Changes in Facial Soft Tissue Asymmetry in Class II Patients One Year After Orthognathic Surgery
Abstract
1. Introduction
2. Materials and Methods
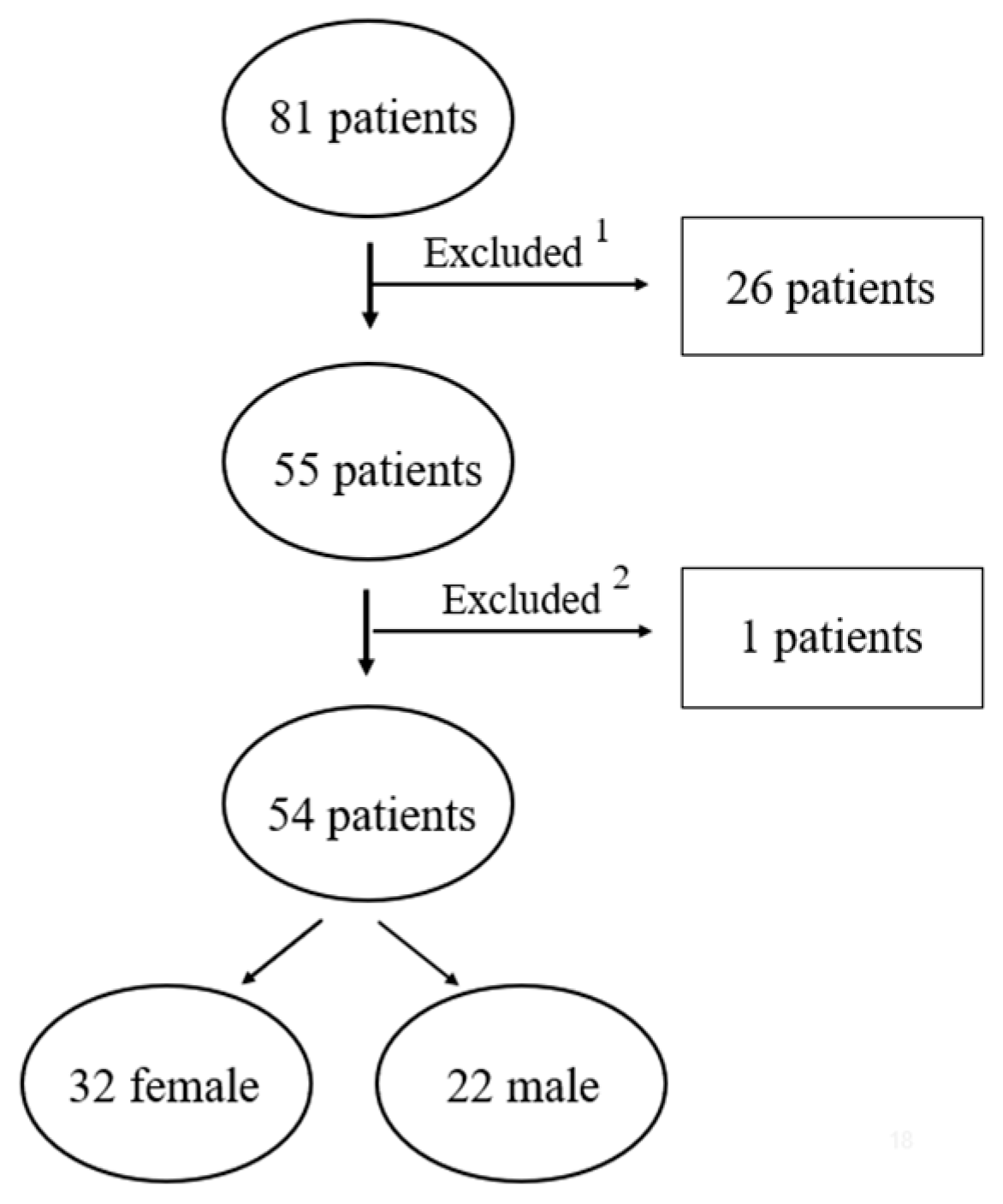
2.1. Study Design and Samples
2.2. Ethical Considerations and Study Registration
2.3. Inclusion and Exclusion Criteria
2.4. Stereophotogrammetry System and Image Acquisition
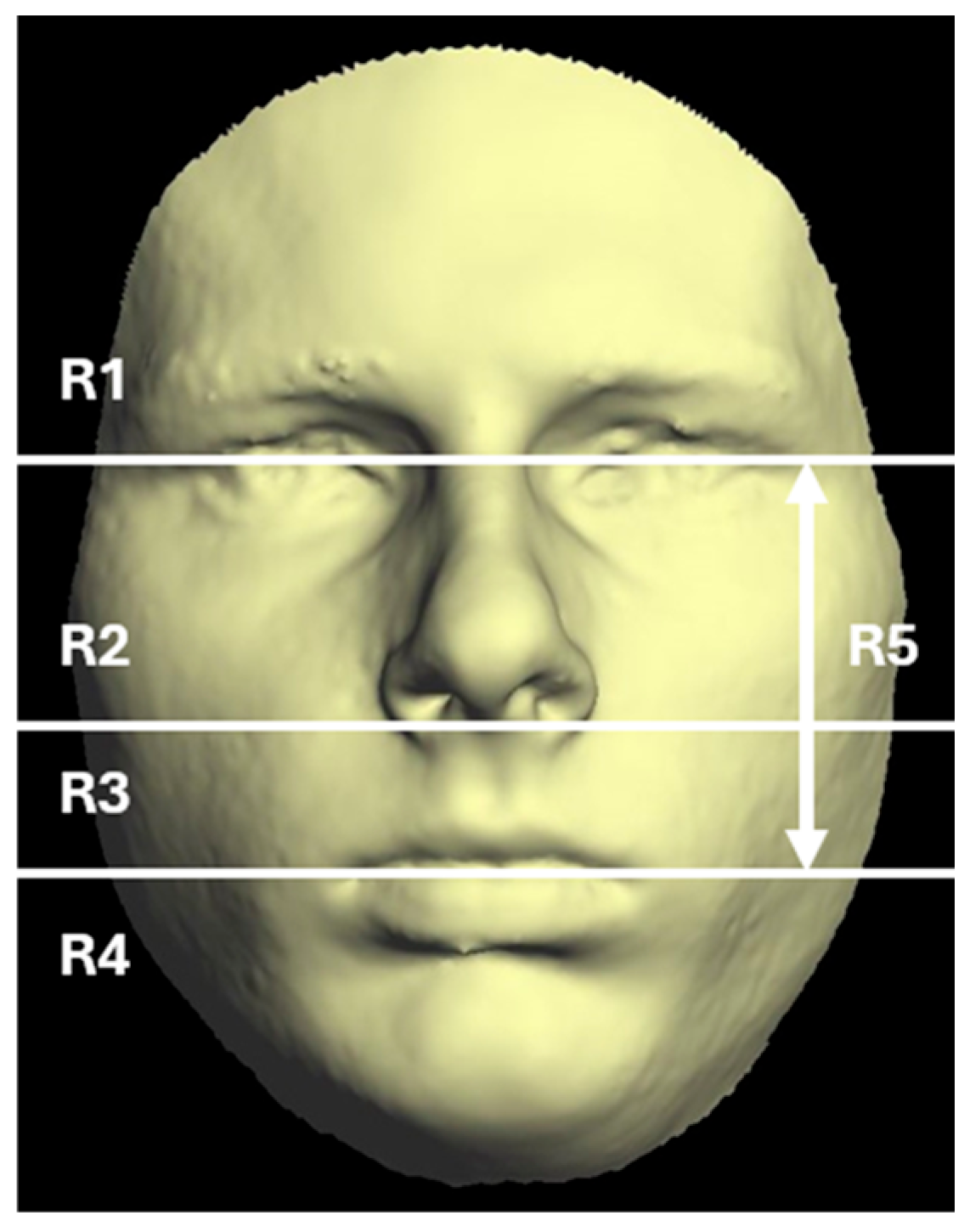
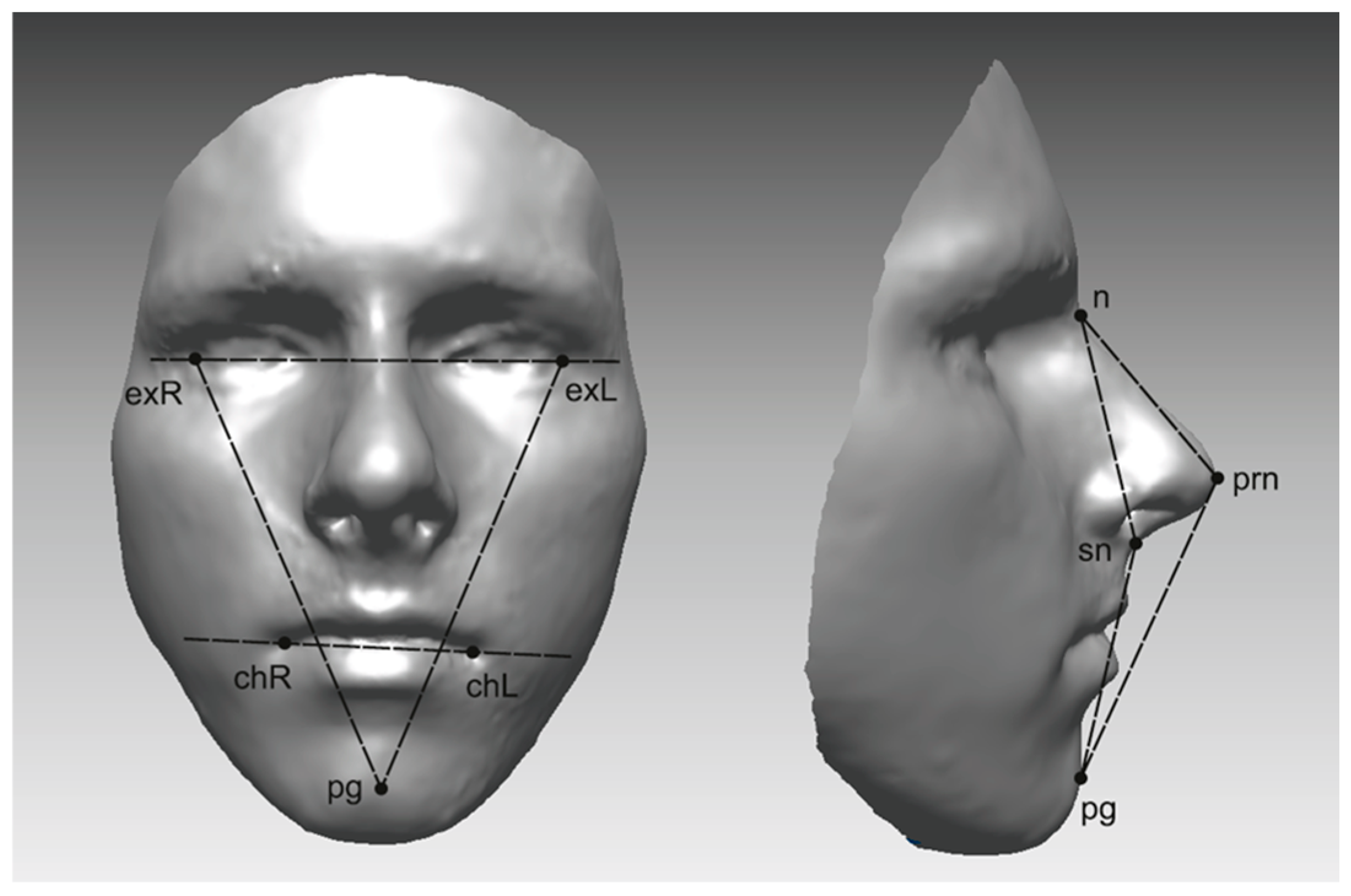
2.5. Quantification of Asymmetry
2.6. Error of Method Analysis
2.7. Statistical Analysis
3. Results
3.1. Landmark Accuracy
3.2. Primary Outcome
3.3. Subgroups
3.4. Deviation of the Pogonion Point
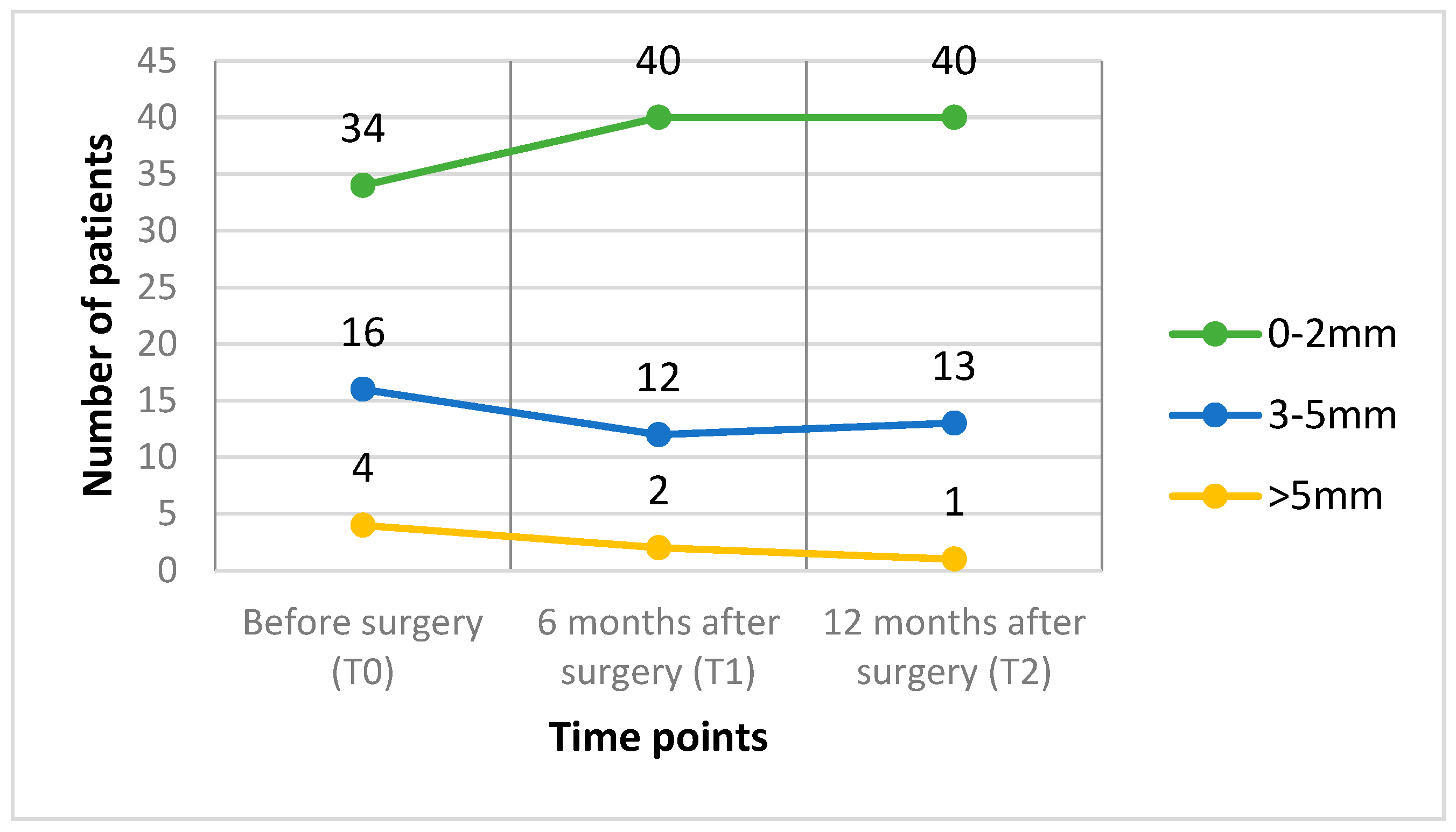
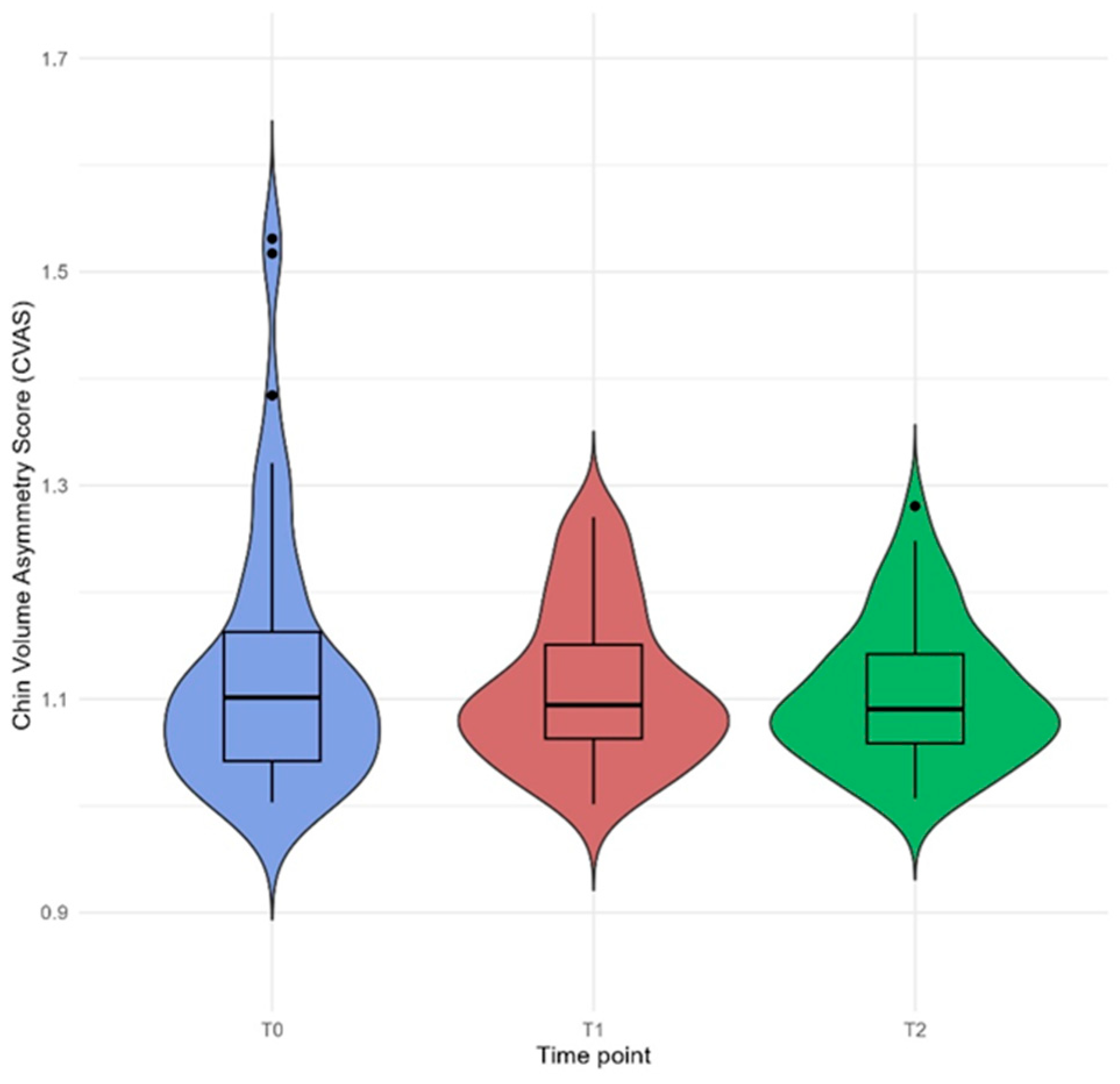
3.5. Chin Volume Asymmetry Score
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3D | Three-dimensional |
| BSSO | Bilateral sagittal split osteotomy |
| CVAS | Chin Volume Asymmetry Score |
| CBCT | Cone-beam computed tomogram |
| MnD | Mean distance |
| MD | Maximum distance |
| SD | Standard deviation |
| T0 | Before surgery |
| T1 | 6 months after surgery |
| T2 | 12 months after surgery |
Appendix A
| Landmark | Abbreviation | Definition |
|---|---|---|
| Glabella | Glab | The most anterior midpoint on the fronto-orbital soft tissue contour |
| Nasion | Nasi | The deepest point of nasal bridge (of the frontonasal suture) |
| Endocanthion | EN_R | The soft tissue point located at the inner commissure of each eye fissure |
| Exocanthion | EX_R | The soft tissue point located at the outer commissure of each eye fissure |
| Endocanthion | EN_L | The soft tissue point located at the inner commissure of each eye fissure |
| Exocanthion | EX_L | The soft tissue point located at the outer commissure of each eye fissure |
| Palpebrale superius | psLe | The highest point in the mid-position of the free margin of each upper eyelid |
| Palpebrale superius | psRi | The highest point in the mid-position of the free margin of each upper eyelid |
| Palpebrale inferius | piRi | The lowest point in the mid-position of the free margin of each lower eyelid |
| Palpebrale inferius | piLe | The lowest point in the mid-position of the free margin of each lower eyelid |
| Pronasale | Pron | The most anterior midpoint of the nasal tip |
| Subnasale | Subn | The midpoint on the nasolabial soft tissue contour between the columella crest and the upper lip |
| Alare right | AL_R | The most lateral point on right alar contour |
| Alare left | AL_L | The most lateral point on left alar contour |
| Labiale superius | LaSu | The midpoint of the vermilion line of the upper lip |
| Labiale inferius | LaIn | The midpoint of the vermilion line of the lower lip |
| Christa philtri left | cphL | The point at left crossing of the vermilion line and the elevated margin of the philtrum |
| Christa philtri right | cphR | The point at right crossing of the vermilion line and the elevated margin of the philtrum |
| Cheilion left | chLe | The point located at the left labial commissure |
| Cheilion right | chRi | The point located at the right labial commissure |
| Pogonion | Pogo | Most anterior midpoint of the chin |
References
- Hajeer, M.Y.; Ayoub, A.F.; Millett, D.T. Three-dimensional assessment of facial soft-tissue asymmetry before and after orthognathic surgery. Br. J. Oral Maxillofac. Surg. 2004, 42, 396–404. [Google Scholar] [CrossRef]
- Moragas, J.S.M.; Oth, O.; Büttner, M.; Mommaerts, M.Y. A systematic review on soft-to-hard tissue ratios in orthognathic surgery part II: Chin procedures. J. Cranio-Maxillofac. Surg. 2015, 43, 1530–1540. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Xu, Y.; Wang, J.; Lu, Z.; Qi, K. 3-dimensional analysis of hard- and soft-tissue symmetry in a Chinese population. BMC Oral Health 2023, 23, 432. [Google Scholar] [CrossRef] [PubMed]
- Thiesen, G.; Gribel, B.F.; Freitas, M.P.M. Facial asymmetry: A current review. Dent. Press J. Orthod. 2015, 20, 110–125. [Google Scholar] [CrossRef] [PubMed]
- Ko, E.W.C.; Huang, C.S.; Chen, Y.R. Characteristics and Corrective Outcome of Face Asymmetry by Orthognathic Surgery. J. Oral Maxillofac. Surg. 2009, 67, 2201–2209. [Google Scholar] [CrossRef]
- Kurt, G.K.; Uysal, T.; Sisman, Y.; Sabri Ramoglu, I. Mandibular Asymmetry in Class II Subdivision Malocclusion. Angle Orthod. 2008, 78, 32–37. [Google Scholar] [CrossRef]
- Kaipainen, A.E.; Sieber, K.R.; Nada, R.M.; Maal, T.J.; Katsaros, C.; Fudalej, P.S. Regional facial asymmetries and attractiveness of the face. Eur. J. Orthod. 2016, 38, 602–608. [Google Scholar] [CrossRef]
- Çoban, G.; Yavuz, İ.; Demirbaş, A.E. Three-dimensional changes in the location of soft tissue landmarks following bimaxillary orthognathic surgery. J. Orofac. Orthop. 2021, 82, 257–265. [Google Scholar] [CrossRef]
- Harnádková, K.; Kočandrlová, K.; Jaklová, L.K.; Dupej, J.; Velemínská, J. The effect of sex and age on facial shape directional asymmetry in adults: A 3D landmarks-based method study. PLoS ONE 2023, 18, e0288702. [Google Scholar] [CrossRef]
- Chen, Y.F.; Liao, Y.F.; Chen, Y.A.; Chen, Y.R. Treatment outcome of bimaxillary surgery for asymmetric skeletal class II deformity. Clin. Oral Investig. 2019, 23, 623–632. [Google Scholar] [CrossRef]
- Onică, N.; Onică, C.A.; Budală, D.G.; Gelețu, G.L.; Balan, M.; Baciu, E.-R.; Murariu, A.; Pertea, M. The Use of 3D Technology in the Management of Residual Asymmetry following Orthognathic Surgery: A Case Report. Healthcare 2023, 11, 2172. [Google Scholar] [CrossRef]
- Gill, D.S.; Lloyd, T.; East, C.; Naini, F.B. The Facial Soft Tissue Effects of Orthognathic Surgery. Facial Plast. Surg. 2017, 33, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Cassi, D.; Battistoni, G.; Magnifico, M.; Di Blasio, C.; Pedrazzi, G.; Di Blasio, A. Three-dimensional evaluation of facial asymmetry in patients with hemifacial microsomia using stereophotogrammetry. J. Cranio-Maxillofac. Surg. 2019, 47, 179–184. [Google Scholar] [CrossRef]
- Hong, C.; Choi, K.; Kachroo, Y.; Kwon, T.; Nguyen, A.; McComb, R.; Moon, W. Evaluation of the 3dMDface system as a tool for soft tissue analysis. Orthod. Craniofac. Res. 2017, 20, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Djordjevic, J.; Toma, A.M.; Zhurov, A.I.; Richmond, S. Three-dimensional quantification of facial symmetry in adolescents using laser surface scanning. Eur. J. Orthod. 2014, 36, 125–132. [Google Scholar] [CrossRef]
- Farkas, L.G.; Deutsch, C.K. Anthropometric determination of craniofacial morphology. Am. J. Med. Genet. 1996, 65, 1–4. [Google Scholar] [CrossRef]
- Toma, A.; Zhurov, A.; Playle, R.; Ong, E.; Richmond, S. Reproducibility of facial soft tissue landmarks on 3D laser-scanned facial images. Orthod. Craniofacial Res. 2009, 12, 33–42. [Google Scholar]
- Kau, C.H.; Richmond, S. Three-Dimensional Imaging for Orthodontics and Maxillofacial Surgery; Wiley-Blackwell: Hoboken, NJ, USA, 2010. [Google Scholar]
- Djordjevic, J.; Pirttiniemi, P.; Harila, V.; Heikkinen, T.; Toma, A.M.; Zhurov, A.I.; Richmond, S. Three-dimensional longitudinal assessment of facial symmetry in adolescents. Eur. J. Orthod. 2013, 35, 143–151. [Google Scholar] [CrossRef]
- Verhoeven, T.; Xi, T.; Schreurs, R.; Bergé, S.; Maal, T. Quantification of facial asymmetry: A comparative study of landmark-based and surface-based registrations. J. Cranio-Maxillofac. Surg. 2016, 44, 1131–1136. [Google Scholar] [CrossRef]
- Taylor, H.O.; Morrison, C.S.; Linden, O.M.; Phillips, B.; Chang, J.; Byrne, M.E.; Sullivan, S.R.; Forrest, C.R.M. Quantitative facial asymmetry: Using three-dimensional photogrammetry to measure baseline facial surface symmetry. J. Craniofacial Surg. 2014, 25, 124–128. [Google Scholar] [CrossRef]
- Codari, M.; Pucciarelli, V.; Stangoni, F.; Zago, M.; Tarabbia, F.; Biglioli, F.; Sforza, C. Facial thirds–based evaluation of facial asymmetry using stereophotogrammetric devices: Application to facial palsy subjects. J. Cranio-Maxillofac. Surg. 2017, 45, 76–81. [Google Scholar] [CrossRef]
- Heikkinen, E.V.; Vuollo, V.; Harila, V.; Sidlauskas, A.; Heikkinen, T. Facial asymmetry and chewing sides in twins. Acta Odontol. Scand. 2022, 80, 197–202. [Google Scholar] [CrossRef]
- Nike, E.; Radzins, O.; Pirttiniemi, P.; Vuollo, V.; Slaidina, A.; Abeltins, A. Evaluation of facial soft tissue asymmetric changes in Class III patients after orthognathic surgery using three-dimensional stereophotogrammetry. Int. J. Oral Maxillofac. Surg. 2023, 52, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Marcotty, P.; Stellzig-Eisenhauer, A.; Bareis, U.; Hartmann, J.; Kochel, J. Three-dimensional perception of facial asymmetry. Eur. J. Orthod. 2011, 33, 647–653. [Google Scholar] [CrossRef]
- Richmond, S.; I Zhurov, A.; Ali, A.B.M.; Pirttiniemi, P.; Heikkinen, T.; Harila, V.; Silinevica, S.; Jakobsone, G.; Urtane, I. Exploring the midline soft tissue surface changes from 12 to 15 years of age in three distinct country population cohorts. Eur. J. Orthod. 2020, 42, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Wermker, K.; Kleinheinz, J.; Jung, S.; Dirksen, D. Soft tissue response and facial symmetry after orthognathic surgery. J. Cranio-Maxillofac. Surg. 2014, 42, e339–e345. [Google Scholar] [CrossRef]
- Da Pozzo, F.; Gibelli, D.; Beltramini, G.A.; Dolci, C.; Giannì, A.B.; Sforza, C. The Effect of Orthognathic Surgery on Soft-Tissue Facial Asymmetry: A Longitudinal Three-Dimensional Analysis. J. Craniofacial Surg. 2020, 31, 1578–1582. [Google Scholar] [CrossRef]
- Linden, O.E.; He, J.K.; Morrison, C.S.; Sullivan, S.R.; Taylor, H.O.B. The relationship between age and facial asymmetry. Plast. Reconstr. Surg. 2018, 142, 1145–1152. [Google Scholar] [CrossRef]
- Cao, R.; Chen, B.; Xu, H.; Cai, Y.; Liu, W. Accuracy of three-dimensional optical devices for facial soft-tissue measurement in clinical practice of stomatology: A PRISMA systematic review. Medicine 2022, 101, e31922. [Google Scholar] [CrossRef]
- Azevedo, A.R.P.; Janson, G.; Henriques, J.F.C.; De Freitas, M.R. Evaluation of asymmetries between subjects with Class II subdivision and apparent facial asymmetry and those with normal occlusion. Am. J. Orthod. Dentofac. Orthop. 2006, 129, 376–383. [Google Scholar] [CrossRef]
- Janson, G.R.P.; Metaxas, A.; Woodside, D.G.; De Freitas, M.R.; Pinzan, A. Three-dimensional evaluation of skeletal and dental asymmetries in Class II subdivision malocclusions. Am. J. Orthod. Dentofac. Orthop. 2001, 119, 406–418. [Google Scholar] [CrossRef]
- Olate, S.; Zaror, C.; Mommaerts, M.Y. A systematic review of soft-to-hard tissue ratios in orthognathic surgery. Part IV: 3D analysis—Is there evidence? J. Cranio-Maxillofac. Surg. 2017, 45, 1278–1286. [Google Scholar] [CrossRef] [PubMed]
- Verdenik, M.; Hren, N.I. Differences in three dimensional soft tissue changes after upper, lower, or both jaw orthognathic surgery in skeletal class III patients. Int. J. Oral Maxillofac. Surg. 2014, 43, 1345–1351. [Google Scholar] [PubMed]
- Kim, Y.K.; Moon, S.W.; Yun, P.Y.; Lee, Y.S.; Larson, B.E.; Lee, N.K. Evaluation of soft tissue changes around the lips after mandibular setback surgery with minimal orthodontics using three-dimensional stereophotogrammetry. J. Oral Maxillofac. Surg. 2016, 74, 1044–1054. [Google Scholar]
- Kau, C.H.; Richmond, S.; Incrapera, A.; English, J.; Xia, J.J. Three-dimensional surface acquisition systems for the study of facial morphology and their application to maxillofacial surgery. Int. J. Med. Robot. Comput. Assist. Surg. 2007, 3, 97–110. [Google Scholar]


| Age (Years) | |
|---|---|
| Range | 22–60 |
| Mean ± SD | 33.2 ± 7.72 |
| Sex, n (%) | |
| Women | 32 (59.3%) |
| Men | 22 (40.7%) |
| Surgery type | |
| Bimaxillary | 30 (55.6%) |
| BSSO | 24 (44.4%) |
| T0 | T1 | T2 | ||
|---|---|---|---|---|
| Median (mm) | Median (mm) | Median (mm) | p-Value | |
| Whole face | ||||
| MnD | 0.71 | 0.74 | 0.75 | 0.769 |
| MD | 3.29 | 3.26 | 3.43 | 0.659 |
| Region 2 | ||||
| MnD | 0.55 | 0.61 | 0.58 | 0.151 |
| MD | 2.19 | 2.39 | 2.35 | 0.337 |
| Region 3 | ||||
| MnD | 0.70 | 0.77 | 0.77 | 0.696 |
| MD | 2.49 | 2.69 | 2.83 | 0.636 |
| Region 4 | ||||
| MnD | 0.96 | 1.07 | 1.04 | 0.650 |
| MD | 3.27 | 3.11 | 3.36 | 0.375 |
| Region 5 | ||||
| MnD | 0.60 | 0.65 | 0.68 | 0.497 |
| MD | 2.56 | 2.86 | 2.91 | 0.433 |
| 3D Angle Measurements | T0 | T1 | T2 | |
|---|---|---|---|---|
| Median (Degrees) | Median (Degrees) | Median (Degrees) | p-Value | |
| n-sn-pg | 154.24 | 157.13 | 158.80 | 0.001 * |
| n-prn-pg | 122.33 | 125.20 | 125.39 | 0.001 * |
| exR-exL-pg | 65.98 | 65.84 | 65.89 | 0.351 |
| exL-exR-pg | 66.23 | 66.31 | 66.39 | 0.790 |
| exRexL-chRchL | 2.37 | 2.30 | 2.40 | 0.729 |
| Sex | T0 | T1 | T2 | ||
|---|---|---|---|---|---|
| Median (mm) | Median (mm) | Median (mm) | p-Value | p-Value (Between Sex) | |
| Male | |||||
| MnD | 0.72 | 0.77 | 0.77 | 0.929 | |
| MD | 3.68 | 3.39 | 3.46 | 0.429 | 0.945 |
| Female | |||||
| MnD | 0.67 | 0.70 | 0.73 | 0.772 | |
| MD | 2.99 | 2.97 | 3.41 | 0.328 |
| Sex | T0 | T1 | T2 | |
|---|---|---|---|---|
| Median (Degrees) | Median (Degrees) | Median (Degrees) | p-Value | |
| Male | ||||
| n-sn-pg | 153.32 | 156.12 | 157.75 | 0.001 * |
| n-prn-pg | 120.73 | 124.00 | 123.75 | 0.001 * |
| Female | ||||
| n-sn-pg | 154.98 | 159.22 | 160.49 | 0.001 * |
| n-prn-pg | 122.68 | 126.41 | 125.95 | 0.001 * |
| T0 | T1 | T2 | |||
|---|---|---|---|---|---|
| Surgery Type | Median (mm) | Median (mm) | Median (mm) | p-Value | p-Value (Between Operation Types) |
| BSSO | |||||
| MnD | 0.67 | 0.72 | 0.71 | 0.960 | |
| MD | 3.24 | 3.15 | 3.08 | 0.450 | |
| n-sn-pg | 154.09 | 157.05 | 158.19 | 0.001 * | |
| n-prn-pg | 121.90 | 124.04 | 124.17 | 0.001 * | 0.756 |
| LF + BSSO | |||||
| MnD | 0.72 | 0.78 | 0.77 | 0.692 | |
| MD | 3.56 | 3.39 | 3.64 | 0.369 | |
| n-sn-pg | 154.41 | 157.29 | 158.93 | 0.001 * | |
| n-prn-pg | 122.78 | 126.41 | 126.61 | 0.001 * |
| Region 4 | T0 | T1 | T2 | |
|---|---|---|---|---|
| Median (mm) | Median (mm) | Median (mm) | p-Value | |
| 0–2 | ||||
| MnD | 0.65 | 0.70 | 0.73 | 0.936 |
| MD | 2.79 | 2.89 | 3.20 | 0.160 |
| 3–5 | ||||
| MnD | 0.77 | 0.89 | 0.78 | 0.868 |
| MD | 3.79 | 4.08 | 3.88 | 0.777 |
| >5 | ||||
| MnD | 1.18 | 1.07 | 0.94 | - |
| MD | 4.94 | 4.96 | 4.88 | - |
| 0–2 | ||||
| n-sn-pg | 154.24 | 157.51 | 158.73 | 0.001 * |
| n-prn-pg | 121.94 | 124.89 | 124.73 | 0.001 * |
| 3–5 | ||||
| n-sn-pg | 154.72 | 156.55 | 158.67 | 0.199 |
| n-prn-pg | 123.36 | 124.96 | 126.01 | 0.114 |
| >5 | ||||
| n-sn-pg | 153.37 | 164.07 | 161.12 | - |
| n-prn-pg | 123.65 | 130.99 | 127.84 | - |
| T0 | T1 | T2 | ||
|---|---|---|---|---|
| CVAS | Median (mm) | Median (mm) | Median (mm) | p-Value |
| (a) Time | 1.10 | 1.09 | 1.09 | 0.901 |
| (b) Sex | ||||
| Male | 1.09 | 1.08 | 1.09 | 0.555 |
| Female | 1.11 | 1.10 | 1.09 | 0.186 |
| (c) Surgery type | ||||
| LF + BSSO | 1.11 | 1.10 | 1.09 | 0.350 |
| BSSO | 1.08 | 1.05 | 1.09 | 0.273 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nike, E.; Radzins, O.; Vuollo, V.; Slaidina, A.; Abeltins, A. Changes in Facial Soft Tissue Asymmetry in Class II Patients One Year After Orthognathic Surgery. J. Clin. Med. 2025, 14, 2912. https://doi.org/10.3390/jcm14092912
Nike E, Radzins O, Vuollo V, Slaidina A, Abeltins A. Changes in Facial Soft Tissue Asymmetry in Class II Patients One Year After Orthognathic Surgery. Journal of Clinical Medicine. 2025; 14(9):2912. https://doi.org/10.3390/jcm14092912
Chicago/Turabian StyleNike, Edmonda, Oskars Radzins, Ville Vuollo, Anda Slaidina, and Andris Abeltins. 2025. "Changes in Facial Soft Tissue Asymmetry in Class II Patients One Year After Orthognathic Surgery" Journal of Clinical Medicine 14, no. 9: 2912. https://doi.org/10.3390/jcm14092912
APA StyleNike, E., Radzins, O., Vuollo, V., Slaidina, A., & Abeltins, A. (2025). Changes in Facial Soft Tissue Asymmetry in Class II Patients One Year After Orthognathic Surgery. Journal of Clinical Medicine, 14(9), 2912. https://doi.org/10.3390/jcm14092912







