PCSK9 Inhibitors “Fast Track” Use Versus “Stepwise” Lipid-Lowering Therapy in Patients with Acute Coronary Syndrome: A Retrospective Single-Center Study in a “Real-World” Population
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Study Treatment
2.3. Study Endpoints
2.4. Statistical Analysis
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van der Bijl, P.; Abou, R.; Goedemans, L.; Gersh, B.J.; Holmes, D.R., Jr.; Marsan, N.A.; Delgado, V.; Bax, J.J. Left Ventricular Post-Infarct Remodeling: Implications for Systolic Function Improvement and Outcomes in the Modern Era. JACC Heart Fail. 2020, 8, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Sabatine, M.S.; De Ferrari, G.M.; Giugliano, R.P.; Huber, K.; Lewis, B.S.; Ferreira, J.; Kuder, J.F.; Murphy, S.A.; Wiviott, S.D.; Kurtz, C.E.; et al. Clinical Benefit of Evolocumab by Severity and Extent of Coronary Artery Disease: Analysis From FOURIER. Circulation 2018, 138, 756–766. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, G.G.; Steg, P.G.; Szarek, M.; Bhatt, D.L. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome. N. Engl. J. Med. 2018, 379, 2097–2107. [Google Scholar] [CrossRef] [PubMed]
- Laufs, U.; Catapano, A.L.; De Caterina, R.; Schiele, F.; Sionis, A.; Zaman, A.; Jukema, J.W. The effect of the 2019 ESC/EAS dyslipidaemia guidelines on low-density lipoprotein cholesterol goal achievement in patients with acute coronary syndromes: The ACS EuroPath IV project. Vasc. Pharmacol. 2023, 148, 107141. [Google Scholar] [CrossRef]
- Ferlini, M.; Munafò, A.; Varbella, F.; Delnevo, F.; Solli, M.; Trabattoni, D.; Piccaluga, E.; Cardile, A.; Canova, P.; Rossini, R.; et al. Achievement of target LDL-cholesterol level in patients with acute coronary syndrome undergoing percutaneous coronary intervention: The JET-LDL registry. Int. J. Cardiol. 2024, 397, 131659. [Google Scholar] [CrossRef]
- Krychtiuk, K.A.; Ahrens, I.; Drexel, H.; Halvorsen, S.; Hassager, C.; Huber, K. Acute LDL-C reduction post ACS: Strike early and strike strong: From evidence to clinical practice. A clinical consensus statement of the Association for Acute CardioVascular Care (ACVC), in collaboration with the European Association of Preventive Cardiology (EAPC) and the European Society of Cardiology Working Group on Cardiovascular Pharmacotherapy. Eur. Heart J. Acute Cardiovasc. Care 2022, 11, 939–949. [Google Scholar] [CrossRef]
- Fogacci, F.; Giovannini, M.; Grandi, E.; Imbalzano, E. Management of High-Risk Hypercholesterolemic Patients and PCSK9 Inhibitors Reimbursement Policies: Data from a Cohort of Italian Hypercholesterolemic Outpatients. J. Clin. Med. 2022, 11, 4701. [Google Scholar] [CrossRef]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef]
- Leucker, T.M.; Blaha, M.J.; Jones, S.R.; Vavuranakis, M.A.; Williams, M.S.; Lai, H.; Schindler, T.H.; Latina, J.; Schulman, S.P.; Gerstenblith, G. Effect of Evolocumab on Atherogenic Lipoproteins During the Peri- and Early Postinfarction Period: A Placebo-Controlled, Randomized Trial. Circulation 2020, 142, 419–421. [Google Scholar] [CrossRef]
- Mehta, S.R.; Pare, G.; Lonn, E.M.; Jolly, S.S.; Natarajan, M.K.; Pinilla-Echeverri, N.; Schwalm, J.D.; Sheth, T.N.; Sibbald, M.; Tsang, M.; et al. Effects of routine early treatment with PCSK9 inhibitors in patients undergoing primary percutaneous coronary intervention for ST-segment elevation myocardial infarction: A randomised, double-blind, sham-controlled trial. EuroIntervention 2022, 18, e888–e896. [Google Scholar] [CrossRef]
- Räber, L.; Ueki, Y.; Otsuka, T.; Losdat, S.; Häner, J.D.; Lonborg, J.; Fahrni, G.; Iglesias, J.F.; van Geuns, R.J.; Ondracek, A.S.; et al. Effect of Alirocumab Added to High-Intensity Statin Therapy on Coronary Atherosclerosis in Patients With Acute Myocardial Infarction: The PACMAN-AMI Randomized Clinical Trial. JAMA 2022, 327, 1771–1781. [Google Scholar] [CrossRef] [PubMed]
- Koskinas, K.C.; Windecker, S.; Pedrazzini, G.; Mueller, C.; Cook, S.; Matter, C.M.; Muller, O.; Häner, J.; Gencer, B.; Crljenica, C.; et al. Evolocumab for Early Reduction of LDL Cholesterol Levels in Patients with Acute Coronary Syndromes (EVOPACS). J. Am. Coll. Cardiol. 2019, 74, 2452–2462. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Kataoka, Y.; Nissen, S.E.; Prati, F.; Windecker, S.; Puri, R.; Hucko, T.; Aradi, D.; Herrman, J.R.; Hermanides, R.S.; et al. Effect of Evolocumab on Coronary Plaque Phenotype and Burden in Statin-Treated Patients Following Myocardial Infarction. JACC Cardiovasc. Imaging 2022, 15, 1308–1321. [Google Scholar] [CrossRef] [PubMed]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Serruys, P.W.; Onuma, Y.; Garg, S.; Sarno, G.; van den Brand, M.; Kappetein, A.P.; Van Dyck, N.; Mack, M.; Holmes, D.; Feldman, T.; et al. Assessment of the SYNTAX score in the Syntax study. EuroIntervention 2009, 5, 50–56. [Google Scholar] [CrossRef]
- Ference, B.A.; Ginsberg, H.N.; Graham, I.; Ray, K.K.; Packard, C.J.; Bruckert, E.; Hegele, R.A.; Krauss, R.M.; Raal, F.J.; Schunkert, H.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2017, 38, 2459–2472. [Google Scholar] [CrossRef] [PubMed]
- Muccioli, S.; Giglio, C.; Annibali, G.; Cerutti, E.; Civera, S.; Casati, R.; Delnevo, F.; De Rosa, C.; Bongioanni, S.; Colopi, M.; et al. The importance of intensive lipid-lowering therapy after acute coronary syndrome: Changing the paradigm to improve the achievement of targets. Eur. Heart J. 2022, 23, 553–561. [Google Scholar] [CrossRef]
- Yamashita, S.; Sakamoto, A.; Shoji, S. Feasibility of Short-Term Aggressive Lipid-Lowering Therapy with the PCSK9 Antibody in Acute Coronary Syndrome. J. Cardiovasc. Dev. Dis. 2023, 10, 204. [Google Scholar] [CrossRef]
- Deng, Y.; Ma, Y.; Zhang, Y.; Gao, J.; Sun, X.; He, S.; Zhu, L.; Zhang, J. The impact of early in-hospital use of PCSK9 inhibitors on cardiovascular outcomes in acute coronary syndrome patients: A systematic review and meta-analysis. Int. J. Cardiol. 2024, 399, 131775. [Google Scholar] [CrossRef]
- Biccirè, F.G.; Häner, J.; Losdat, S.; Ueki, Y.; Shibutani, H.; Otsuka, T.; Kakizaki, R.; Hofbauer, T.M.; van Geuns, R.J.; Stortecky, S.; et al. Concomitant Coronary Atheroma Regression and Stabilization in Response to Lipid-Lowering Therapy. J. Am. Coll. Cardiol. 2023, 82, 1737–1747. [Google Scholar] [CrossRef]
- Wu, C.; Lin, D.; Ji, J.; Jiang, Y.; Jiang, F.; Wang, Y. PCSK9 Inhibition Regulates Infarction-Induced Cardiac Myofibroblast Transdifferentiation via Notch1 Signaling. Cell Biochem. Biophys. 2023, 81, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Franchi, F.; Ortega-Paz, L.; Rollini, F.; Been, L.; Rivas, A.; Maaliki, N.; Zhou, X.; Pineda, A.M.; Suryadevara, S.; Soffer, D.; et al. Impact of evolocumab on the pharmacodynamic profiles of clopidogrel in patients with atherosclerotic cardiovascular disease: A randomised, double-blind, placebo-controlled study. EuroIntervention 2023, 18, 1254–1265. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Zhao, Y.M.; He, N.Q.; Gao, R.; Xu, W.X.; Zhuo, X.J.; Ren, Z.; Wu, C.Y.; Liu, L.S. PCSK9: A emerging participant in heart failure. Biomed. Pharmacother.=Biomed. Pharmacother. 2023, 158, 114106. [Google Scholar] [CrossRef]
- Tiller, C.; Holzknecht, M. Association of Circulating PCSK9 with Ischemia-Reperfusion Injury in Acute ST-Elevation Myocardial Infarction. Circ. Cardiovasc. Imaging 2024, 17, e016482. [Google Scholar] [CrossRef]
- Benedetti, A. Is PCSK9 the Key Player in the Ischemia-Reperfusion Match? Circ. Cardiovasc. Imaging 2024, 17, e017210. [Google Scholar] [CrossRef]
- Gao, J.; Liu, J.Y.; Lu, P.J.; Xiao, J.Y.; Gao, M.D.; Li, C.P.; Cui, Z.; Liu, Y. Effects of Evolocumab Added to Moderate-Intensity Statin Therapy in Chinese Patients With Acute Coronary Syndrome: The EMSIACS Trial Study Protocol. Front. Physiol. 2021, 12, 750872. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Wang, X.; Zhou, J.; Wang, D.; Pang, Y.; Xu, X.; Sang, Z.; Zhang, Y.; Zhang, J.; Wu, S.; et al. Impact of early PCSK9 inhibitor treatment on heart after percutaneous coronary intervention in patients with STEMI: Design and rationale of the PERFECT II trial. Front. Cardiovasc. Med. 2022, 9, 1009674. [Google Scholar] [CrossRef]
- Dias, C.S.; Shaywitz, A.J.; Wasserman, S.M.; Smith, B.P.; Gao, B.; Stolman, D.S.; Crispino, C.P.; Smirnakis, K.V.; Emery, M.G.; Colbert, A.; et al. Effects of AMG 145 on low-density lipoprotein cholesterol levels: Results from 2 randomized, double-blind, placebo-controlled, ascending-dose phase 1 studies in healthy volunteers and hypercholesterolemic subjects on statins. J. Am. Coll. Cardiol. 2012, 60, 1888–1898. [Google Scholar] [CrossRef]
- Johannesen, C.D.L.; Mortensen, M.B.; Langsted, A.; Nordestgaard, B.G. Apolipoprotein B and Non-HDL Cholesterol Better Reflect Residual Risk Than LDL Cholesterol in Statin-Treated Patients. J. Am. Coll. Cardiol. 2021, 77, 1439–1450. [Google Scholar] [CrossRef]
- Hagström, E.; Steg, P.G.; Szarek, M.; Bhatt, D.L.; Bittner, V.A.; Danchin, N.; Diaz, R.; Goodman, S.G.; Harrington, R.A.; Jukema, J.W.; et al. Apolipoprotein B, Residual Cardiovascular Risk After Acute Coronary Syndrome, and Effects of Alirocumab. Circulation 2022, 146, 657–672. [Google Scholar] [CrossRef]
- Schwartz, G.G.; Szarek, M.; Bittner, V.A.; Diaz, R.; Goodman, S.G.; Jukema, J.W.; Landmesser, U.; López-Jaramillo, P.; Manvelian, G.; Pordy, R.; et al. Lipoprotein(a) and Benefit of PCSK9 Inhibition in Patients With Nominally Controlled LDL Cholesterol. J. Am. Coll. Cardiol. 2021, 78, 421–433. [Google Scholar] [CrossRef] [PubMed]

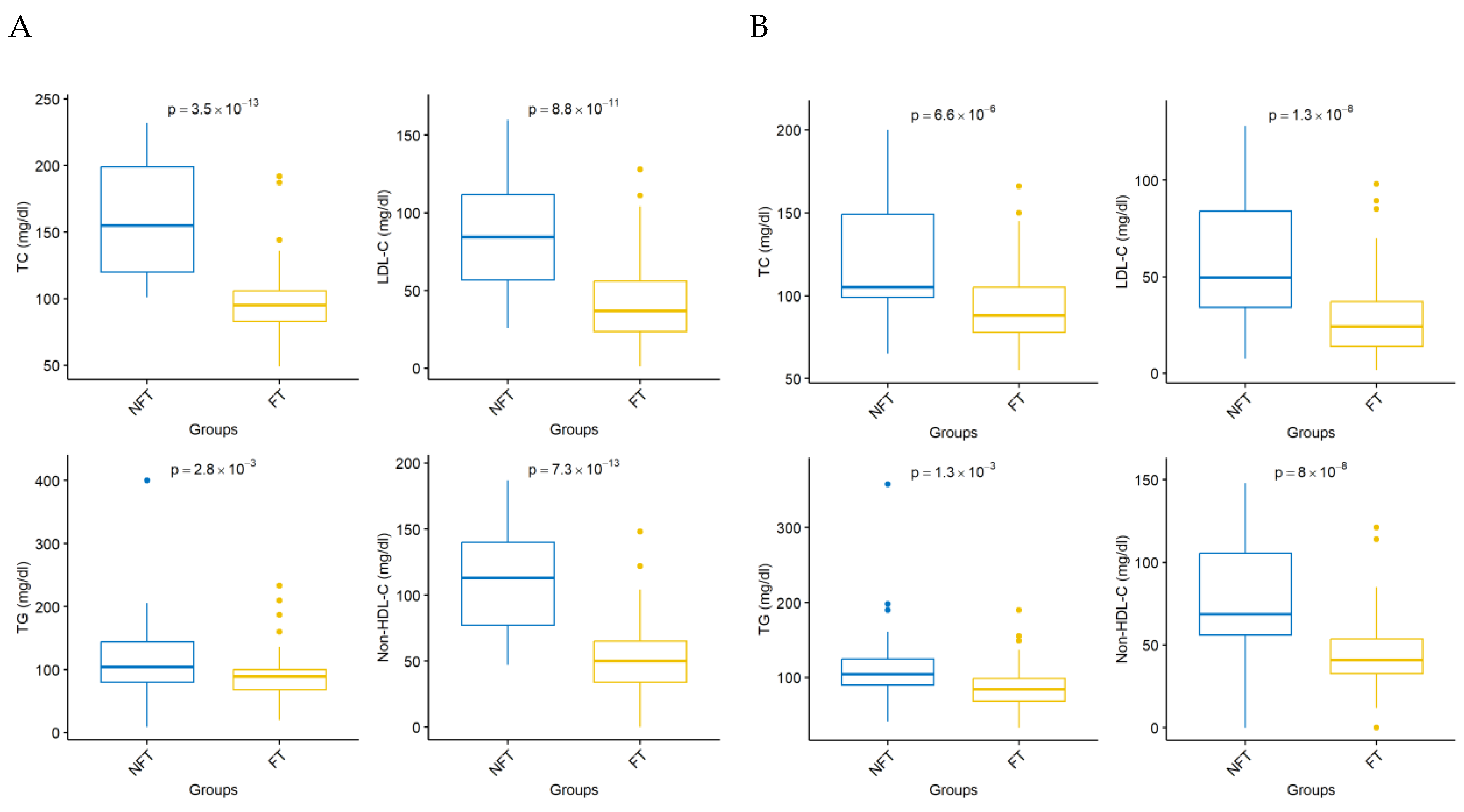
| Variable | PCSK9i FT | PCSK9i NFT | p-Value |
|---|---|---|---|
| Sex, n (%) | 0.25 | ||
| Male | 47 (73.4) | 50 (82.0) | |
| Female | 17 (26.6) | 11 (18.0) | |
| Familiarity, n (%) | 0.02 | ||
| no | 35 (54.7) | 46 (75.4) | |
| yes | 29 (45.3) | 15 (24.6) | |
| Diabetes mellitus, n (%) | 0.21 | ||
| no | 54 (84.4) | 46 (75.4) | |
| yes | 10 (15.6) | 15 (24.6) | |
| Smoker, n (%) | 0.82 | ||
| no | 19 (29.7) | 20 (32.8) | |
| current | 43 (67.2) | 40 (65.6) | |
| former | 2 (3.1) | 1 (1.6) | |
| Arterial Hypertension, n (%) | 0.02 | ||
| no | 11 (17.2) | 22 (36.1) | |
| yes | 53 (82.8) | 39 (63.9) | |
| Diagnosis, n (%) | 0.02 | ||
| Unstable angina | 4 (6.2) | 0 (0.0) | |
| NSTEMI | 7 (10.9) | 1 (1.7) | |
| STEMI | 53 (82.8) | 57 (98.3) | |
| LLT naive, n (%) | 0.23 | ||
| yes | 10 (15.3) | 16 (25.4) | |
| no | 55 (84.6) | 47 (74.6) | |
| CASS score, n (%) | 0.86 | ||
| 0 | 1 (1.6) | 1 (1.8) | |
| 1 | 23 (35.9) | 18 (31.6) | |
| 2 | 20 (31.2) | 22 (38.6) | |
| 3 | 20 (31.2) | 16 (28.1) | |
| Proximal segment, n (%) | 0.47 | ||
| no | 25 (39.1) | 19 (32.8) | |
| yes | 39 (60.9) | 39 (67.2) | |
| OCT, n (%) | 0.3 | ||
| no | 59 (92.2) | 56 (96.6) | |
| yes | 5 (7.8) | 2 (3.4) | |
| IVUS, n (%) | 0.2 | ||
| no | 55 (85.9) | 54 (93.1) | |
| yes | 9 (14.1) | 4 (6.9) |
| Variable | PCSK9i FT | PCSK9i NFT | |||||
|---|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | p-Value | |
| BMI | 64 | 28.028 | 4.589 | 58 | 28.444 | 4.642 | 0.560 |
| Age | 64 | 57.094 | 10.033 | 61 | 62.180 | 11.275 | 0.008 |
| Stent total lenght | 64 | 70.375 | 41.177 | 57 | 68.579 | 40.008 | 0.780 |
| Stent maximum diameter | 64 | 3.523 | 0.654 | 58 | 3.759 | 0.647 | 0.032 |
| LVEF at admission | 63 | 43.714 | 7.408 | 60 | 43.250 | 6.964 | 0.860 |
| LVEF at discharge | 63 | 47.429 | 7.411 | 60 | 46.017 | 9.438 | 0.550 |
| Glucose blood level at admission | 63 | 119.429 | 67.956 | 60 | 132.333 | 69.353 | 0.220 |
| Glucose blood level at discharge | 63 | 92.889 | 21.350 | 60 | 105.983 | 50.613 | 0.400 |
| TC day 0 | 64 | 200.969 | 42.636 | 62 | 192.806 | 47.382 | 0.370 |
| LDL-C day 0 | 64 | 128.444 | 40.112 | 62 | 121.526 | 43.525 | 0.310 |
| TG day 0 | 64 | 133.719 | 56.501 | 62 | 135.113 | 62.916 | 0.950 |
| HDL day 0 | 64 | 45.781 | 11.182 | 62 | 44.258 | 10.504 | 0.410 |
| Non-HDL-C day 0 | 64 | 155.188 | 44.272 | 62 | 148.548 | 46.656 | 0.530 |
| TC day 30 | 53 | 98.208 | 27.862 | 57 | 156.246 | 39.723 | |
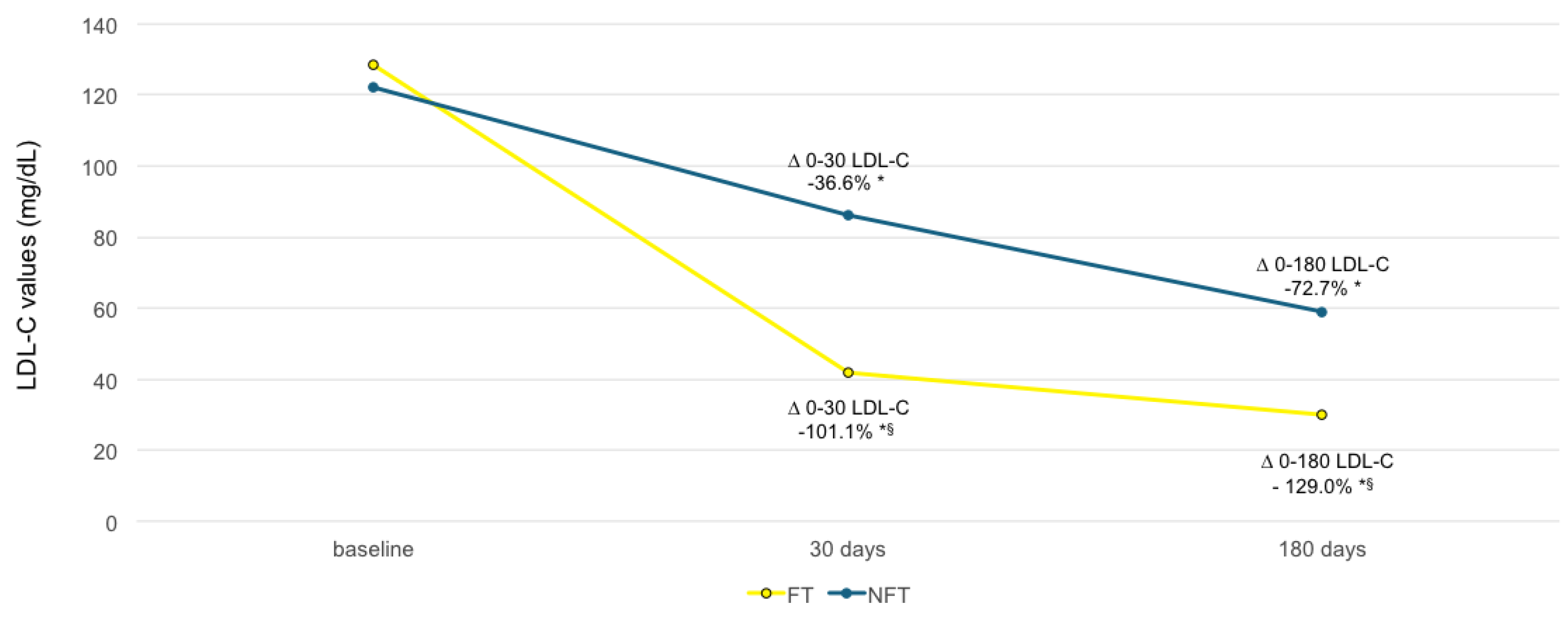
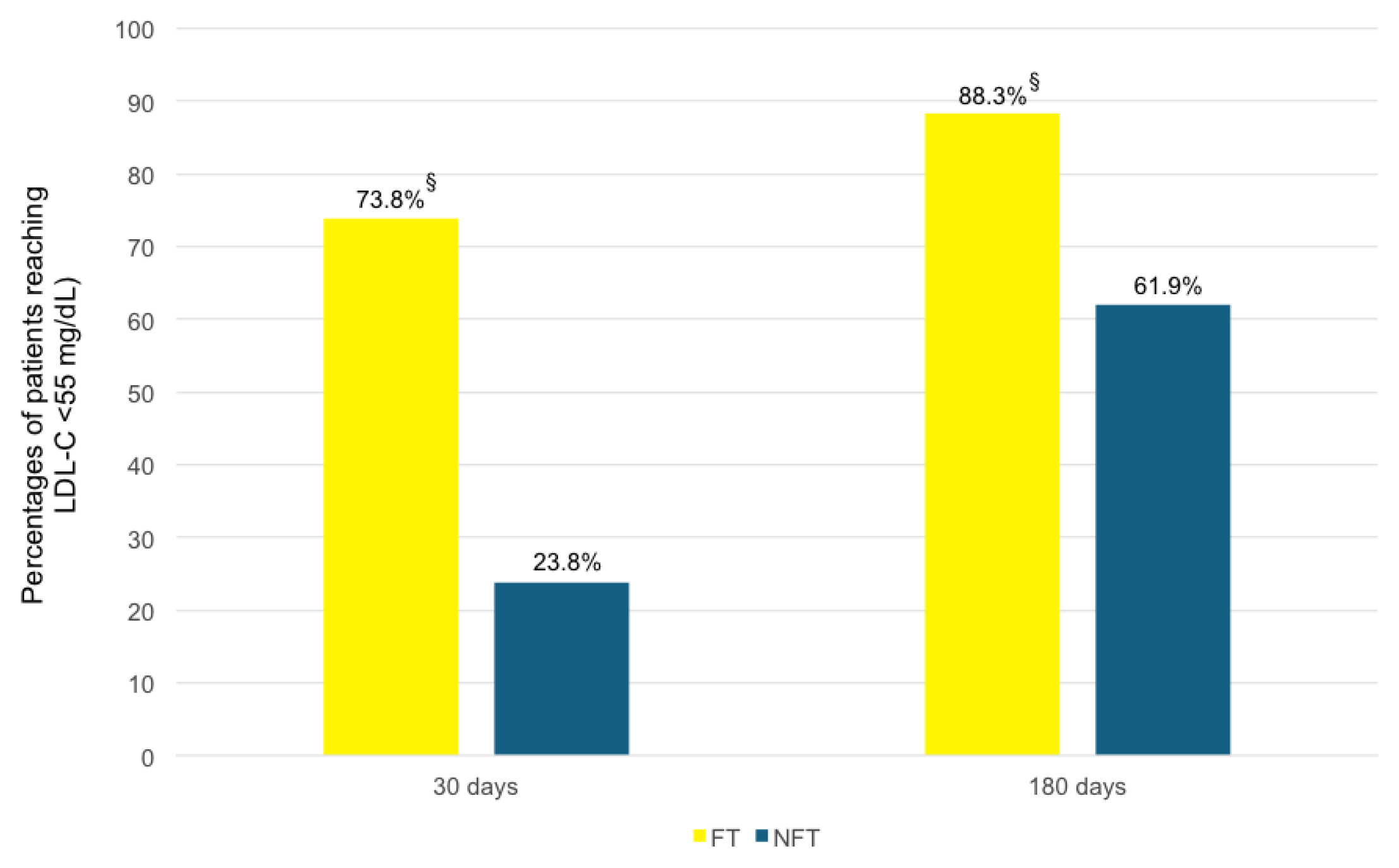
| LDL-C day 30 | 60 | 41.563 | 27.573 | 62 | 85.590 | 35.896 | <0.001 |
| TG day 30 | 53 | 90.679 | 39.675 | 57 | 117.404 | 57.631 | 0.003 |
| HDL day 30 | 53 | 42.830 | 10.493 | 57 | 45.544 | 10.712 | 0.140 |
| Non-HDL-C day 30 | 55 | 53.364 | 28.731 | 57 | 111.491 | 38.068 | <0.001 |
| TC day 180 | 51 | 92.588 | 23.417 | 53 | 122.226 | 36.227 | <0.001 |
| LDL-C day 180 | 59 | 29.617 | 21.026 | 61 | 59.049 | 32.405 | <0.001 |
| TG day 180 | 47 | 89.319 | 33.620 | 51 | 110.882 | 47.908 | 0.001 |
| HDL day 180 | 49 | 48.653 | 11.300 | 53 | 43.453 | 11.150 | 0.019 |
| Non-HDL-C day 180 | 52 | 44.962 | 22.913 | 54 | 77.315 | 35.193 | <0.001 |
| MACEs, Adverse Reactions, and Discontinuation of Therapy | PCSK9i FT | PCSK9i NFT | |
|---|---|---|---|
| In-hospital | Death | 0 | 1 |
| Myocardial infarction | 0 | 0 | |
| Ischemia-driven revascularization | 0 | 0 | |
| Stent thrombosis | 0 | 0 | |
| Adverse reactions (injection-site reaction or pain, fatigue, headache, influenza, and illness) | 0 | 0 | |
| Discontinuation of therapy | 0 | 0 | |
| During follow-up | Death | 0 | 1 |
| Myocardial infarction | 1 | 2 | |
| Ischemia-driven revascularization | 1 | 0 | |
| Stent thrombosis | 0 | 1 | |
| Adverse reactions (injection-site reaction or pain, fatigue, headache, influenza, and illness) | 0 | 0 | |
| Discontinuation of therapy | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Andrea, D.; Capone, V.; Bellis, A.; Castaldo, R.; Franzese, M.; Carpinella, G.; Furbatto, F.; La Rocca, F.; Marsico, F.; Marfella, R.; et al. PCSK9 Inhibitors “Fast Track” Use Versus “Stepwise” Lipid-Lowering Therapy in Patients with Acute Coronary Syndrome: A Retrospective Single-Center Study in a “Real-World” Population. J. Clin. Med. 2025, 14, 2992. https://doi.org/10.3390/jcm14092992
D’Andrea D, Capone V, Bellis A, Castaldo R, Franzese M, Carpinella G, Furbatto F, La Rocca F, Marsico F, Marfella R, et al. PCSK9 Inhibitors “Fast Track” Use Versus “Stepwise” Lipid-Lowering Therapy in Patients with Acute Coronary Syndrome: A Retrospective Single-Center Study in a “Real-World” Population. Journal of Clinical Medicine. 2025; 14(9):2992. https://doi.org/10.3390/jcm14092992
Chicago/Turabian StyleD’Andrea, Davide, Valentina Capone, Alessandro Bellis, Rossana Castaldo, Monica Franzese, Gerardo Carpinella, Fulvio Furbatto, Fulvio La Rocca, Fabio Marsico, Raffaele Marfella, and et al. 2025. "PCSK9 Inhibitors “Fast Track” Use Versus “Stepwise” Lipid-Lowering Therapy in Patients with Acute Coronary Syndrome: A Retrospective Single-Center Study in a “Real-World” Population" Journal of Clinical Medicine 14, no. 9: 2992. https://doi.org/10.3390/jcm14092992
APA StyleD’Andrea, D., Capone, V., Bellis, A., Castaldo, R., Franzese, M., Carpinella, G., Furbatto, F., La Rocca, F., Marsico, F., Marfella, R., Paolisso, G., Paolisso, P., Fumagalli, C., Cappiello, M., Bossone, E., & Mauro, C. (2025). PCSK9 Inhibitors “Fast Track” Use Versus “Stepwise” Lipid-Lowering Therapy in Patients with Acute Coronary Syndrome: A Retrospective Single-Center Study in a “Real-World” Population. Journal of Clinical Medicine, 14(9), 2992. https://doi.org/10.3390/jcm14092992








