The Effects of a Pre-Extubation Single Recruitment Maneuver on Ultrasonographic Lung Conditions in Patients Undergoing Lateral Decubitus Surgery: A Randomized Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
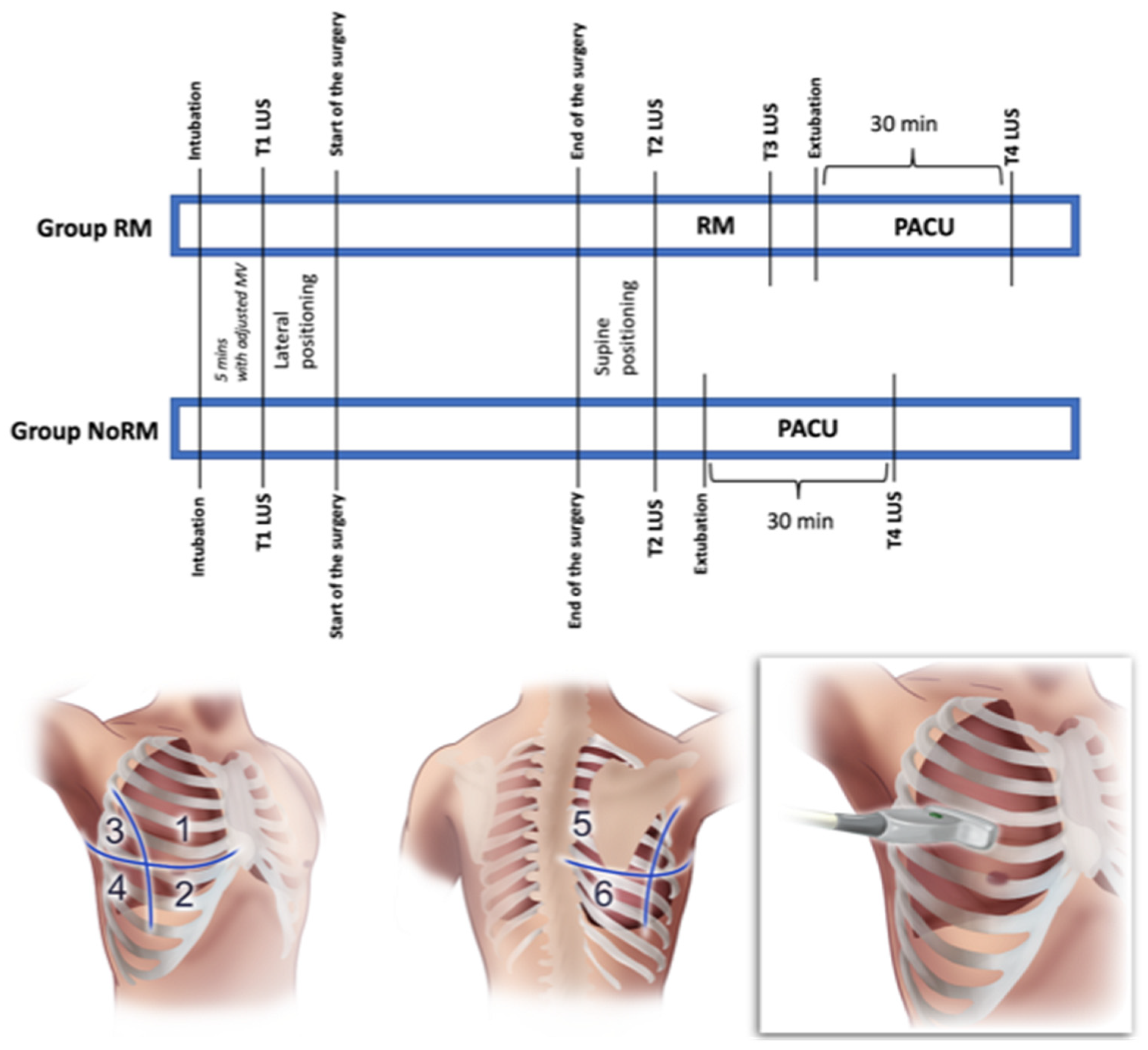
2.1. Study Design
2.2. Perioperative Anesthesiologic Care of the Patients
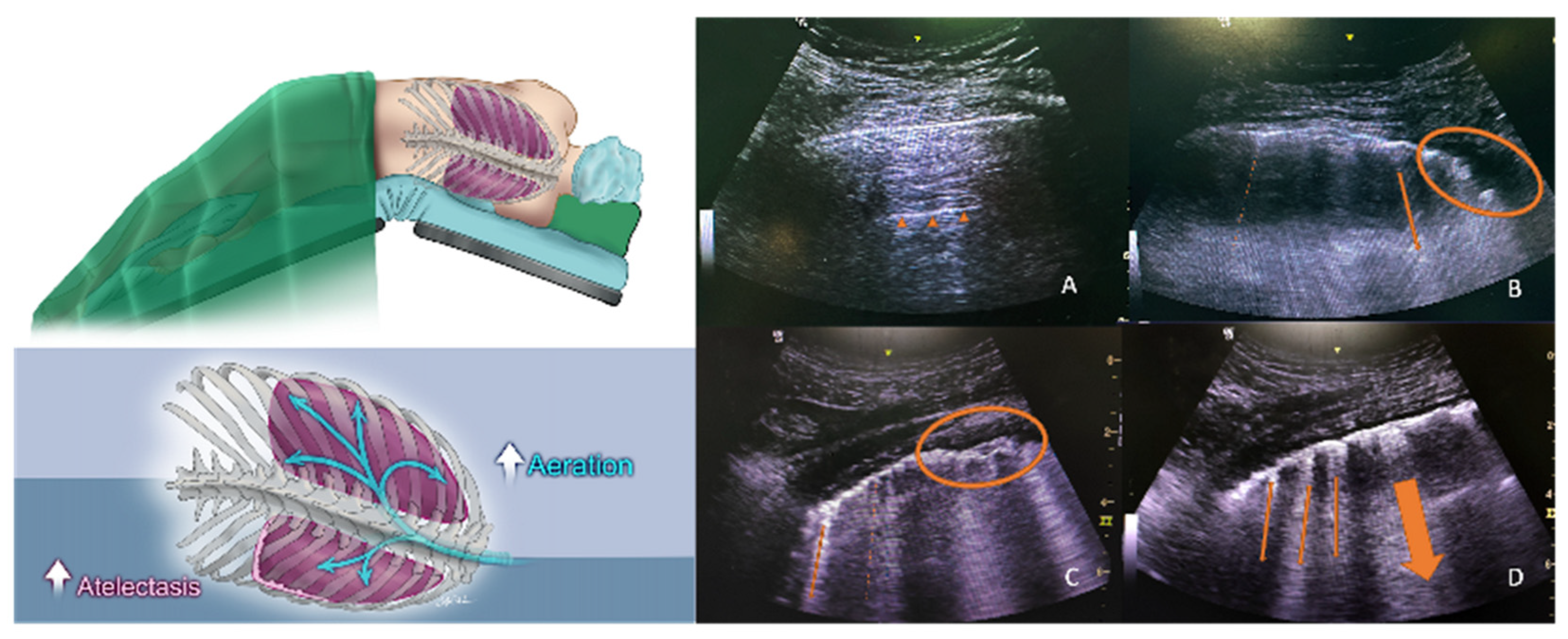
2.3. Recruitment Maneuver and Lung Ultrasound Score (LUS) Assessment
2.4. Outcome Measures
2.5. Statistical Analyses
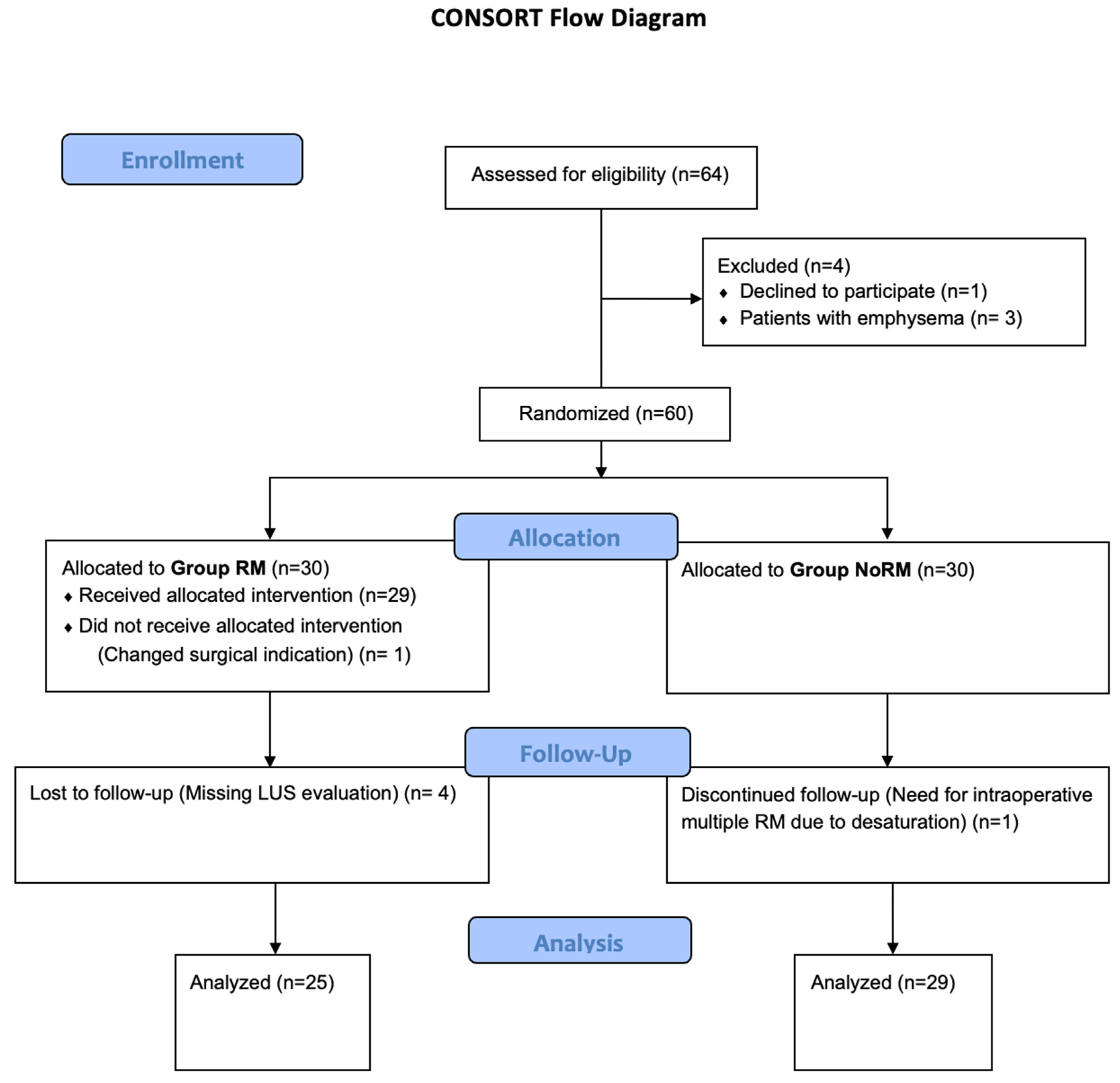
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RM | recruitment maneuver |
| LUS | Lung Ultrasound Score |
| PPC | postoperative pulmonary complication |
| PEEP | positive end-expiratory pressure |
| ARISCAT | Analysis of Risk Index for Surgical Comorbidities and Anesthetic Technique |
| MAC | minimum alveolar concentration |
| PACU | Post-Anesthesia Care Unit |
| ASA | American Society of Anesthesiologists Physical Status |
| IV | intravenous |
References
- Duggan, M.; Kavanagh, B.P. Pulmonary atelectasis: A pathogenic perioperative entity. Anesthesiology 2005, 102, 838–854. [Google Scholar] [CrossRef]
- Jo, Y.Y.; Kim, J.Y.; Kwak, Y.L.; Kim, Y.B.; Kwak, H.J. The effect of pressure-controlled ventilation on pulmonary mechanics in the prone position during posterior lumbar spine surgery: A comparison with volume-controlled ventilation. J. Neurosurg. Anesthesiol. 2012, 24, 14–18. [Google Scholar]
- Lynch, S.; Brand, L.; Levy, A. Changes in lung thorax compliance during orthopedic surgery. Anesthesiology 1959, 20, 278–282. [Google Scholar] [PubMed]
- Larsson, A.; Jonmarker, C.; Lindahl, S.G.; Werner, O. Lung function in the supine and lateral decubitus positions in anaesthetized infants and children. Br. J. Anaesth. 1989, 62, 378–384. [Google Scholar] [CrossRef]
- Lagier, D.; Zeng, C.; Fernandez-Bustamante, A.; Vidal Melo, M.F. Perioperative Pulmonary Atelectasis: Part II. Clinical Implications. Anesthesiology 2022, 136, 206–236. [Google Scholar] [PubMed]
- Futier, E.; Marret, E.; Jaber, S. Perioperative positive pressure ventilation: An integrated approach to improve pulmonary care. Anesthesiology 2014, 121, 400–408. [Google Scholar]
- Mojoli, F.; Bouhemad, B.; Mongodi, S.; Lichtenstein, D. Lung Ultrasound for Critically Ill Patients. Am. J. Respir. Crit. Care Med. 2019, 199, 701–714. [Google Scholar] [PubMed]
- Roshdy, A. Respiratory Monitoring During Mechanical Ventilation: The Present and the Future. J. Intensive Care Med. 2023, 38, 407–417. [Google Scholar]
- Mongodi, S.; De Luca, D.; Colombo, A.; Stella, A.; Santangelo, E.; Corradi, F.; Gargani, L.; Rovida, S.; Volpicelli, G.; Bouhemad, B.; et al. Quantitative Lung Ultrasound: Technical Aspects and Clinical Applications. Anesthesiology 2021, 134, 949–965. [Google Scholar] [CrossRef]
- Bouhemad, B.; Mongodi, S.; Via, G.; Rouquette, I. Ultrasound for “lung monitoring” of ventilated patients. Anesthesiology 2015, 122, 437–447. [Google Scholar]
- Senturk, M.; Slinger, P.; Cohen, E. Intraoperative mechanical ventilation strategies for one-lung ventilation. Best Pract. Res. Clin. Anaesthesiol. 2015, 29, 357–369. [Google Scholar]
- Kiss, T.; Wittenstein, J.; Becker, C.; Birr, K.; Cinnella, G.; Cohen, E.; El Tahan, M.R.; Falcão, L.F.; Gregoretti, C.; Granell, M.; et al. Protective ventilation with high versus low positive end-expiratory pressure during one-lung ventilation for thoracic surgery (PROTHOR): Study protocol for a randomized controlled trial. Trials 2019, 20, 213. [Google Scholar]
- Jammer, I.B.; Wickboldt, N.; Sander, M.; Smith, A.; Schultz, M.J.; Pelosi, P.; Leva, B.; Rhodes, A.; Hoeft, A.; Walder, B.; et al. Standards for definitions and use of outcome measures for clinical effectiveness research in perioperative medicine: European Perioperative Clinical Outcome (EPCO) definitions: A statement from the ESA-ESICM joint taskforce on perioperative outcome measures. Eur. J. Anaesthesiol. 2015, 32, 88–105. [Google Scholar] [PubMed]
- Park, S.K.; Yang, H.; Yoo, S.; Kim, W.H.; Lim, Y.J.; Bahk, J.H.; Kim, J.T. Ultrasound-guided versus conventional lung recruitment manoeuvres in laparoscopic gynaecological surgery: A randomised controlled trial. Eur. J. Anaesthesiol. 2021, 38, 275–284. [Google Scholar]
- Hansell, L.; Milross, M.; Delaney, A.; Tian, D.H.; Ntoumenopoulos, G. Lung ultrasound has greater accuracy than conventional respiratory assessment tools for the diagnosis of pleural effusion, lung consolidation and collapse: A systematic review. J. Physiother. 2021, 67, 41–48. [Google Scholar] [PubMed]
- Sett, A.; Rogerson, S.R.; Foo, G.W.; Keene, J.; Thomas, N.; Kee, P.P.; Zayegh, A.; Donath, S.M.; Tingay, D.G.; Davis, P.G.; et al. Estimating Preterm Lung Volume: A Comparison of Lung Ultrasound, Chest Radiography, and Oxygenation. J. Pediatr. 2023, 259, 113437. [Google Scholar]
- Maw, A.M.; Hassanin, A.; Ho, P.M.; McInnes, M.D.; Moss, A.; Juarez-Colunga, E.; Soni, N.J.; Miglioranza, M.H.; Platz, E.; DeSanto, K.; et al. Diagnostic Accuracy of Point-of-Care Lung Ultrasonography and Chest Radiography in Adults with Symptoms Suggestive of Acute Decompensated Heart Failure: A Systematic Review and Meta-analysis. JAMA Netw. Open 2019, 2, e190703. [Google Scholar] [PubMed]
- Wang, D.; Qi, Y. Lung ultrasound score and in-hospital mortality of adults with acute respiratory distress syndrome: A meta-analysis. BMC Pulm. Med. 2024, 24, 62. [Google Scholar]
- Genereux, V.; Chasse, M.; Girard, F.; Massicotte, N.; Chartrand-Lefebvre, C.; Girard, M. Effects of positive end-expiratory pressure/recruitment manoeuvres compared with zero end-expiratory pressure on atelectasis during open gynaecological surgery as assessed by ultrasonography: A randomised controlled trial. Br. J. Anaesth. 2020, 124, 101–109. [Google Scholar]
- Monastesse, A.; Girard, F.; Massicotte, N.; Chartrand-Lefebvre, C.; Girard, M. Lung Ultrasonography for the Assessment of Perioperative Atelectasis: A Pilot Feasibility Study. Anesth. Analg. 2017, 124, 494–504. [Google Scholar]
- Mezidi, M.; Guerin, C. Effects of patient positioning on respiratory mechanics in mechanically ventilated ICU patients. Ann. Transl. Med. 2018, 6, 384. [Google Scholar] [CrossRef] [PubMed]
- Canet, J.; Gallart, L.; Gomar, C.; Paluzie, G.; Valles, J.; Castillo, J.; Sabate, S.; Mazo, V.; Briones, Z.; Sanchis, J.; et al. Prediction of postoperative pulmonary complications in a population-based surgical cohort. Anesthesiology 2010, 113, 1338–1350. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zhang, Y.W.; Gao, J.; Fu, H.M.; Si, L.; Gao, Y.T. Effects of lung-protective ventilation strategy on lung aeration loss and postoperative pulmonary complications in moderate-risk patients undergoing abdominal surgery. Minerva Anestesiol. 2021, 87, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Szabo, M.; Bozo, A.; Darvas, K.; Soos, S.; Ozse, M.; Ivanyi, Z.D. The role of ultrasonographic lung aeration score in the prediction of postoperative pulmonary complications: An observational study. BMC Anesthesiol. 2021, 21, 19. [Google Scholar] [CrossRef]
- Touw, H.R.; Parlevliet, K.L.; Beerepoot, M.; Schober, P.; Vonk, A.; Twisk, J.W.; Elbers, P.W.; Boer, C.; Tuinman, P.R. Lung ultrasound compared with chest X-ray in diagnosing postoperative pulmonary complications following cardiothoracic surgery: A prospective observational study. Anaesthesia 2018, 73, 946–954. [Google Scholar] [CrossRef]

| Group RM (+) (n = 25) | Group NoRM (–) (n = 29) | p | |
|---|---|---|---|
| Age (years) | 52 (48, 57) | 57 (51, 64.5) | 0.3 a |
| Gender | 0.2 b | ||
| Female | 14, 56% | 11, 38% | |
| Male | 11, 44% | 18, 62% | |
| BMI (kg/m2) | 29 (26.5, 29) | 27 (24, 29.5) | 0.1 a |
| BMI > 30 (n, %) | 5, 20% | 7, 24% | 0.7 b |
| ASA-PS | 0.06 b | ||
| ASA I | 11, 44% | 5, 17% | |
| ASA II | 13, 52% | 19, 66% | |
| ASA III | 1, 4% | 5, 17% | |
| Functional capacity | 0.1 c | ||
| I | 24, 96% | 23, 79% | |
| II | 1, 4% | 6, 21% | |
| ARISCAT score | 38 (38, 41) | 41 (34, 41) | 0.8 a |
| Comorbidities | |||
| Hypertension | 10, 40% | 14, 48% | 0.5 b |
| Diabetes mellitus | 4, 16% | 4, 19% | 1 c |
| Chronic obstructive pulmonary disease | 1, 4% | 1, 3% | 1 c |
| Smoking | 10, 40% | 9, 31% | 0.5 b |
| Ischemic heart disease | 1, 4% | 3, 14% | 0.4 c |
| Renal failure | 0, 0% | 2, 7% | 0.5 c |
| Endocrinologic disease | 1, 4% | 4, 14% | 0.4 c |
| Postoperative Pulmonary Complications | 6, 24% | 5, 17% | 0.5 b |
| New requirement for O2 or respiratory support | 0 | 1, 3% | |
| Aspiration pneumonitis | 0 | 0 | |
| Pneumonia | 2, 4% | 2, 6% | |
| ARDS | 0 | 0 | |
| Pneumothorax | 0 | 0 | |
| Atelectasis | 4, 8% | 2, 6% | |
| Bronchospasm | 0 | 0 | |
| PACU room air test (+) | 6, 24% | 11, 38% | 0.3 b |
| Surgery duration (min) | 215 (175, 275) | 220 (190, 260) | 0.8 a |
| Anesthesia duration (min) | 240 (210, 300) | 240 (225, 300) | 0.7 a |
| Total fluid administered intraoperatively (mL) | 2500 (2000, 3300) | 2500 (1800, 3100) | 0.4 a |
| Intraoperative first hour PaO2/FiO2 | 255 (245, 320) | 266 (264, 362) | 0.1 a |
| PACU PaO2/FiO2 | 416 (410, 480) | 296 (245, 412) | 0.5 a |
| Lung Ultrasound Score Assessment (LUS) | Group RM | Group NoRM | p |
|---|---|---|---|
| T1 LUS | |||
| Dependent lung lower zones (zones 2+4+6) | 3 (2, 4) | 2 (1, 3) | 0.1 |
| Dependent lung upper zones (zones 1+3+5) | 1 (0, 2) | 1 (0, 2) | 0.8 |
| Dependent lung (total) | 3 (2, 6) | 3 (2, 4) | 0.2 |
| Global (right lung + left lung) | 8 (5, 11.5) | 6 (4, 8.5) | 0.2 |
| T2 LUS | |||
| Dependent lung lower zones (zones 2+4+6) | 8 (6, 8) | 8 (7, 8) | 0.8 |
| Dependent lung upper zones (zones 1+3+5) | 7 (4, 8) | 6 (5, 8) | 0.4 |
| Dependent lung (Total) | 14 (10, 16) | 14 (12, 17) | 0.5 |
| Global (right lung + left lung) | 21 (17, 22.5) | 18 (17, 20) | 0.09 |
| Pre-extubation LUS measurements (T3 in Group RM vs. T2 in Group NoRM) | |||
| Dependent lung lower zones (zones 2+4+6) | 6 (4, 7) | 8 (7, 8) | 0.003 * |
| Dependent lung upper zones (zones 1+3+5) | 4 (2, 6) | 6 (5, 8) | 0.002 * |
| Dependent lung (total) | 10 (6, 13.5) | 14 (12, 17) | 0.002 * |
| Global (right lung + left lung) | 16 (12.5, 17) | 18 (17, 20) | <0.001 * |
| T4 LUS | |||
| Dependent lung lower zones (zones 2+4+6) | 4 (4, 5.5) | 5 (3, 6) | 0.8 |
| Dependent lung upper zones (zones 1+3+5) | 2 (1, 3.5) | 4 (3, 4.5) | 0.01 * |
| Dependent lung (total) | 6 (5, 9.5) | 8 (7, 10) | 0.1 |
| Global (right lung + left lung) | 13 (11, 15.5) | 12 (10, 15.5) | 0.4 |
| Lung Ultrasound Score assessment (LUS) | PPC (+) | PPC (-) | p |
| T1 LUS | |||
| Dependent lung lower zones (zones 2+4+6) | 3 (2, 4) | 2 (1, 3) | 0.1 |
| Dependent lung upper zones (zones 1+3+5) | 1 (0, 3) | 1 (0, 2) | 0.8 |
| Dependent lung (total) | 3 (2, 7) | 3 (2, 4) | 0.4 |
| Global (right lung + left lung) | 7 (4, 13) | 6 (5, 9) | 0.7 |
| T2 LUS (in both groups) | |||
| Dependent lung lower zones (zones 2+4+6) | 7 (5, 8) | 7 (5, 8) | 0.3 |
| Dependent lung upper zones (zones 1+3+5) | 5 (1, 8) | 7 (5, 8) | 0.3 |
| Dependent lung (total) | 12 (6, 16) | 14 (12, 16) | 0.3 |
| Global (right lung + left lung) | 21 (20, 23) | 18 (17, 21) | 0.04 * |
| T4 LUS | |||
| Dependent lung lower zones (zones 2+4+6) | 5 (4, 6) | 4 (3, 5) | 0.3 |
| Dependent lung upper zones (zones 1+3+5) | 3 (1, 6) | 3 (2, 4) | 0.7 |
| Dependent lung (total) | 8 (5, 12) | 7 (6, 10) | 0.4 |
| Global (right lung + left lung) | 16 (14, 19) | 12 (10, 14) | 0.01 * |
| LUS change due to single recruitment maneuver in Group RM | T2 | T3 | |
| Dependent lung lower zones (2+4+6) | 8 (6, 8) | 6 (4, 7) | <0.001 ** |
| Dependent lung upper zones (1+3+5) | 7 (4, 8) | 4 (2, 6) | <0.001 ** |
| Dependent lung (total) | 14 (10, 16) | 10 (6, 13.5) | <0.001 ** |
| Global (right lung + left lung) | 21 (17, 22.5) | 16 (12.5, 17) | <0.001 ** |
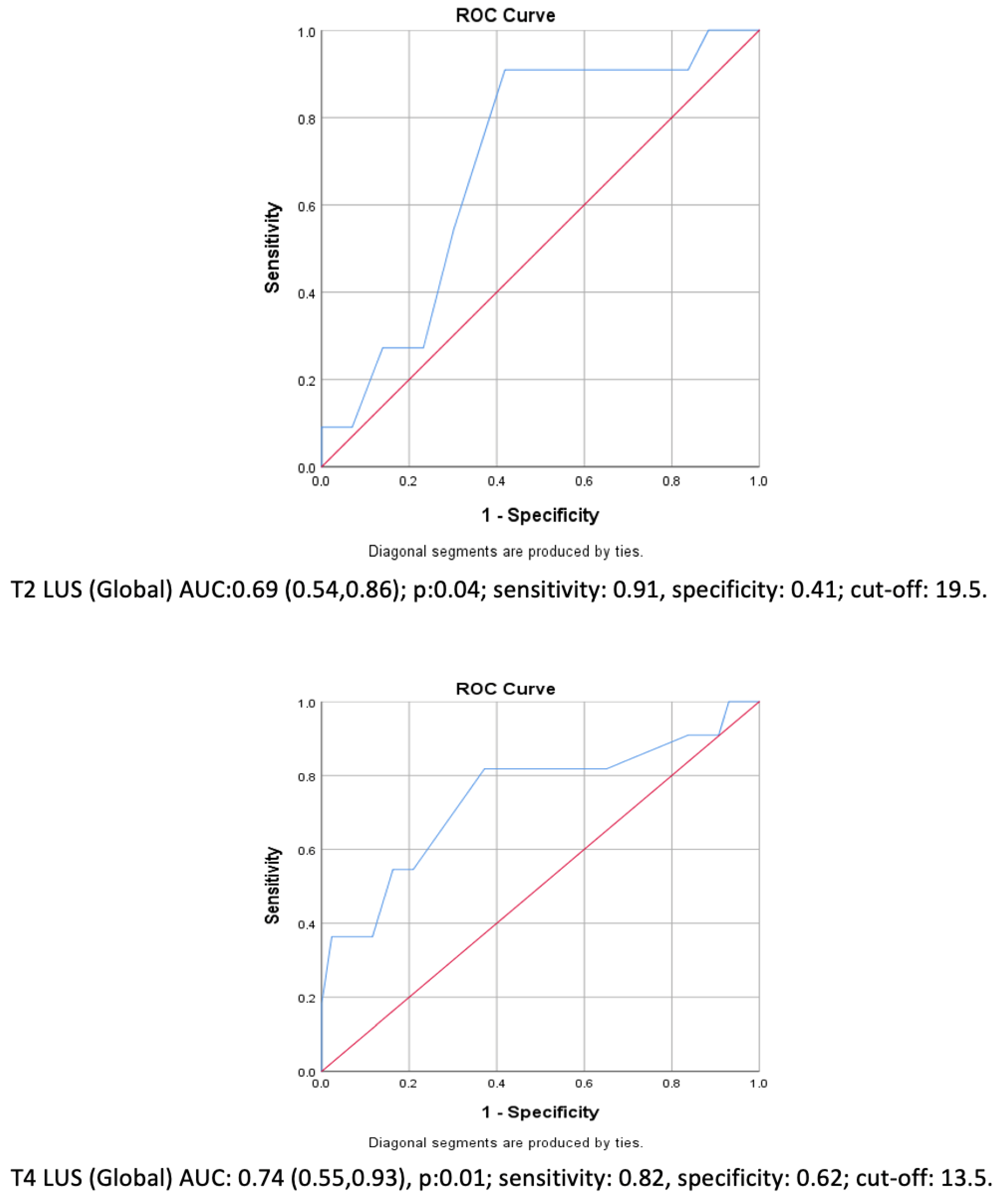
| Whole-Study-Group Regression Analysis (PPC occurrence) | OR (95% CI) | ||
| T2 LUS (in Group NoRM) + T3 LUS (in Group RM) Global > 19 | 10.2 (1.14, 91.2) | 0.04 *** | |
| T4 LUS Global > 13 | 5.2 (0.93, 29.9) | 0.06 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bingül, E.S.; Savran Karadeniz, M.; Canbaz, M.; Şentürk, E.; Uzuntürk, C.; Erdem, S.; Şentürk, N.M. The Effects of a Pre-Extubation Single Recruitment Maneuver on Ultrasonographic Lung Conditions in Patients Undergoing Lateral Decubitus Surgery: A Randomized Clinical Trial. J. Clin. Med. 2025, 14, 2969. https://doi.org/10.3390/jcm14092969
Bingül ES, Savran Karadeniz M, Canbaz M, Şentürk E, Uzuntürk C, Erdem S, Şentürk NM. The Effects of a Pre-Extubation Single Recruitment Maneuver on Ultrasonographic Lung Conditions in Patients Undergoing Lateral Decubitus Surgery: A Randomized Clinical Trial. Journal of Clinical Medicine. 2025; 14(9):2969. https://doi.org/10.3390/jcm14092969
Chicago/Turabian StyleBingül, Emre Sertaç, Meltem Savran Karadeniz, Mert Canbaz, Emre Şentürk, Cansu Uzuntürk, Selçuk Erdem, and Nüzhet M. Şentürk. 2025. "The Effects of a Pre-Extubation Single Recruitment Maneuver on Ultrasonographic Lung Conditions in Patients Undergoing Lateral Decubitus Surgery: A Randomized Clinical Trial" Journal of Clinical Medicine 14, no. 9: 2969. https://doi.org/10.3390/jcm14092969
APA StyleBingül, E. S., Savran Karadeniz, M., Canbaz, M., Şentürk, E., Uzuntürk, C., Erdem, S., & Şentürk, N. M. (2025). The Effects of a Pre-Extubation Single Recruitment Maneuver on Ultrasonographic Lung Conditions in Patients Undergoing Lateral Decubitus Surgery: A Randomized Clinical Trial. Journal of Clinical Medicine, 14(9), 2969. https://doi.org/10.3390/jcm14092969






