra-articular polynucleotides and hyaluronic acid versus hyaluronic acid alone in the treatment of knee osteoarthritis: Two-year follow-up. BMC Musculoskelet. Disord. 2021, 22, 773. [Google Scholar] [CrossRef]
- Guelfi, M.; Fabbrini, R.; Guelfi, M.G. Intra-articular treatment of knee and ankle osteoarthritis with polynucleotides: Prospective case record cohort vs historical controls. J. Biol. Regul. Homeost. Agents 2020, 34, 1949–1953. [Google Scholar] [CrossRef]
- Migliore, A.; Graziano, E.; Martín, L.S.M.; Sorbino, A.; Raichi, M.; Bon, G. Three-year management of hip osteoarthritis with intra-articular polynucleotides: A real-life cohort retrospective study. J. Biol. Regul. Homeost. Agents 2021, 35, 1189–1194. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef] [PubMed]
- Lequesne, M.G. The algofunctional indices for hip and knee osteoarthritis. J. Rheumatol. 1997, 24, 779–781. [Google Scholar] [PubMed]
- Jensen, M.P.; Karoly, P. Self-Report Scales and Procedures for Assessing Pain in Adults. In Handbook of Pain Assessment, 3rd ed.; Turk, D.C., Melzack, R., Eds.; Guilford Press: New York, NY, USA, 2011; pp. 19–44. [Google Scholar]
- Bellamy, N.; Buchanan, W.W.; Goldsmith, C.H.; Campbell, J.; Stitt, L.W. Validation study of WOMAC: A health status instrument for measuring clinically important patient-relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J. Rheumatol. 1988, 15, 1833–1840. [Google Scholar]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.-G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef]
- Knights, A.J.; Redding, S.J.; Maerz, T. Inflammation in osteoarthritis: The latest progress and ongoing challenges. Curr. Opin. Rheumatol. 2023, 35, 128–134. [Google Scholar] [CrossRef]
- Yao, Q.; Wu, X.; Tao, C.; Gong, W.; Chen, M.; Qu, M.; Zhong, Y.; He, T.; Chen, S.; Xiao, G. Osteoarthritis: Pathogenic signaling pathways and therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 56. [Google Scholar] [CrossRef]
- Primorac, D.; Molnar, V.; Rod, E.; Jelec, Z.; Cukelj, F.; Matisic, V.; Vrdoljak, T.; Hudetz, D.; Hajsok, H.; Borićet, I. Knee osteoarthritis: A review of pathogenesis and state-of-the-art non-operative therapeutic considerations. Genes 2020, 11, 854. [Google Scholar] [CrossRef]
- Bannuru, R.R.; Osani, M.C.; Vaysbrot, E.E.; Arden, N.K.; Bennell, K.; Bierma-Zeinstra, S.M.A.; Kraus, V.B.; Lohmander, L.S.; Abbott, J.H.; Bhandari, M.; et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr. Cartil. 2019, 27, 1578–1589. [Google Scholar] [CrossRef]
- Lotto, M.L.; Wright, E.J.; Appleby, D.; Zelicof, S.B.; Lemos, M.J.; Lubowitz, J.H. Ex vivo comparison of mechanical versus thermal chondroplasty: Assessment of tissue effect at the surgical endpoint. Arthroscopy 2008, 24, 410–415. [Google Scholar] [CrossRef]
- Tuan, R.S. A second-generation autologous chondrocyte implantation approach to the treatment of focal articular cartilage defects. Arthritis. Res. Ther. 2007, 9, 109. [Google Scholar] [CrossRef]
- Nagle, J.A. Knee joint preservation with autologous cartilage implantation. AORN J. 2007, 86, 550–562. [Google Scholar] [CrossRef] [PubMed]
- Riegger-Krugh, C.L.; McCarty, E.C.; Robinson, M.S.; Wegzyn, D.A. Autologous chondrocyte implantation: Current surgery and rehabilitation. Med. Sci. Sports Exerc. 2008, 40, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Gorensek, M.; Jaksimović, C.; Kregar-Velikonja, N.; Gorensek, M.; Knezevic, M.; Jeras, M.; Pavlovcic, V.; Cör, A. Nucleus pulposus repair with cultured autologous elastic cartilage-derived chondrocytes. Cell Mol. Biol. Lett. 2004, 9, 363–373. [Google Scholar]
- Cecchi, F.; Mannoni, A.; Molino-Lova, R.; Ceppatelli, S.; Benvenuti, E.; Bandinelli, S.; Lauretani, F.; Macchi, C.; Ferrucci, L. Hip and knee pain epidemiology in a community-based sample of Italian persons aged 65 and older. Osteoarthr. Cartil. 2008, 16, 1039–1046. [Google Scholar] [CrossRef]
- Gennero, L.; Denysenko, T.; Calisti, G.F.; Vercelli, A.; Vercelli, C.M.; Amedeo, S.; Mioletti, S.; Parino, E.; Montanaro, M.; Melcarne, A.; et al. Protective effects of polynucleotides on cartilage degradation in experimental cultures. Cell Biochem. Funct. 2013, 31, 214–227. [Google Scholar] [CrossRef]
- Cenzato, N.; Crispino, R.; Russillo, A.; Del Fabbro, M.; Tartaglia, G.M. Clinical effectiveness of polynucleotide TMJ injection compared with physiotherapy: A 3-month randomised clinical trial. Br. J. Oral. Maxillofac. Surg. 2024, 62, 807–812. [Google Scholar] [CrossRef]
- Cavallini, M.; Bartoletti, E.; Maioli, L.; Massirone, A.; Palmieri, I.P.; Papagni, M.; Priori, M.; Trocchi, G. Consensus report on the use of PN-HPT™ (polynucleotides highly purified technology) in aesthetic medicine. J. Cosmet. Dermatol. 2021, 20, 922–928. [Google Scholar] [CrossRef]
- Cavallini, M.; Bartoletti, E.; Maioli, E. on behalf of The Polynucleotides HPT™ Priming Board. Value and benefits of the Polynucleotides HPT™ Dermal Priming Paradigm. A consensus on practice guidance for aesthetic medicine practitioners and future research. Clin. Exp. Dermatol. Ther. 2024, 9, 224. [Google Scholar]





| Pain or discomfort | Maximum distance walked (can walk with pain) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
| Age (years old, *) | Mean ± SEM, all patients | 66.1 ± 12.8 |
| Range | 34–90 | |
| Gender (**) | Female | 28 (53.8%) |
| Male | 24 (46.2%) | |
| Weight (kg) (***) | Mean ± SEM, all patients | 74.0 ± 12.6 |
| Range | 50–110 | |
| Height (cm) (***) | Mean ± SEM, all patients | 168.1 (8.6) |
| Range | 150–182 | |
| Which knee? (****) | Right knee | 23 (43.4%) |
| Left knee | 18 (34.0%) | |
| Both knees | 12 (22.6%) |
| Evaluation | Investigator’s Assessment | Patient’s Self-Assessment | ||
|---|---|---|---|---|
| Patients | % | Patients | % | |
| Worsened | 0 | 0 | 0 | 0 |
| No change | 7 | 10.9 | 7 | 10.9 |
| Slightly improved | 16 | 25.1 | 20 | 31.3 |
| Improved | 20 | 31.2 | 27 | 42.2 |
| Optimal results | 21 | 32.8 | 10 | 15.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barcaro, F.; Cerino, A.; Cervini, A.F.; Gaffuri, M.; Vaso, N.; Vela, M. Combined Intra-Articular PN HPT™ and Hyaluronic Acid: Regeneration Medicine in Knee Osteoarthritis. J. Clin. Med. 2025, 14, 3043. https://doi.org/10.3390/jcm14093043
Barcaro F, Cerino A, Cervini AF, Gaffuri M, Vaso N, Vela M. Combined Intra-Articular PN HPT™ and Hyaluronic Acid: Regeneration Medicine in Knee Osteoarthritis. Journal of Clinical Medicine. 2025; 14(9):3043. https://doi.org/10.3390/jcm14093043
Chicago/Turabian StyleBarcaro, Francesco, Alessandro Cerino, Armando Francesco Cervini, Mario Gaffuri, Nikoleta Vaso, and Mario Vela. 2025. "Combined Intra-Articular PN HPT™ and Hyaluronic Acid: Regeneration Medicine in Knee Osteoarthritis" Journal of Clinical Medicine 14, no. 9: 3043. https://doi.org/10.3390/jcm14093043
APA StyleBarcaro, F., Cerino, A., Cervini, A. F., Gaffuri, M., Vaso, N., & Vela, M. (2025). Combined Intra-Articular PN HPT™ and Hyaluronic Acid: Regeneration Medicine in Knee Osteoarthritis. Journal of Clinical Medicine, 14(9), 3043. https://doi.org/10.3390/jcm14093043







