How to Evaluate Kidney Function in Elite Endurance Athletes: Pros and Cons of Different Creatinine-Based Formulas
Abstract
1. Introduction
2. Materials and Methods
- (1)
- Skills: archery, equestrian, golf, shooting, figure skating, sailing, curling, diving, surfing and equestrian sports.
- (2)
- Endurance: cycling, rowing, canoeing, triathlon, long-distance running, long-distance swimming (over 800 m), cross-country skiing, pentathlon, biathlon, Nordic combined and long-distance skating.
- -
- -
- MDRD: eGFR = 175 × (serum creatinine−1.154) × (age−0.203) × 1.212 (if black) or\and × 0.742 (if female) [15]
- -
- MCQE: eGFR = exp {1.911 + (5.249/serum creatinine) − (2.114/serum creatinine2) − 0.00686 × age (−0.205 if female)} [19]
- -
- CKD-EPI (Chronic Kidney Disease Epidemiology): eGFR = 141 × min (Scr/κ,1)α × max (Scr/κ, 1)−1.209 × 0.993Age × 1.018 [if female] × 1.159 [if black]; Scr is serum creatinine (mg/dL), κ is 0.7 for females and 0.9 for males, α is −0.329 for females and −0.411 for males, min indicates the minimum of Scr/κ or 1, and max indicates the maximum of Scr/κ or 1 [21].
3. Statistical Analysis
4. Results
4.1. Kidney Function
4.2. Gender Differences
4.3. EGFR Calculated with CG in Female Athletes
5. Discussion
6. Limitations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tidmas, V.; Brazier, J.; Bottoms, L.; Muniz, D.; Desai, T.; Hawkins, J.; Sridharan, S.; Farrington, K. Ultra-Endurance Participation and Acute Kidney Injury: A Narrative Review. Int. J. Environ. Res. Public Health 2022, 19, 16887. [Google Scholar] [CrossRef]
- Lecina, M.; Castellar-Otín, C.; López-Laval, I.; Carrasco Páez, L.; Pradas, F. Acute Kidney Injury and Hyponatremia in Ultra-Trail Racing: A Systematic Review. Medicina 2022, 58, 569. [Google Scholar] [CrossRef]
- Scheer, V.; Tiller, N.B.; Doutreleau, S.; Khodaee, M.; Knechtle, B.; Pasternak, A.; Rojas-Valverde, D. Potential Long-Term Health Problems Associated with Ultra-Endurance Running: A Narrative Review. Sports Med. 2022, 52, 725–740. [Google Scholar] [CrossRef]
- Lipman, G.S.; Krabak, B.J.; Waite, B.L.; Logan, S.B.; Menon, A.; Chan, G.K. A Prospective Cohort Study of Acute Kidney Injury in Multi-Stage Ultramarathon Runners: The Biochemistry in Endurance Runner Study (BIERS). Res. Sports Med. 2014, 22, 185–192. [Google Scholar] [CrossRef]
- Lippi, G.; Schena, F.; Salvagno, G.L.; Tarperi, C.; Montagnana, M.; Gelati, M.; Banfi, G.; Guidi, G.C. Acute Variation of Estimated Glomerular Filtration Rate Following a Half-Marathon Run. Int. J. Sports Med. 2008, 29, 948–951. [Google Scholar] [CrossRef]
- Kao, W.-F.; Hou, S.-K.; Chiu, Y.-H.; Chou, S.-L.; Kuo, F.-C.; Wang, S.-H.; Chen, J.-J. Effects of 100-Km Ultramarathon on Acute Kidney Injury. Clin. J. Sport Med. 2015, 25, 49–54. [Google Scholar] [CrossRef]
- Wołyniec, W.; Ratkowski, W.; Kasprowicz, K.; Jastrzębski, Z.; Małgorzewicz, S.; Witek, K.; Grzywacz, T.; Żmijewski, P.; Renke, M. Glomerular Filtration Rate Is Unchanged by Ultramarathon. J. Strength. Cond. Res. 2018, 32, 3207–3215. [Google Scholar] [CrossRef]
- Bl, K. Laboratory Assessment of Renal Disease: Clearance, Urinalysis, and Renal Biopsy. Kidney 1996, 56, 1137–1174. [Google Scholar]
- Cirillo, M. Rationale, pros and cons of GFR estimation: The Cockcroft-Gault and MDRD equations. G. Ital. Nefrologia 2009, 26, 310–317. [Google Scholar]
- Lin, J.; Knight, E.L.; Hogan, M.L.; Singh, A.K. A Comparison of Prediction Equations for Estimating Glomerular Filtration Rate in Adults without Kidney Disease. J. Am. Soc. Nephrol. 2003, 14, 2573–2580. [Google Scholar] [CrossRef]
- Perrone, R.D.; Madias, N.E.; Levey, A.S. Serum Creatinine as an Index of Renal Function: New Insights into Old Concepts. Clin. Chem. 1992, 38, 1933–1953. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, M.; Anastasio, P.; De Santo, N.G. Relationship of Gender, Age, and Body Mass Index to Errors in Predicted Kidney Function. Nephrol. Dial Transpl. 2005, 20, 1791–1798. [Google Scholar] [CrossRef]
- Levey, A.S.; Inker, L.A.; Coresh, J. GFR Estimation: From Physiology to Public Health. Am. J. Kidney Dis. 2014, 63, 820–834. [Google Scholar] [CrossRef] [PubMed]
- Cockcroft, D.W.; Gault, M.H. Prediction of Creatinine Clearance from Serum Creatinine. Nephron 1976, 16, 31–41. [Google Scholar] [CrossRef]
- Levey, A.S.; Coresh, J.; Greene, T.; Stevens, L.A.; Zhang, Y.L.; Hendriksen, S.; Kusek, J.W.; Van Lente, F. Chronic Kidney Disease Epidemiology Collaboration Using Standardized Serum Creatinine Values in the Modification of Diet in Renal Disease Study Equation for Estimating Glomerular Filtration Rate. Ann. Intern. Med. 2006, 145, 247–254. [Google Scholar] [CrossRef]
- Pierrat, A.; Gravier, E.; Saunders, C.; Caira, M.-V.; Aït-Djafer, Z.; Legras, B.; Mallié, J.-P. Predicting GFR in Children and Adults: A Comparison of the Cockcroft-Gault, Schwartz, and Modification of Diet in Renal Disease Formulas. Kidney Int. 2003, 64, 1425–1436. [Google Scholar] [CrossRef]
- Verhave, J.C.; Fesler, P.; Ribstein, J.; du Cailar, G.; Mimran, A. Estimation of Renal Function in Subjects with Normal Serum Creatinine Levels: Influence of Age and Body Mass Index. Am. J. Kidney Dis. 2005, 46, 233–241. [Google Scholar] [CrossRef]
- Myers, G.L.; Miller, W.G.; Coresh, J.; Fleming, J.; Greenberg, N.; Greene, T.; Hostetter, T.; Levey, A.S.; Panteghini, M.; Welch, M. Recommendations for Improving Serum Creatinine Measurement: A Report from the Laboratory Working Group of the National Kidney Disease Education Program. Clin. Chem. 2006, 52, 5–18. [Google Scholar] [CrossRef]
- Rule, A.D.; Larson, T.S.; Bergstralh, E.J.; Slezak, J.M.; Jacobsen, S.J.; Cosio, F.G. Using Serum Creatinine to Estimate Glomerular Filtration Rate: Accuracy in Good Health and in Chronic Kidney Disease. Ann. Intern. Med. 2004, 141, 929–937. [Google Scholar] [CrossRef]
- Saleem, M.; Florkowski, C.M.; George, P.M. Comparison of the Mayo Clinic Quadratic Equation with the Modification of Diet in Renal Disease Equation and Radionuclide Glomerular Filtration Rate in a Clinical Setting. Nephrology 2008, 13, 684–688. [Google Scholar] [CrossRef]
- Inker, L.A.; Eneanya, N.D.; Coresh, J.; Tighiouart, H.; Wang, D.; Sang, Y.; Crews, D.C.; Doria, A.; Estrella, M.M.; Froissart, M.; et al. New Creatinine- and Cystatin C-Based Equations to Estimate GFR without Race. N. Engl. J. Med. 2021, 385, 1737–1749. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A New Equation to Estimate Glomerular Filtration Rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Montañés Bermúdez, R.; Bover Sanjuán, J.; Oliver Samper, A.; Ballarín Castán, J.A.; Gràcia García, S. Assessment of the new CKD-EPI equation to estimate the glomerular filtration rate. Nefrologia 2010, 30, 185–194. [Google Scholar] [CrossRef]
- Stevens, L.A.; Padala, S.; Levey, A.S. Advances in Glomerular Filtration Rate-Estimating Equations. Curr. Opin. Nephrol. Hypertens. 2010, 19, 298–307. [Google Scholar] [CrossRef]
- Stevens, L.A.; Schmid, C.H.; Greene, T.; Zhang, Y.L.; Beck, G.J.; Froissart, M.; Hamm, L.L.; Lewis, J.B.; Mauer, M.; Navis, G.J.; et al. Comparative Performance of the CKD Epidemiology Collaboration (CKD-EPI) and the Modification of Diet in Renal Disease (MDRD) Study Equations for Estimating GFR Levels above 60 mL/Min/1.73 M2. Am. J. Kidney Dis. 2010, 56, 486–495. [Google Scholar] [CrossRef]
- Carter, J.L.; Stevens, P.E.; Irving, J.E.; Lamb, E.J. Estimating Glomerular Filtration Rate: Comparison of the CKD-EPI and MDRD Equations in a Large UK Cohort with Particular Emphasis on the Effect of Age. QJM Int. J. Med. 2011, 104, 839–847. [Google Scholar] [CrossRef]
- Van den Brand, J.A.J.G.; van Boekel, G.A.J.; Willems, H.L.; Kiemeney, L.A.L.M.; den Heijer, M.; Wetzels, J.F.M. Introduction of the CKD-EPI Equation to Estimate Glomerular Filtration Rate in a Caucasian Population. Nephrol. Dial. Transpl. 2011, 26, 3176–3181. [Google Scholar] [CrossRef]
- Alaini, A.; Malhotra, D.; Rondon-Berrios, H.; Argyropoulos, C.P.; Khitan, Z.J.; Raj, D.S.C.; Rohrscheib, M.; Shapiro, J.I.; Tzamaloukas, A.H. Establishing the Presence or Absence of Chronic Kidney Disease: Uses and Limitations of Formulas Estimating the Glomerular Filtration Rate. World J. Methodol. 2017, 7, 73–92. [Google Scholar] [CrossRef]
- Pelliccia, A.; Sharma, S.; Gati, S.; Bäck, M.; Börjesson, M.; Caselli, S.; Collet, J.-P.; Corrado, D.; Drezner, J.A.; Halle, M.; et al. 2020 ESC Guidelines on Sports Cardiology and Exercise in Patients with Cardiovascular Disease: The Task Force on Sports Cardiology and Exercise in Patients with Cardiovascular Disease of the European Society of Cardiology (ESC). Eur. Heart J. 2021, 42, 17–96. [Google Scholar] [CrossRef]
- Di Gioia, G.; Buzzelli, L.; Maestrini, V.; Nenna, A.; Monosilio, S.; Squeo, M.R.; Lemme, E.; Pelliccia, A. Lipid Profile in Olympic Athletes: Proposal for a “Lipid Athlete Score” as a Clinical Tool to Identify High-Risk Athletes. J. Clin. Med. 2023, 12, 7449. [Google Scholar] [CrossRef]
- Mosteller, R.D. Simplified Calculation of Body-Surface Area. N. Engl. J. Med. 1987, 317, 1098. [Google Scholar] [CrossRef] [PubMed]
- Levin, A.; Stevens, P.E.; Bilous, R.W.; Coresh, J.; De Francisco, A.L.; De Jong, P.E.; Griffith, K.E.; Hemmelgarn, B.R.; Iseki, K.; Lamb, E.J.; et al. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group: KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. Suppl. 2013, 3, 1–150. [Google Scholar] [CrossRef]
- Banfi, G.; Del Fabbro, M. Serum Creatinine Values in Elite Athletes Competing in 8 Different Sports: Comparison with Sedentary People. Clin. Chem. 2006, 52, 330–331. [Google Scholar] [CrossRef] [PubMed]
- Milić, R.; Banfi, G.; Del Fabbro, M.; Dopsaj, M. Serum Creatinine Concentrations in Male and Female Elite Swimmers. Correlation with Body Mass Index and Evaluation of Estimated Glomerular Filtration Rate. Clin. Chem. Lab. Med. 2011, 49, 285–289. [Google Scholar] [CrossRef]
- Vikse, B.E.; Vollset, S.E.; Tell, G.S.; Refsum, H.; Iversen, B.M. Distribution and Determinants of Serum Creatinine in the General Population: The Hordaland Health Study. Scand. J. Clin. Lab. Investig. 2004, 64, 709–722. [Google Scholar] [CrossRef]
- Banfi, G.; Del Fabbro, M.; Lippi, G. Relation between Serum Creatinine and Body Mass Index in Elite Athletes of Different Sport Disciplines. Br. J. Sports Med. 2006, 40, 675–678, discussion 678. [Google Scholar] [CrossRef]
- Banfi, G.; Del Fabbro, M.; Lippi, G. Creatinine Values during a Competitive Season in Elite Athletes Involved in Different Sport Disciplines. J. Sports Med. Phys. Fit. 2008, 48, 479–482. [Google Scholar]
- Swaminathan, R.; Ho, C.S.; Chu, L.M.; Donnan, S. Relation between Plasma Creatinine and Body Size. Clin. Chem. 1986, 32, 371–373. [Google Scholar] [CrossRef]
- Swaminathan, R.; Major, P.; Snieder, H.; Spector, T. Serum Creatinine and Fat-Free Mass (Lean Body Mass). Clin. Chem. 2000, 46, 1695–1696. [Google Scholar] [CrossRef]
- Lippi, G.; Brocco, G.; Franchini, M.; Schena, F.; Guidi, G. Comparison of Serum Creatinine, Uric Acid, Albumin and Glucose in Male Professional Endurance Athletes Compared with Healthy Controls. Clin. Chem. Lab. Med. 2004, 42, 644–647. [Google Scholar] [CrossRef]
- Lippi, G.; Banfi, G.; Salvagno, G.L.; Franchini, M.; Guidi, G.C. Glomerular Filtration Rate in Endurance Athletes. Clin. J. Sport Med. 2008, 18, 286–288. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Banfi, G.; Luca Salvagno, G.; Montagnana, M.; Franchini, M.; Cesare Guidi, G. Comparison of Creatinine-Based Estimations of Glomerular Filtration Rate in Endurance Athletes at Rest. Clin. Chem. Lab. Med. 2008, 46, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Kratz, A.; Lewandrowski, K.B.; Siegel, A.J.; Chun, K.Y.; Flood, J.G.; Van Cott, E.M.; Lee-Lewandrowski, E. Effect of Marathon Running on Hematologic and Biochemical Laboratory Parameters, Including Cardiac Markers. Am. J. Clin. Pathol. 2002, 118, 856–863. [Google Scholar] [CrossRef]
- Gerth, J.; Ott, U.; Fünfstück, R.; Bartsch, R.; Keil, E.; Schubert, K.; Hübscher, J.; Scheucht, S.; Stein, G. The Effects of Prolonged Physical Exercise on Renal Function, Electrolyte Balance and Muscle Cell Breakdown. Clin. Nephrol. 2002, 57, 425–431. [Google Scholar] [CrossRef]
- Saengsirisuwan, V.; Phadungkij, S.; Pholpramool, C. Renal and Liver Functions and Muscle Injuries during Training and after Competition in Thai Boxers. Br. J. Sports Med. 1998, 32, 304–308. [Google Scholar] [CrossRef]
- Colombini, A.; Corsetti, R.; Graziani, R.; Lombardi, G.; Lanteri, P.; Banfi, G. Evaluation of Creatinine, Cystatin C and eGFR by Different Equations in Professional Cyclists during the Giro d’Italia 3-Weeks Stage Race. Scand. J. Clin. Lab. Investig. 2012, 72, 114–120. [Google Scholar] [CrossRef]
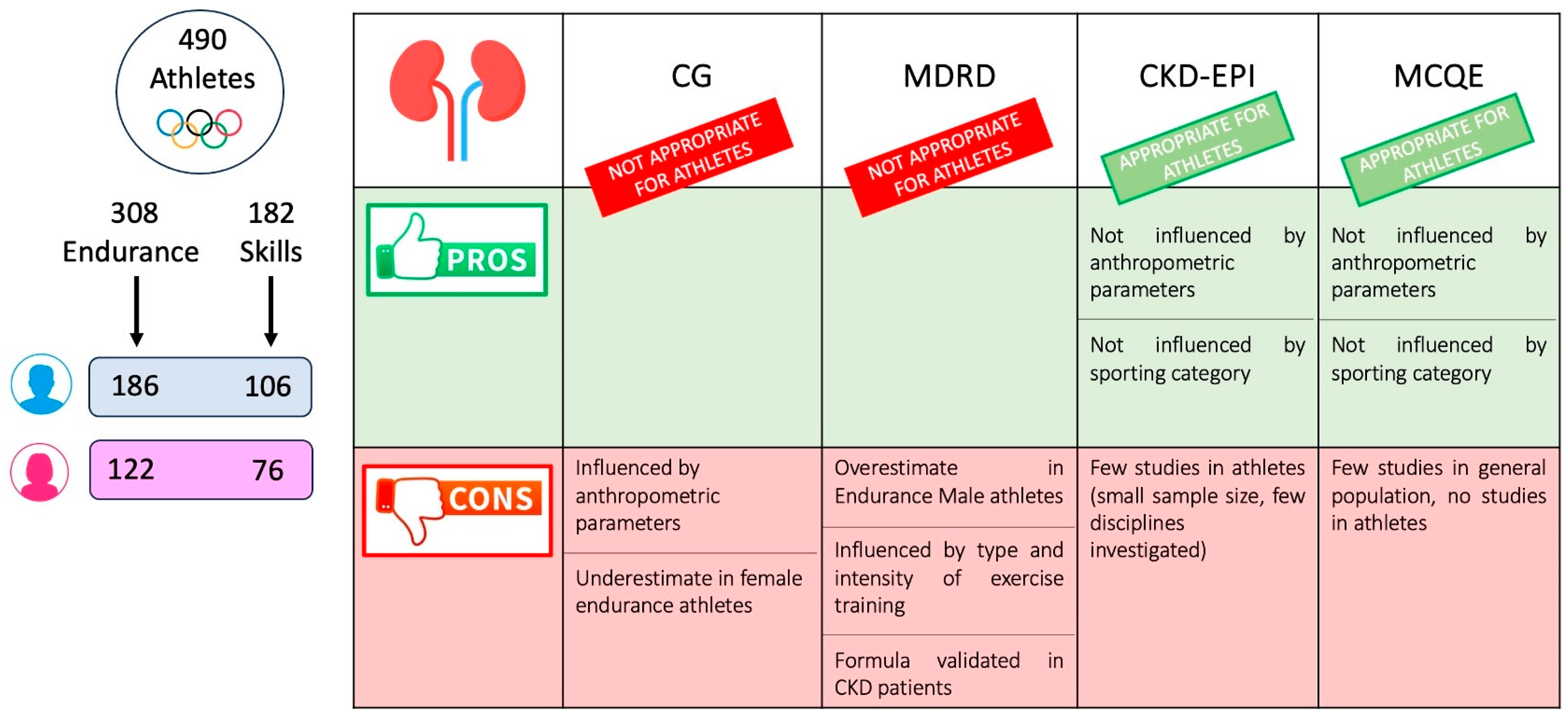
| Skills | Endurance | p-Value | |
|---|---|---|---|
| n, (%) | 182 (37.1) | 308 (62.9) | |
| Male, n (%) | 106 (58.2) | 186 (60.4) | 0.639 |
| Age, mean | 27.5 ± 5.5 | 26.6 ± 4.6 | 0.086 |
| Weight, kg | 72.5.4 ± 11.5 | 69.1 ± 14.6 | 0.013 |
| BMI, kg\m2 | 23.9 ± 3.1 | 21.9 ± 3.1 | <0.0001 |
| BSA | 1.85 ± 0.21 | 1.82 ± 0.22 | 0.171 |
| Fat mass, % | 20.3 ± 7.8 | 13.2 ± 5.3 | <0.0001 |
| Smokers, n (%) | 25 (13.7) | 1 (0.3) | <0.0001 |
| Family history for CVD, n (%) | 44 (24.2) | 58 (18.8) | 0.159 |
| SPB, mmHg | 109.0 ± 24.2 | 107.7 ± 16.9 | 0.433 |
| DBP, mmHg | 68.7 ± 10.9 | 67.1 ± 10.7 | 0.152 |
| Obesity, n (%); BMI > 30 kg/m2 | 11 (6) | 0 (0) | <0.0001 |
| Kcal | 2231.9 ± 482.2 | 2807.4 ± 738 | <0.0001 |
| Protein, % | 19.6 ± 4.0 | 18.8 ± 3.5 | 0.131 |
| Fat, % | 30.2 ± 5.4 | 29.2 ± 3.3 | 0.087 |
| Carbohydrate, % | 49.3 ± 5.2 | 51.8 ± 5 | 0.001 |
| CPK, U/L | 178.3 ± 211 | 260.9 ± 325.3 | 0.002 |
| AST, U/L | 21.3 ± 6.5 | 29.3 ± 15.5 | <0.0001 |
| ALT, U/L | 20.7 ± 9.7 | 24 ± 12.5 | 0.002 |
| Creatinine, mg/dL | 0.88 ± 0.13 | 0.91 ± 0.14 | 0.014 |
| CKD-EPI, mL/min × 1.73 m2 | 121.7 ± 7.9 | 121 ± 7.1 | 0.321 |
| G2: eGFR 60–89.9 mL/min × 1.73 m2 | 0 (0) | 0 (0) | |
| G1: eGFR ≥ 90 mL/min × 1.73 m2 | 182 (100) | 308 (100) | 1.000 |
| CG, mL/min × 1.73 m2 | 122.6 ± 30.8 | 113.6 ± 27 | 0.0008 |
| G2: eGFR 60–89.9 mL/min × 1.73 m2 | 24 (13.2) | 57 (18.5) | 0.125 |
| G1: eGFR ≥ 90 mL/min × 1.73 m2 | 158 (86.8) | 251 (81.5) | |
| MCQE, mL/min × 1.73 m2 | 134.5 ± 12.9 | 133.8 ± 14.4 | 0.593 |
| G2: eGFR 60–89.9 mL/min × 1.73 m2 | 1 (0.5) | 3 (1) | 0.614 |
| G1: eGFR ≥ 90 mL/min × 1.73 m2 | 181 (99.5) | 305 (99) | |
| MDRD, mL/min × 1.73 m2 | 122.6 ± 24 | 129.3 ± 25.8 | 0.004 |
| G2: eGFR 60–89.9 mL/min × 1.73 m2 | 11 (6) | 18 (5.8) | 0.927 |
| G1: eGFR ≥ 90 mL/min × 1.73 m2 | 171 (94) | 290 (94.2) |
| Male, n = 292 | Female, n = 198 | Skills | Endurance | |||||
|---|---|---|---|---|---|---|---|---|
| Skills | Endurance | p-Value | Skills | Endurance | p-Value | Male vs. Female | Male vs. Female | |
| n, (%) | 106 (36.3) | 186 (63.7) | 76 (38.4) | 122 (61.6) | ||||
| Age, mean | 28.2 ± 6.3 | 26.8 ± 4.5 | 0.032 | 26.6 ± 5.8 | 26.4 ± 4.8 | 0.871 | 0.089 | 0.542 |
| Weight, kg | 78.9 ± 12.8 | 75.7 ± 11.9 | 0.035 | 63.5 ± 10.3 | 59.1 ± 12.4 | 0.010 | <0.0001 | <0.0001 |
| BMI, Kg\m2 | 24.6 ± 3.2 | 22.7 ± 2.6 | <0.0001 | 23 ± 3 | 20.9 ± 3.4 | <0.0001 | 0.0006 | <0.0001 |
| BSA | 1.96 ± 0.18 | 1.94 ± 0.19 | 0.360 | 1.69 ± 0.15 | 1.64 ± 0.13 | 0.012 | <0.0001 | <0.0001 |
| Fat mass, % | 16.7 ± 7 | 10 ± 3.3 | <0.0001 | 25 ± 6.1 | 18 ± 4 | <0.0001 | <0.0001 | <0.0001 |
| Creatinine, mg/dL | 0.93 ± 0.1 | 0.97 ± 0.1 | 0.048 | 0.81 ± 0.1 | 0.84 ± 0.12 | 0.132 | <0.0001 | <0.0001 |
| K+, mEqu/L | 4.47 ± 0.3 | 4.56 ± 0.3 | 0.033 | 4.49 ± 0.4 | 4.52 ± 0.3 | 0.549 | 0.702 | 0.400 |
| CKD-EPI, mL/min × 1.73 m2 | 118.4 ± 6.2 | 118.3 ± 6.2 | 0.891 | 126.2 ± 7.8 | 125.1 ± 6.4 | 0.266 | <0.0001 | <0.0001 |
| G2: eGFR 60–89.9 mL/min × 1.73 m2 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||||
| G1: eGFR ≥ 90 mL/min × 1.73 m2 | 106 (100) | 186 (100) | 1.000 | 76 (100) | 122 (100) | 1.000 | ||
| CG, mL/min × 1.73 m2 | 133.2 ± 27.4 | 125 ± 20.7 | 0.047 | 107.8 ± 29 | 96 ± 26.1 | 0.003 | <0.0001 | <0.0001 |
| G2: eGFR 60–89.9 mL/min × 1.73 m2 | 0 (0) | 4 (2.1) | 0.132 | 24 (31.6) | 53 (43.3) | 0.095 | <0.0001 | <0.0001 |
| G1: eGFR ≥ 90 mL/min × 1.73 m2 | 106 (100) | 182 (97.9) | 52 (68.4) | 69 (54.7) | ||||
| MCQE, mL/min × 1.73 m2 | 133.3 ± 12.4 | 130.5 ± 15.4 | 0.120 | 136.3 ± 13.3 | 138.8 ± 11.1 | 0.145 | 0.124 | <0.0001 |
| G2: eGFR 60–89.9 mL/min × 1.73 m2 | 0 (0) | 3 (1.6) | 0.187 | 1 (1.3) | 0 (0) | 0.204 | 0.263 | 0.158 |
| G1: eGFR ≥ 90 mL/min × 1.73 m2 | 106 (100) | 183 (98.4) | 75 (98.7) | 122 (100) | ||||
| MDRD, mL/min × 1.73 m2 | 130.3 ± 21.8 | 137.5 ± 24.9 | 0.015 | 111.7 ± 22.6 | 116.8 ± 21.8 | 0.116 | <0.0001 | <0.0001 |
| G2: eGFR 60–89.9 mL/min × 1.73 m2 | 2 (1.9) | 5 (2.7) | 0.666 | 9 (11.8) | 13 (10.7) | 0.796 | 0.005 | 0.003 |
| G1: eGFR ≥ 90 mL/min × 1.73 m2 | 104 (98.1) | 181 (97.3) | 67 (88.2) | 109 (89.3) | ||||
| Total Female, n = 198 | Skills Female, n = 76 | Endurance Female, n = 122 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| G2 | G1 | p-Value | G2 | G1 | p-Value | G2 | G1 | p-Value | |
| n, (%) | 67 (33.8) | 131 (66.2) | 24 (31.6) | 52 (68.4) | 53 (43.4) | 69 (56.6) | |||
| Age, mean | 28 ± 5 | 25.6 ± 5.1 | 0.001 | 28.3 ± 5.8 | 25.8 ± 5.7 | 0.077 | 27.8 ± 4.6 | 25.4 ± 4.6 | 0.005 |
| Weight, kg | 54.2 ± 6.2 | 65 ± 12.6 | <0.0001 | 55.2 ± 6.1 | 67.4 ± 9.6 | <0.0001 | 53.7 ± 6.1 | 63.3 ± 14.3 | <0.0001 |
| BMI, Kg\m2 | 19.9 ± 1.8 | 22.8 ± 3.7 | <0.0001 | 20.7 ± 1.5 | 24 ± 3 | <0.0001 | 19.6 ± 1.8 | 21.8 ± 4 | 0.0003 |
| BSA | 1.57 ± 0.1 | 1.72 ± 0.1 | <0.0001 | 1.58 ± 0.1 | 1.74 ± 0.1 | <0.0001 | 1.56 ± 0.1 | 1.70 ± 0.1 | <0.0001 |
| Fat mass, % | 18.1 ± 4.4 | 22.8 ± 3.7 | <0.0001 | 21.1 ± 4.5 | 26.7 ± 5.9 | 0.0001 | 16.7 ± 3.6 | 19.1 ± 3.9 | 0.0009 |
| Smokers, n (%) | 4 (6) | 3 (2.3) | 0.184 | 4 (16.7) | 3 (5.8) | 0.126 | 0 (0) | 0 (0) | 1.000 |
| Family history for CVD, n (%) | 12 (17.9) | 25 (19.1) | 0.841 | 6 (25) | 11 (21.1) | 0.708 | 6 (11.3) | 14 (20.3) | 0.184 |
| SPB, mmHg | 99.8 ± 9.8 | 105.1 ± 9.1 | 0.0005 | 98.7 ± 7.9 | 106.2 ± 7.6 | 0.0007 | 100.2 ± 10.5 | 104.3 ± 10.1 | 0.049 |
| DBP, mmHg | 64.5 ± 6 | 65.9 ± 7.1 | 0.209 | 64.2 ± 5.5 | 67.1 ± 6.4 | 0.091 | 64.6 ± 6.2 | 64.9 ± 7.5 | 0.857 |
| Kcal | 2238.3 ± 386 | 2234.5 ± 417 | 0.965 | 1996.9 ± 338 | 2030 ± 385 | 0.807 | 2381.5 ± 338 | 2348.1 ± 390 | 0.743 |
| Protein, % | 18.5 ± 2.5 | 19.3 ± 5.4 | 0.349 | 20.6 ± 2.3 | 19.3 ± 6.3 | 0.462 | 17.3 ± 1.6 | 19.4 ± 4.9 | 0.037 |
| Fat, % | 29.2 ± 3.3 | 28.4 ± 6 | 0.453 | 29.6 ± 4.2 | 28.5 ± 8.2 | 0.652 | 29 ± 2.7 | 28.3 ± 4.4 | 0.536 |
| Carbs, % | 52 ± 3.9 | 50.5 ± 6.7 | 0.206 | 49.6 ± 4 | 48.5 ± 6.1 | 0.549 | 53.5 ± 3 | 51.7 ± 6.7 | 0.213 |
| CPK, U/L | 162.3 ± 104.8 | 172.9 ± 234.2 | 0.710 | 151.3 ± 78.5 | 110.5 ± 62.3 | 0.018 | 167.3 ± 114.4 | 220 ± 296.9 | 0.227 |
| AST, U/L | 25.1 ± 8.9 | 22.2 ± 7.8 | 0.015 | 21.4 ± 5.7 | 18.1 ± 4.6 | 0.019 | 26.8 ± 9.5 | 25.3 ± 8.3 | 0.361 |
| ALT, U/L | 21.7 ± 9.9 | 18.2 ± 8.6 | 0.009 | 19 ± 8.1 | 15.7 ± 5.4 | 0.044 | 23 ± 10.4 | 20.1 ± 10 | 0.130 |
| Creatinine, mg/dL | 0.91 ± 0.1 | 0.78 ± 0.1 | <0.0001 | 0.93 ± 0.12 | 0.76 ± 0.09 | <0.0001 | 0.90 ± 0.1 | 0.80 ± 0.1 | <0.0001 |
| CG, mL/min × 1.73 m2 | 79.2 ± 7 | 114.1 ± 27.7 | <0.0001 | 78.8 ± 6.7 | 121.2 ± 25.4 | <0.0001 | 79.4 ± 7.1 | 108.8 ± 28.1 | <0.0001 |
| Urine gravity, g/mL | 1020.3 ± 6.5 | 1021.4 ± 8.1 | 0.515 | 1018 ± 6.2 | 1022.5 ± 8.4 | 0.124 | 1021.3 ± 6.3 | 1020.7 ± 7.8 | 0.767 |
| CG | CKD-EPI | MCQE | MDRD | |
|---|---|---|---|---|
| Skills vs. endurance | ↓ endurance | = | = | ↑ endurance |
| KDIGO category, global | 16.5% G2 | 0% G2 | 0.8% G2 | 5.9% G2 |
| KDIGO category, males | 1.4% G2 | 0% G2 | 1% G2 | 2.4% G2 |
| KDIGO category, females | 38.9% G2 | 0% G2 | 0.5% G2 | 11.1% G2 |
| Male vs. female | ↑ males | ↓ males | ↓ male endurance | ↑ males |
| Male skills vs. endurance | ↓ endurance | = | = | ↑ endurance |
| Female skills vs. endurance | ↓ endurance | = | = | = |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Gioia, G.; Ferrera, A.; Serdoz, A.; Spinelli, A.; Fiore, R.; Buzzelli, L.; Zampaglione, D.; Squeo, M.R. How to Evaluate Kidney Function in Elite Endurance Athletes: Pros and Cons of Different Creatinine-Based Formulas. J. Clin. Med. 2025, 14, 2955. https://doi.org/10.3390/jcm14092955
Di Gioia G, Ferrera A, Serdoz A, Spinelli A, Fiore R, Buzzelli L, Zampaglione D, Squeo MR. How to Evaluate Kidney Function in Elite Endurance Athletes: Pros and Cons of Different Creatinine-Based Formulas. Journal of Clinical Medicine. 2025; 14(9):2955. https://doi.org/10.3390/jcm14092955
Chicago/Turabian StyleDi Gioia, Giuseppe, Armando Ferrera, Andrea Serdoz, Alessandro Spinelli, Roberto Fiore, Lorenzo Buzzelli, Domenico Zampaglione, and Maria Rosaria Squeo. 2025. "How to Evaluate Kidney Function in Elite Endurance Athletes: Pros and Cons of Different Creatinine-Based Formulas" Journal of Clinical Medicine 14, no. 9: 2955. https://doi.org/10.3390/jcm14092955
APA StyleDi Gioia, G., Ferrera, A., Serdoz, A., Spinelli, A., Fiore, R., Buzzelli, L., Zampaglione, D., & Squeo, M. R. (2025). How to Evaluate Kidney Function in Elite Endurance Athletes: Pros and Cons of Different Creatinine-Based Formulas. Journal of Clinical Medicine, 14(9), 2955. https://doi.org/10.3390/jcm14092955







