The Predictive Value of ALBI Score for No-Reflow in Non-ST Elevation Acute Coronary Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Laboratory Analysis and ALBI Score Calculation
2.3. Coronary Angiography
2.4. Statistical Preparation
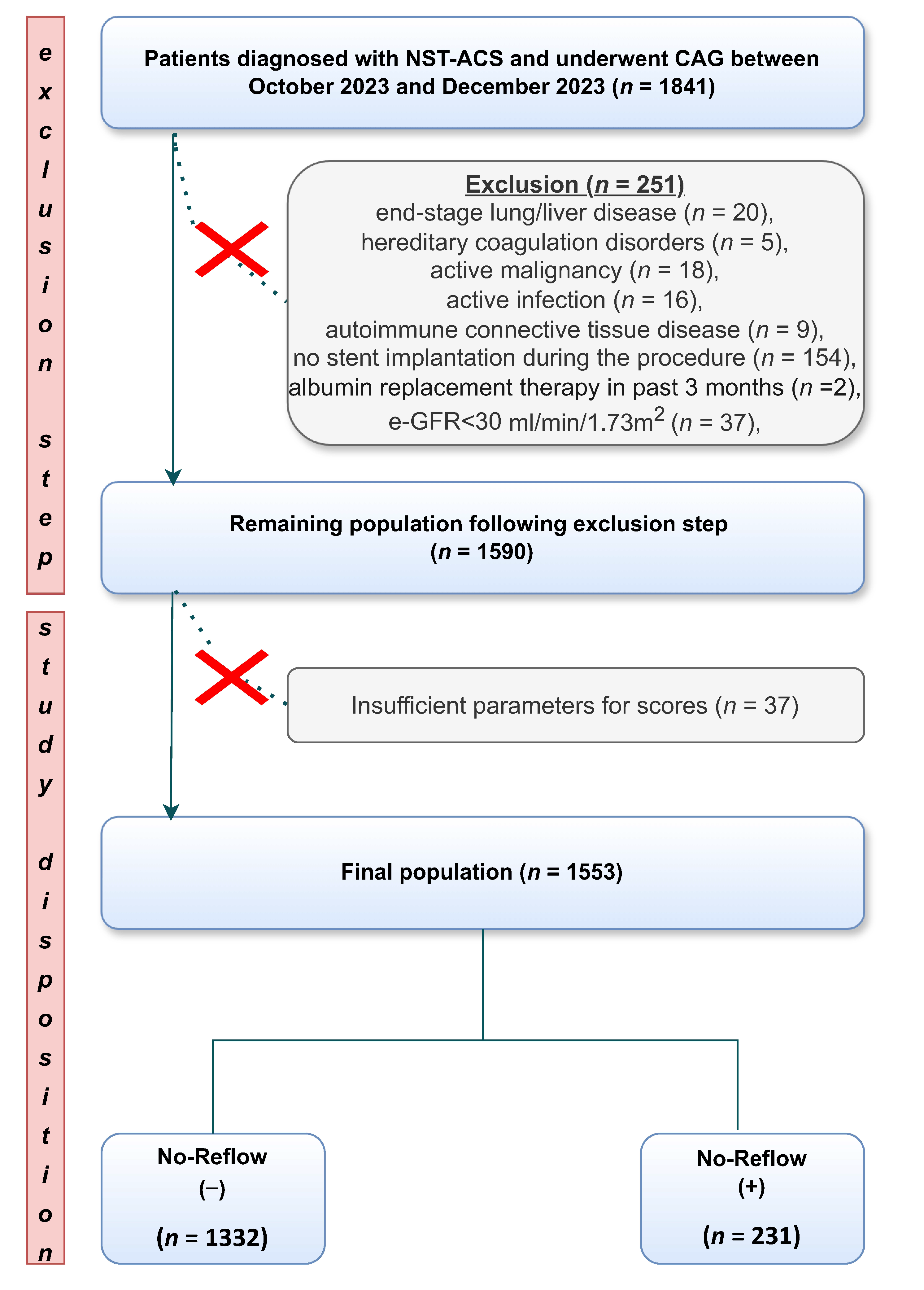
3. Results
3.1. Baseline Characreristics, Procedural Data, and Outcomes
3.2. Feature Selection and Modeling with the XGBoost Machine Learning Algorithm
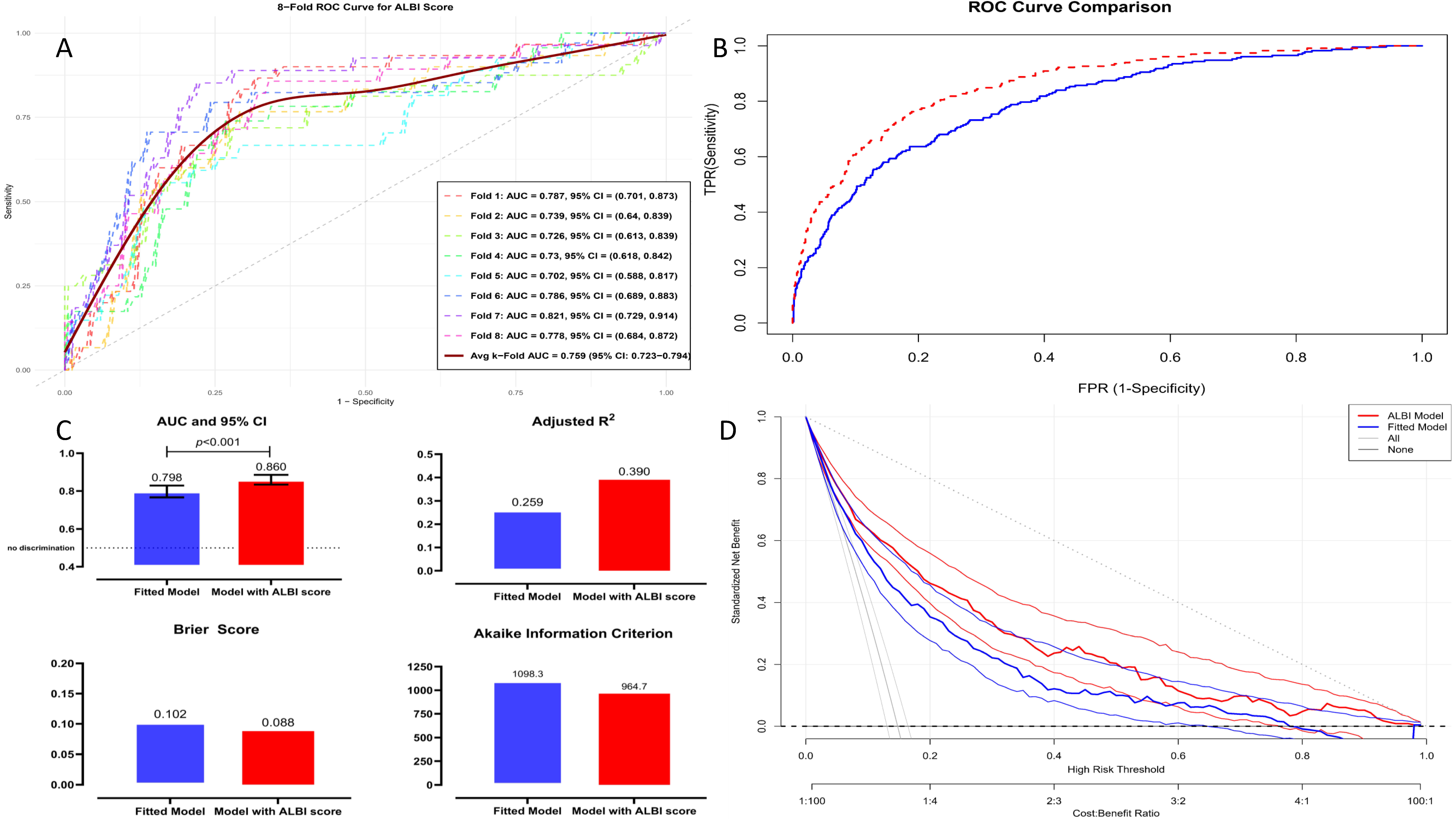
3.3. Discrimination, Calibration, and Performance Evaluation of the XGBoost Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Spadafora, L.; Betti, M.; D’Ascenzo, F.; De Ferrari, G.; De Filippo, O.; Gaudio, C.; Collet, C.; Sabouret, P.; Agostoni, P.; Zivelonghi, C.; et al. Impact of In-Hospital Bleeding on Post-Discharge Therapies and Prognosis in Acute Coronary Syndromes. J. Cardiovasc. Pharmacol. 2025. epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Fajar, J.K.; Heriansyah, T.; Rohman, M.S. The predictors of no reflow phenomenon after percutaneous coronary intervention in patients with ST elevation myocardial infarction: A meta-analysis. Indian Heart J. 2018, 70, S406–S418. [Google Scholar] [CrossRef]
- Ndrepepa, G.; Kastrati, A. Coronary No-Reflow after Primary Percutaneous Coronary Intervention—Current Knowledge on Pathophysiology, Diagnosis, Clinical Impact and Therapy. J. Clin. Med. 2023, 12, 5592. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, R.; Charron, T.; Puley, G.; Dick, A.; Strauss, B.H. Microvascular obstruction and the no-reflow phenomenon after percutaneous coronary intervention. Circulation 2008, 117, 3152–3156. [Google Scholar] [CrossRef]
- Annibali, G.; Scrocca, I.; Aranzulla, T.C.; Meliga, E.; Maiellaro, F.; Musumeci, G. “No-Reflow” Phenomenon: A Contemporary Review. J. Clin. Med. 2022, 11, 2233. [Google Scholar] [CrossRef] [PubMed]
- Harbalıoğlu, H.; Genç, Ö.; Alıcı, G.; Quisi, A.; Yıldırım, A. Impact of HAT2CH2 Score on the development of no-reflow phenomenon in patients with ST-segment elevation myocardial infarction. Angiology 2024, 75, 44–53. [Google Scholar] [CrossRef]
- Pantea-Roșan, L.R.; Pantea, V.A.; Bungau, S.; Tit, D.M.; Behl, T.; Vesa, C.M.; Bustea, C.; Moleriu, R.D.; Rus, M.; Popescu, M.I. No-reflow after PPCI—A predictor of short-term outcomes in STEMI patients. J. Clin. Med. 2020, 9, 2956. [Google Scholar] [CrossRef]
- Johnson, P.J.; Berhane, S.; Kagebayashi, C.; Satomura, S.; Teng, M.; Reeves, H.L.; O’Beirne, J.; Fox, R.; Skowronska, A.; Palmer, D.; et al. Assessment of liver function in patients with hepatocellular carcinoma: A new evidence-based approach-the ALBI grade. J. Clin. Oncol. 2015, 33, 550–558. [Google Scholar] [CrossRef]
- Yamada, S.; Kaneshiro, T.; Yoshihisa, A.; Nodera, M.; Amami, K.; Nehashi, T.; Takeishi, Y. Albumin-bilirubin score for prediction of outcomes in heart failure patients treated with cardiac resynchronization therapy. J. Clin. Med. 2021, 10, 5378. [Google Scholar] [CrossRef]
- Jiang, M.; Wei, X.-b.; Huang, J.-l.; Lin, Y.-w.; Yu, D.-q. Albumin-bilirubin score to predict outcomes in patients with idiopathic dilated cardiomyopathy. Arq. Bras. Cardiol. 2022, 118, 1108–1115. [Google Scholar] [CrossRef]
- Evlice, M.; Bedir, Ö.; Coşkun, M.; Paçacı, E.; Cerşit, S.; Öcal, L.; Gürsoy, M.O.; Şen, Ö.; Kurt, İ.H. The relationship between echocardiographic parameters and albumin bilirubin (ALBI) score in patients with isolated secundum type atrial septal defect. Echocardiography 2023, 40, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, A.; Omer, G.; Mert, E.; Aslan, E.; Emre, P.; Ahmet, O.; Gokhan, Y.M.; Emre, S.; Yeliz, G.; Omer, S.; et al. Predictive power of ALBI score-based nomogram for 30-day mortality following transcatheter aortic valve implantation. Biomark. Med. 2025. epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.-A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes: Developed by the task force on the management of acute coronary syndromes of the European Society of Cardiology (ESC). Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef]
- Levey, A.S.; Coresh, J.; Greene, T.; Stevens, L.A.; Zhang, Y.; Hendriksen, S.; Kusek, J.W.; Van Lente, F.; Chronic Kidney Disease Epidemiology Collaboration. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann. Intern. Med. 2006, 145, 247–254. [Google Scholar] [CrossRef]
- Group, T.S. The Thrombolysis in Myocardial Infarction (TIMI) trial, phase I findings. N. Engl. J. Med. 1985, 312, 932–936. [Google Scholar]
- Gibson, C.M.; Cannon, C.P.; Daley, W.L.; Dodge Jr, J.T.; Alexander, B.; Marble, S.J.; McCabe, C.H.; Raymond, L.; Fortin, T.; Poole, W.K. TIMI frame count: A quantitative method of assessing coronary artery flow. Circulation 1996, 93, 879–888. [Google Scholar] [CrossRef]
- Gibson, C.M.; de Lemos, J.A.; Murphy, S.A.; Marble, S.J.; McCabe, C.H.; Cannon, C.P.; Antman, E.M.; Braunwald, E. Combination therapy with abciximab reduces angiographically evident thrombus in acute myocardial infarction: A TIMI 14 substudy. Circulation 2001, 103, 2550–2554. [Google Scholar] [CrossRef]
- Chen, T.; Guestrin, C. Xgboost: A scalable tree boosting system. In Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining 2016, San Francisco, CA, USA, 13–17 August 2016; pp. 785–794. [Google Scholar]
- Pencina, M.J.; D’Agostino Sr, R.B.; Steyerberg, E.W. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat. Med. 2011, 30, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Genç, Ö.; Yıldırım, A.; Erdoğan, A. A biomarker for estimating no-reflow phenomenon in PCI-treated non-ST-segment elevation myocardial infarction patients: Serum Cystatin C. Anatol. Curr. Med. J. 2024, 6, 108–115. [Google Scholar] [CrossRef]
- Fedai, H.; Sariisik, G.; Toprak, K.; Taşcanov, M.B.; Efe, M.M.; Arğa, Y.; Doğanoğulları, S.; Gez, S.; Demirbağ, R. A Machine Learning Model for the Prediction of No-Reflow Phenomenon in Acute Myocardial Infarction Using the CALLY Index. Diagnostics 2024, 14, 2813. [Google Scholar] [CrossRef]
- Armillotta, M.; Bergamaschi, L.; Paolisso, P.; Belmonte, M.; Angeli, F.; Sansonetti, A.; Stefanizzi, A.; Bertolini, D.; Bodega, F.; Amicone, S.; et al. Prognostic Relevance of Type 4a Myocardial Infarction and Periprocedural Myocardial Injury in Patients with Non-ST-Segment-Elevation Myocardial Infarction. Circulation 2025, 151, 760–772. [Google Scholar] [CrossRef]
- Sanfilippo, A.; La Manna, A.; Tamburino, C. Prognostic impact of no-reflow following acute myocardial infarction. E-J. ESC Counc. Cardiol. Pract. 2011, 9, 20. [Google Scholar]
- Yarlioglues, M.; Karacali, K.; Ilhan, B.C.; Oner, D.Y. A retrospective study: Association of C-reactive protein and uric acid to albumin ratio with the no-reflow phenomenon in patients with ST elevation myocardial infarction. Int. J. Cardiol. 2024, 397, 131621. [Google Scholar] [CrossRef]
- Gresele, P.; Deckmyn, H.; Huybrechts, E.; Vermylen, J. Serum albumin enhances the impairment of platelet aggregation with thromboxane synthase inhibition by increasing the formation of prostaglandin D2. Biochem. Pharmacol. 1984, 33, 2083–2088. [Google Scholar] [CrossRef] [PubMed]
- Roche, M.; Rondeau, P.; Singh, N.R.; Tarnus, E.; Bourdon, E. The antioxidant properties of serum albumin. FEBS Lett. 2008, 582, 1783–1787. [Google Scholar] [CrossRef]
- Arques, S. Serum albumin and cardiovascular disease: State-of-the-art review. Ann. Cardiol. d’Angéiol. 2020, 69, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Hashizume, N.; Kanzaki, Y.; Maruyama, T.; Kozuka, A.; Yahikozawa, K. Prognostic significance of serum albumin in patients with stable coronary artery disease treated by percutaneous coronary intervention. PLoS ONE 2019, 14, e0219044. [Google Scholar] [CrossRef]
- Çınar, T.; Şaylık, F.; Hayıroğlu, M.İ.; Asal, S.; Selçuk, M.; Çiçek, V.; Tanboğa, İ.H. The association of serum uric acid/albumin ratio with no-reflow in patients with ST elevation myocardial infarction. Angiology 2023, 74, 381–386. [Google Scholar] [CrossRef]
- Karabağ, Y.; Çağdaş, M.; Rencuzogullari, I.; Karakoyun, S.; Artaç, İ.; İliş, D.; Yesin, M.; Çağdaş, Ö.S.; Altıntaş, B.; Burak, C. Usefulness of the C-reactive protein/albumin ratio for predicting no-reflow in ST-elevation myocardial infarction treated with primary percutaneous coronary intervention. Eur. J. Clin. Investig. 2018, 48, e12928. [Google Scholar] [CrossRef]
- Oduncu, V.; Erkol, A.; Karabay, C.Y.; Kurt, M.; Akgün, T.; Bulut, M.; Pala, S.; Kirma, C. The prognostic value of serum albumin levels on admission in patients with acute ST-segment elevation myocardial infarction undergoing a primary percutaneous coronary intervention. Coron. Artery Dis. 2013, 24, 88–94. [Google Scholar] [CrossRef]
- Serdar, Z.; Serdar, A.; Altin, A.; Eryilmaz, U.; Albayrak, S. The relation between oxidant and antioxidant parameters and severity of acute coronary syndromes. Acta Cardiol. 2007, 62, 373–380. [Google Scholar] [CrossRef]
- Fearon, I.M.; Faux, S.P. Oxidative stress and cardiovascular disease: Novel tools give (free) radical insight. J. Mol. Cell. Cardiol. 2009, 47, 372–381. [Google Scholar] [CrossRef]
- Cathcart, M.K. Regulation of superoxide anion production by NADPH oxidase in monocytes/macrophages: Contributions to atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Rader, D.J.; Daugherty, A. Translating molecular discoveries into new therapies for atherosclerosis. Nature 2008, 451, 904–913. [Google Scholar] [CrossRef] [PubMed]
- Hadi, H.A.; Carr, C.S.; Al Suwaidi, J. Endothelial dysfunction: Cardiovascular risk factors, therapy, and outcome. Vasc. Health Risk Manag. 2005, 1, 183–198. [Google Scholar]
- Freedman, J.E. Oxidative stress and platelets. Arterioscler. Thromb. Vasc. Biol. 2008, 28, s11–s16. [Google Scholar] [CrossRef]
- Esenboğa, K.; Kurtul, A.; Yamantürk, Y.Y.; Tan, T.S.; Tutar, D.E. Systemic immune-inflammation index predicts no-reflow phenomenon after primary percutaneous coronary intervention. Acta Cardiol. 2022, 77, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Neuzil, J.; Stocker, R. Free and albumin-bound bilirubin are efficient co-antioxidants for alpha-tocopherol, inhibiting plasma and low density lipoprotein lipid peroxidation. J. Biol. Chem. 1994, 269, 16712–16719. [Google Scholar] [CrossRef]
- Nakayama, M.; Takahashi, K.; Komaru, T.; Fukuchi, M.; Shioiri, H.; Sato, K.-i.; Kitamuro, T.; Shirato, K.; Yamaguchi, T.; Suematsu, M. Increased expression of heme oxygenase-1 and bilirubin accumulation in foam cells of rabbit atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1373–1377. [Google Scholar] [CrossRef]
- Okuhara, K.; Kisaka, T.; Ozono, R.; Kurisu, S.; Inoue, I.; Soga, J.; Yano, Y.; Oshima, T.; Kihara, Y.; Yoshizumi, M. Change in bilirubin level following acute myocardial infarction is an index for heme oxygenase activation. South. Med. J. 2010, 103, 876–881. [Google Scholar] [CrossRef]
- Wu, T.-W.; Wu, J.; Li, R.-K.; Mickle, D.; Carey, D. Albumin-bound bilirubins protect human ventricular myocytes against oxyradical damage. Biochem. Cell Biol. 1991, 69, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Marcantoni, E.; Di Francesco, L.; Dovizio, M.; Bruno, A.; Patrignani, P. Novel Insights into the Vasoprotective Role of Heme Oxygenase-1. Int. J. Hypertens. 2012, 2012, 127910. [Google Scholar] [CrossRef] [PubMed]
- Celik, T.; Kaya, M.G.; Akpek, M.; Yarlioglues, M.; Sarli, B.; Topsakal, R.; Gibson, C.M. Does serum bilirubin level on admission predict TIMI flow grade and in-hospital MACE in patients with STEMI undergoing primary PCI. Angiology 2014, 65, 198–204. [Google Scholar] [CrossRef]
- Hamur, H.; Duman, H.; Bakirci, E.M.; Kucuksu, Z.; Demirelli, S.; Kalkan, K.; Degirmenci, H. Bilirubin levels and thrombus burden in patients with ST-segment elevation myocardial infarction. Angiology 2016, 67, 565–570. [Google Scholar] [CrossRef]
- Sahin, O.; Akpek, M.; Elcik, D.; Karadavut, S.; Simsek, V.; Tulmac, M.; Orscelik, O.; Calapkorur, B.; Ergin, A.; Kaya, M.G. Bilirubin levels and the burden of coronary atherosclerosis in patients with STEMI. Angiology 2013, 64, 200–204. [Google Scholar] [CrossRef]
- Allen, L.A.; Felker, G.M.; Pocock, S.; McMurray, J.J.; Pfeffer, M.A.; Swedberg, K.; Wang, D.; Yusuf, S.; Michelson, E.L.; Granger, C.B. Liver function abnormalities and outcome in patients with chronic heart failure: Data from the Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) program. Eur. J. Heart Fail. 2009, 11, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Shakiba, M.; Salari, A.; Mirbolouk, F.; Sotudeh, N.; Nikfarjam, S. Clinical, Laboratory, and Procedural Predictors of No-Reflow in Patients Undergoing Primary Percutaneous Coronary Intervention. J. Tehran Heart Cent. 2020, 15, 50–56. [Google Scholar] [CrossRef]
- Gasior, M.; Gierlotka, M.; Lekston, A.; Wilczek, K.; Zebik, T.; Hawranek, M.; Wojnar, R.; Szkodzinski, J.; Piegza, J.; Dyrbus, K. Comparison of outcomes of direct stenting versus stenting after balloon predilation in patients with acute myocardial infarction (DIRAMI). Am. J. Cardiol. 2007, 100, 798–805. [Google Scholar] [CrossRef]



| Variable | Pooled (n = 1563) | No-Reflow (−) (n = 1332) | No-Reflow (+) (n = 231) | p-Value * | |
|---|---|---|---|---|---|
| Basic characteristics and admission parameters | |||||
| Age, years | 62.3 ± 10.9 | 61.9 ± 10.8 | 65.2 ± 10.8 | <0.001 | |
| Gender, male, n (%) | 733 (46.9) | 604 (45.3) | 129 (55.8) | 0.003 | |
| Systolic blood pressure, mm Hg | 126.9 ± 21.3 | 126.9 ± 20.6 | 127.1 ± 24.8 | 0.899 | |
| Diastolic blood pressure, mm Hg | 82.1 ± 14.5 | 77.6 ± 13.8 | 74.7 ± 15.2 | 0.004 | |
| Heart rate, bpm | 82.1 ± 14.5 | 81.4 ± 13.9 | 86.0 ± 17.4 | <0.001 | |
| Left ventricular ejection fraction, % | 49.1 ± 9.7 | 49.3 ± 9.5 | 47.5 ± 10.3 | 0.009 | |
| Killip classification, n (%) | <0.001 | ||||
| I | 1111 (71.1) | 980 (73.6) | 131 (56.7) | ||
| II | 273 (17.5) | 235 (17.6) | 38 (16.5) | ||
| III | 134 (8.6) | 93 (7.0) | 41 (17.7) | ||
| IV | 45 (2.9) | 24 (1.8) | 21 (9.1) | ||
| Risk factors, n (%) | |||||
| Hypertension | 727 (46.5) | 612 (45.9) | 115 (49.8) | 0.285 | |
| Diabetes mellitus | 692(44.3) | 574 (43.1) | 118 (51.1) | 0.024 | |
| PCI history | 282 (18.0) | 222 (16.7) | 60 (26.0) | <0.001 | |
| CABG | 267 (17.1) | 224 (16.8) | 43 (18.6) | 0.508 | |
| Chronic HF | 194 (12.4) | 146 (11.0) | 48 (20.8) | <0.001 | |
| Hyperlipidemia | 399 (25.5) | 334 (25.1) | 65 (28.1) | 0.327 | |
| Smoking | <0.001 | ||||
| No | 790 (50.5) | 683 (51.3) | 107 (46.3) | ||
| Former | 439 (28.1) | 393 (29.5) | 46 (19.9) | ||
| Current | 334 (21.4) | 256 (19.2) | 78 (33.4) | ||
| Laboratory findings | |||||
| Glucose, mg/dL | 169 ± 88 | 165 ± 85 | 193 ± 100 | <0.001 | |
| Urea, mg/dL | 38.1 ± 17.3 | 36.9 ± 15.7 | 44.8 ± 23.3 | <0.001 | |
| Creatinine, mg/dL | 0.93 ± 0.50 | 0.90 ± 0.48 | 1.08 ± 0.59 | <0.001 | |
| e-GFR, mL/min/1.73 m2 | 85.6 ± 23.1 | 87.3 ± 21.5 | 76.3 ± 29.4 | <0.001 | |
| hs-CRP, mg/L | 5.7 (2.5–12.7) | 5.4 (2.4–11.7) | 8.1 (3.8–18.3) | <0.001 | |
| Albumin, g/dL | 38.6 ± 4.3 | 39.1 ± 4.0 | 35.4 ± 4.9 | <0.001 | |
| ALT, IU/L | 22 (16–31) | 22 (16–31) | 22 (16–35) | 0.652 | |
| AST, IU/L | 30 (22–48) | 30 (22–48) | 30 (22–46) | 0.845 | |
| Total Bilirubin, μmol/L | 9.91 ± 5.89 | 9.46 ± 5.66 | 12.56 ± 6.49 | <0.001 | |
| Sodium, mmol/L | 137.6 ± 3.0 | 137.7 ± 2.9 | 137.1 ± 3.5 | 0.003 | |
| Potassium, mEq/L | 4.3 ± 0.5 | 4.3 ± 0.5 | 4.4 ± 0.5 | 0.010 | |
| White blood cell, ×103/μL | 10.8 ± 3.3 | 10.7 ± 3.2 | 10.9 ± 3.7 | 0.305 | |
| Hemoglobin, g/dL | 13.6 ± 1.9 | 13.7 ± 1.8 | 12.8 ± 2.1 | <0.001 | |
| Platelets, ×103/μL | 260 ± 75 | 260 ± 72 | 265 ± 85 | 0.341 | |
| hs-TnI, ng/L | 857 (225–2798) | 681 (193–2567) | 2552 (720–7071) | <0.001 | |
| CK-MB, ng/mL | 12.9 (6.1–25.8) | 13.0 (6.4–25.8) | 12.3 (5.2–28.2) | 0.275 | |
| HDL-C, mg/dL | 42 ± 12 | 42 ± 12 | 42 ± 11 | 0.948 | |
| LDL-C, mg/dL | 129 ± 34 | 130 ± 33 | 120 ± 35 | <0.001 | |
| Total-C, mg/dL | 193 ± 46 | 194 ± 45 | 184 ± 48 | 0.003 | |
| Triglycerides, mg/dL | 148 (102–206) | 105 (102–207) | 143 (99–203) | 0.556 | |
| Medications taken at the time of admission, n (%) | |||||
| Acetylsalicylic acid | 194 (12.4) | 177 (13.3) | 17 (7.4) | 0.010 | |
| ACEi or ARB | 643 (41.1) | 527 (39.6) | 116 (50.2) | 0.002 | |
| Beta-blockers | 192 (12.3) | 175 (13.1) | 17 (7.4) | 0.012 | |
| Statins | 158 (10.1) | 117 (8.8) | 41 (17.7) | <0.001 | |
| Oral anticoagulants | 42 (2.7) | 40 (3.0) | 2 (0.9) | 0.102 | |
| GRACE Score | 137 ± 30 | 137.5 ± 29 | 137 ± 32 | 0.916 | |
| ALBI Score | −2.66 ± 0.40 | −2.72 ± 0.37 | −2.31 ± 0.44 | <0.001 | |
| Variable | Pooled (n = 1563) | No-Reflow (−) (n = 1332) | No-Reflow (+) (n = 231) | p-Value * | |
|---|---|---|---|---|---|
| Culprit vessel, n (%) | 0.255 | ||||
| LM | 38 (2.4) | 31 (2.3) | 7 (3.0) | ||
| LAD | 609 (39.0) | 523 (39.3) | 86 (37.2) | ||
| LCx | 323 (20.7) | 264 (19.8) | 59 (25.5) | ||
| RCA | 428 (27.4) | 374 (28.1) | 54 (23.4) | ||
| Graft | 165 (10.6) | 140 (10.5) | 25 (10.8) | ||
| Thrombus burden, n (%) | 0.425 | ||||
| Low (0–1–2–3) | 925 (59.2) | 794 (59.6) | 131 (56.7) | ||
| High (4–5) | 638 (40.6) | 538 (40.4) | 100 (43.3) | ||
| GP IIb/IIIa inhibitor infusion, n (%) | 249 (15.9) | 119 (8.9) | 130 (56.3) | <0.001 | |
| In-hospital shock, n (%) | 88 (5.6) | 62 (4.7) | 26 (11.3) | <0.001 | |
| Malignant arrhythmia, n (%) | 114 (7.3) | 66 (5.0) | 48 (20.8) | <0.001 | |
| Pre-dilatation, n (%) | 1122 (71.8) | 989 (74.2) | 133 (57.6) | <0.001 | |
| Multi-vessel disease, n (%) | 312 (20.0) | 252 (18.9) | 60 (26.0) | 0.016 | |
| In-hospital mortality, n (%) | 87 (5.6) | 58 (4.4) | 29 (12.6) | <0.001 | |
| High-dose statin, n (%) | 1471 (94.1) | 1247 (93.6) | 224 (97.0) | 0.048 | |
| Antiplatelet therapy, n (%) | 0.053 | ||||
| Clopidogrel | 235 (15.0) | 208(15.6) | 27 (11.7) | ||
| Ticagrelor | 1264 (80.9) | 1075 (80.7) | 189 (81.9) | ||
| Prasugrel | 64 (4.1) | 49 (3.7) | 15 (6.5) | ||
| Variable | Co-Variate Effect | Fitted Model | ALBI Model | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value * | OR | 95% CI | p-Value * | OR | 95% CI | p-Value * | |
| hs-cTnI | 1.00 | 1.00–1.00 | <0.001 | 1.00 | 1.00–1.00 | <0.001 | 1.00 | 1.00–1.00 | <0.001 |
| e-GFR | 0.98 | 0.98–0.98 | <0.001 | 0.99 | 0.98–1.00 | 0.053 | 0.99 | 0.99–1.00 | 0.236 |
| Pre-dilatation, yes | 0.47 | 0.35–0.63 | <0.001 | 0.24 | 0.14–0.40 | <0.001 | 0.24 | 0.14–0.40 | <0.001 |
| Hemoglobin | 0.78 | 0.72–0.84 | <0.001 | 0.82 | 0.75–0.90 | <0.001 | 0.84 | 0.77–0.93 | <0.001 |
| Glucose | 1.00 | 1.00–1.01 | <0.001 | 1.00 | 1.00–1.00 | 0.056 | 1.00 | 1.00–1.00 | 0.078 |
| CK-MB | 0.99 | 0.99–1.00 | 0.576 | 0.99 | 0.98–0.99 | 0.011 | 0.99 | 0.99–1.00 | 0.043 |
| Malignant arrhythmia, yes | 0.96 | 3.31–7.40 | <0.001 | 4.58 | 2.92–7.17 | <0.001 | 4.61 | 2.81–7.56 | <0.001 |
| Statins use at admission, yes | 2.24 | 1.52–3.30 | <0.001 | 3.45 | 2.18–5.43 | <0.001 | 4.10 | 2.51–6.71 | <0.001 |
| Sodium | 0.93 | 0.90–0.98 | 0.004 | 0.97 | 0.92–1.03 | 0.342 | 0.95 | 0.90–1.01 | 0.101 |
| Triglycerides | 1.00 | 0.99–1.00 | 0.918 | 1.00 | 0.99–1.00 | 0.549 | 1.00 | 1.00–1.00 | 0.238 |
| LVEF | 0.98 | 0.97–1.00 | 0.009 | 1.00 | 0.98–1.02 | 0.872 | 1.00 | 0.98–1.03 | 0.480 |
| GRACE score, continuous | 1.00 | 0.99–1.00 | 0.916 | 0.99 | 0.99–1.01 | 0.835 | 1.00 | 0.99–1.01 | 0.303 |
| Thrombus burden, from 0 to 5, reference: 0 | - | - | <0.001 + | - | - | <0.001 + | - | - | 0.128 + |
| ALBI score, continuous | 10.28 | 7.16–14.79 | <0.001 | - | - | - | 12.10 | 7.75–18.89 | <0.001 |
| Discrimination and Reclassification | ||||||||
|---|---|---|---|---|---|---|---|---|
| Goodness of Fit | Net Reclassification Improvement | Integrated Discrimination Index | Median Improvement | |||||
| C-Index (95% CI) | p-Value * | NRI Index (95% CI) | p-Value * | IDI Index (95% CI) | p-Value * | Median Improvement Index (95% CI) | p-Value * | |
| Fitted model § | 0.799 (0.764–0.829) | <0.001 | ref | - | ref | - | - | - |
| ALBI model ‖ | 0.850 (0.831–0.884) | <0.001 | 0.145 (0.102–0.189) | 0.002 | 0.111 (0.085–0.136) | <0.001 | 0.105 (0.082–0.146) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yildirim, A.; Coskun, M.; Demirtas, A.O. The Predictive Value of ALBI Score for No-Reflow in Non-ST Elevation Acute Coronary Syndrome. J. Clin. Med. 2025, 14, 3035. https://doi.org/10.3390/jcm14093035
Yildirim A, Coskun M, Demirtas AO. The Predictive Value of ALBI Score for No-Reflow in Non-ST Elevation Acute Coronary Syndrome. Journal of Clinical Medicine. 2025; 14(9):3035. https://doi.org/10.3390/jcm14093035
Chicago/Turabian StyleYildirim, Abdullah, Mukremin Coskun, and Abdullah Orhan Demirtas. 2025. "The Predictive Value of ALBI Score for No-Reflow in Non-ST Elevation Acute Coronary Syndrome" Journal of Clinical Medicine 14, no. 9: 3035. https://doi.org/10.3390/jcm14093035
APA StyleYildirim, A., Coskun, M., & Demirtas, A. O. (2025). The Predictive Value of ALBI Score for No-Reflow in Non-ST Elevation Acute Coronary Syndrome. Journal of Clinical Medicine, 14(9), 3035. https://doi.org/10.3390/jcm14093035






