The Association Between Erythropoiesis Resistance Index and Clinical Outcomes in Hemodialysis Patients: A Nationwide Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources and Participants
2.2. Inclusion and Exclusion Criteria
2.3. Study Variables
2.4. Statistical Analyses
3. Results
3.1. Clinical Characteristics
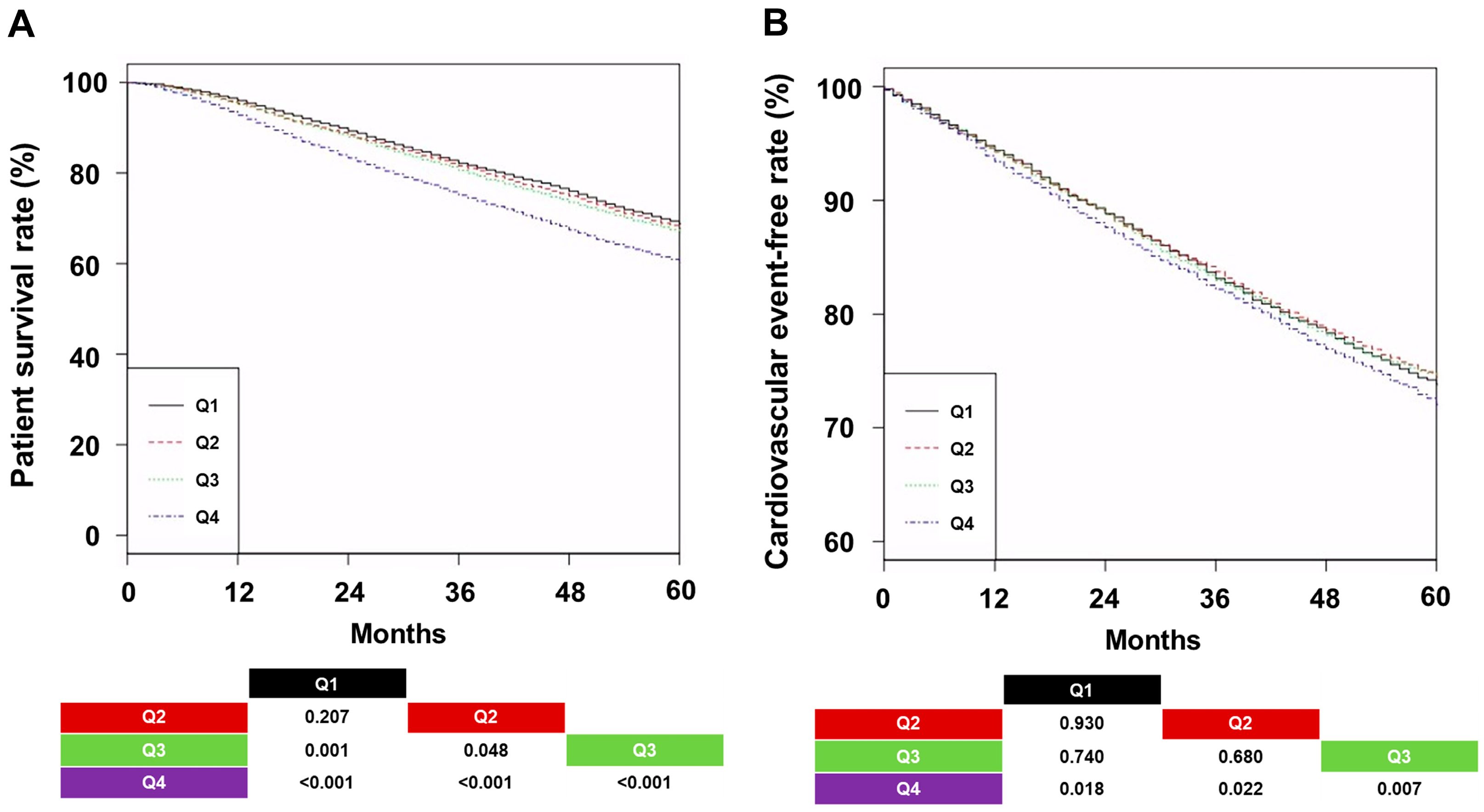
3.2. All-Cause Mortality and CVE According to Groups
3.3. Subgroup Analysis
3.4. Analyses Using the Balanced Cohort
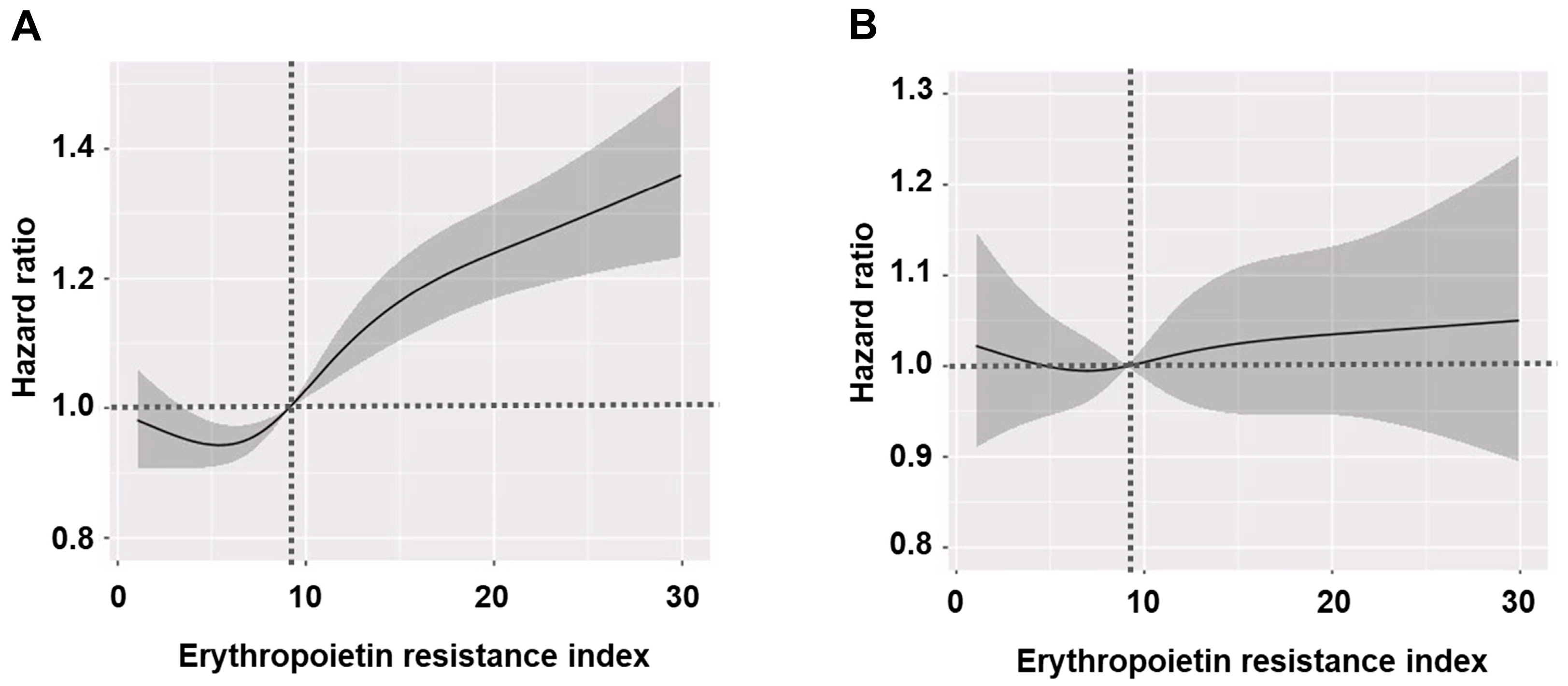
3.5. Clinical Factors Associated with ERI
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, K.M.; Jeong, S.A.; Ban, T.H.; Hong, Y.A.; Hwang, S.D.; Choi, S.R.; Lee, H.; Kim, J.H.; Kim, S.H.; Kim, T.H.; et al. Status and trends in epidemiologic characteristics of diabetic end-stage renal disease: An analysis of the 2021 Korean Renal Data System. Kidney Res. Clin. Pract. 2024, 43, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Bello, A.K.; Okpechi, I.G.; Osman, M.A.; Cho, Y.; Htay, H.; Jha, V.; Wainstein, M.; Johnson, D.W. Epidemiology of haemodialysis outcomes. Nat. Rev. Nephrol. 2022, 18, 378–395. [Google Scholar] [CrossRef] [PubMed]
- Babitt, J.L.; Lin, H.Y. Mechanisms of anemia in CKD. J. Am. Soc. Nephrol. 2012, 23, 1631–1634. [Google Scholar] [CrossRef]
- van der Meer, P.; van Veldhuisen, D.J. Anaemia and renal dysfunction in chronic heart failure. Heart. 2009, 95, 1808–1812. [Google Scholar] [CrossRef]
- KDIGO Anemia Work Group. KDIGO Clinical Practice Guideline for Anemia in Chronic Kidney Disease. Kidney Int. Suppl. 2012, 2, 279–335. [Google Scholar]
- Locatelli, F.; Del Vecchio, L.; Minutolo, R.; De Nicola, L. Anemia: A Connection Between Heart Failure and Kidney Failure. Cardiol. Clin. 2021, 39, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Wang, J.; Onishi, Y.; Nangaku, M. Association between magnesium, erythropoietin resistance and mortality: The Japanese Dialysis Outcomes and Practice Patterns Study (J-DOPPS). Clin. Kidney J. 2024, 17, sfae153. [Google Scholar] [CrossRef]
- Zhao, X.; Gan, L.; Hou, F.F.; Liang, X.; Chen, X.; Chen, Y.; Ni, Z.; Zuo, L. The influencing factors of the erythropoietin resistance index and its association with all-cause mortality in maintenance hemodialysis patients. Ren. Fail. 2024, 46, 2290922. [Google Scholar] [CrossRef]
- Pan, S.; Zhao, D.L.; Li, P.; Sun, X.F.; Zhou, J.H.; Song, K.K.; Wang, Y.; Miao, L.N.; Ni, Z.H.; Lin, H.L.; et al. Relationships among the Dosage of Erythropoiesis-Stimulating Agents, Erythropoietin Resistance Index, and Mortality in Maintenance Hemodialysis Patients. Blood Purif. 2022, 51, 171–181. [Google Scholar] [CrossRef]
- Kang, S.H.; Kim, B.Y.; Son, E.J.; Kim, G.O.; Do, J.Y. Comparison of Patient Survival According to Erythropoiesis-Stimulating Agent Type of Treatment in Maintenance Hemodialysis Patients. J. Clin. Med. 2023, 12, 625. [Google Scholar] [CrossRef]
- Health Insurance Review & Assessment Service. 6th Hemodialysis Quality Assessment Program. Available online: https://www.hira.or.kr/bbsDummy.do?pgmid=HIRAA020002000100&brdScnBltNo=4&brdBltNo=6619#none (accessed on 12 March 2025).
- Daugirdas, J.T. Second generation logarithmic estimates of single-pool variable volume Kt/V: An analysis of error. J. Am. Soc. Nephrol. 1993, 4, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Vega, A.; Abad, S.; Verdalles, U.; Aragoncillo, I.; Velazquez, K.; Quiroga, B.; Escudero, V.; López-Gómez, J.M. Dose equivalence between continuous erythropoietin receptor activator (CERA), Darbepoetin and Epoetin in patients with advanced chronic kidney disease. Hippokratia 2014, 18, 315–318. [Google Scholar] [PubMed]
- Fishbane, S.; Berns, J.S. Hemoglobin cycling in hemodialysis patients treated with recombinant human erythropoietin. Kidney Int. 2005, 68, 1337–1343. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Quan, H.; Sundararajan, V.; Halfon, P.; Fong, A.; Burnand, B.; Luthi, J.C.; Saunders, L.D.; Beck, C.A.; Feasby, T.E.; Ghali, W.A. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med. Care 2005, 43, 1130–1139. [Google Scholar] [CrossRef]
- Kang, S.H.; Kim, B.Y.; Son, E.J.; Kim, G.O.; Do, J.Y. Influence of Different Types of β-Blockers on Mortality in Patients on Hemodialysis. Biomedicines 2023, 11, 2838. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.H.; Su, F.Y.; Chang, H.Y.; Su, P.C.; Chiu, L.Y.; Nowicki, M.; Kao, C.C.; Lin, Y.C. The Effect of Statin on Anemia in Patients with Chronic Kidney Disease and End-Stage Kidney Disease: A Systematic Review and Meta-Analysis. J. Pers. Med. 2022, 12, 1175. [Google Scholar] [CrossRef]
- Cheungpasitporn, W.; Thongprayoon, C.; Chiasakul, T.; Korpaisarn, S.; Erickson, S.B. Renin-angiotensin system inhibitors linked to anemia: A systematic review and meta-analysis. QJM 2015, 108, 879–884. [Google Scholar] [CrossRef]
- Del Vecchio, L.; Locatelli, F. An overview on safety issues related to erythropoiesis-stimulating agents for the treatment of anaemia in patients with chronic kidney disease. Expert Opin. Drug Saf. 2016, 15, 1021–1030. [Google Scholar] [CrossRef]
- Hazzan, A.D.; Shah, H.H.; Hong, S.; Sakhiya, V.; Wanchoo, R.; Fishbane, S. Treatment with erythropoiesis-stimulating agents in chronic kidney disease patients with cancer. Kidney Int. 2014, 86, 34–39. [Google Scholar] [CrossRef][Green Version]
- Watanabe, D.; Suzuma, K.; Matsui, S.; Kurimoto, M.; Kiryu, J.; Kita, M.; Suzuma, I.; Ohashi, H.; Ojima, T.; Murakami, T.; et al. Erythropoietin as a retinal angiogenic factor in proliferative diabetic retinopathy. N. Engl. J. Med. 2005, 353, 782–792. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.H.; Jeon, Y.; Yook, J.M.; Choi, S.Y.; Jung, H.Y.; Choi, J.Y.; Park, S.H.; Kim, C.D.; Kim, Y.L.; Cho, J.H. Medium cut-off dialyzer improves erythropoiesis stimulating agent resistance in a hepcidin-independent manner in maintenance hemodialysis patients: Results from a randomized controlled trial. Sci. Rep. 2020, 10, 16062. [Google Scholar] [CrossRef] [PubMed]
- Yajima, T.; Yajima, K.; Takahashi, H. Association of the erythropoiesis-stimulating agent resistance index and the geriatric nutritional risk index with cardiovascular mortality in maintenance hemodialysis patients. PLoS ONE 2021, 16, e0245625. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, J.; Wang, S.; Yu, Q.; Li, H. High Erythropoiesis Resistance Index Is a Significant Predictor of Cardiovascular and All-Cause Mortality in Chinese Maintenance Hemodialysis Patients. Mediat. Inflamm. 2020, 2020, 1027230. [Google Scholar] [CrossRef]
- Fujikawa, T.; Ikeda, Y.; Fukuhara, S.; Akiba, T.; Akizawa, T.; Kurokawa, K.; Saito, A. Time-dependent resistance to erythropoiesis-stimulating agent and mortality in hemodialysis patients in the Japan Dialysis Outcomes and Practice Patterns Study. Nephron Clin. Pract. 2012, 122, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Shimizu, H.; Kyono, A.; Sato, M.; Yamashita, T.; Otsuka, K.; Nitta, K. Relationship between responsiveness to erythropoiesis-stimulating agent and long-term outcomes in chronic hemodialysis patients: A single-center cohort study. Int. Urol. Nephrol. 2014, 46, 151–159. [Google Scholar] [CrossRef]
- Okazaki, M.; Komatsu, M.; Kawaguchi, H.; Tsuchiya, K.; Nitta, K. Erythropoietin resistance index and the all-cause mortality of chronic hemodialysis patients. Blood Purif. 2014, 37, 106–112. [Google Scholar] [CrossRef]
- Eriguchi, R.; Taniguchi, M.; Ninomiya, T.; Hirakata, H.; Fujimi, S.; Tsuruya, K.; Kitazono, T. Hyporesponsiveness to erythropoiesis-stimulating agent as a prognostic factor in Japanese hemodialysis patients: The Q-Cohort study. J. Nephrol. 2015, 28, 217–225. [Google Scholar] [CrossRef]
- Bae, M.N.; Kim, S.H.; Kim, Y.O.; Jin, D.C.; Song, H.C.; Choi, E.J.; Kim, Y.L.; Kim, Y.S.; Kang, S.W.; Kim, N.H.; et al. Association of Erythropoietin-Stimulating Agent Responsiveness with Mortality in Hemodialysis and Peritoneal Dialysis Patients. PLoS ONE 2015, 10, e0143348. [Google Scholar] [CrossRef]
- Panichi, V.; Rosati, A.; Bigazzi, R.; Paoletti, S.; Mantuano, E.; Beati, S.; Marchetti, V.; Bernabini, G.; Grazi, G.; Rizza, G.M.; et al. Anaemia and resistance to erythropoiesis-stimulating agents as prognostic factors in haemodialysis patients: Results from the RISCAVID study. Nephrol. Dial. Transplant. 2011, 26, 2641–2648. [Google Scholar] [CrossRef]
- Kidney Disease: Improving Global Outcomes (KDIGO) Anemia Work Group. KDIGO 2025 Clinical Practice Guideline for Anemia in Chronic Kidney Disease (CKD): Public Review Draft. November 2024. Available online: https://kdigo.org (accessed on 10 April 2025).
- Susantitaphong, P.; Riella, C.; Jaber, B.L. Effect of ultrapure dialysate on markers of inflamma tion, oxidative stress, nutrition and anemia parameters: A meta-analysis. Nephrol. Dial. Transpl. 2013, 28, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Ayus, J.C.; Mizani, M.R.; Achinger, S.G.; Thadhani, R.; Go, A.S.; Lee, S. Effects of short daily versus conventional hemodialysis on left ventricular hypertrophy and inflammatory markers: A prospective, controlled study. J. Am. Soc. Nephrol. 2005, 16, 2778–2788. [Google Scholar] [CrossRef] [PubMed]
- Akchurin, O.M.; Kaskel, F. Update on inflammation in chronic kidney disease. Blood Purif. 2015, 39, 84–92. [Google Scholar] [CrossRef]
- Morikami, Y.; Fujimori, A.; Okada, S.; Kumei, M.; Mizobuchi, N.; Sakai, M. Comparison of 2-week versus 4-week dosing intervals of epoetin beta pegol on erythropoiesis and iron metabolism in hemodialysis patients. Ther. Apher. Dial. 2014, 18, 414–420. [Google Scholar] [CrossRef] [PubMed]

| Q1 (n = 8979) | Q2 (n = 8978) | Q3 (n = 8978) | Q4 (n = 8978) | p-Value | |
|---|---|---|---|---|---|
| Age (years) | 59.6 ± 12.7 | 60.5 ± 12.8 a | 60.7 ± 12.8 a | 61.8 ± 12.7 abc | <0.001 |
| Sex (male, %) | 6190 (68.9%) | 5600 (62.4%) | 4867 (54.2%) | 4175 (46.5%) | <0.001 |
| HD vintage (months) | 63 ± 66 | 55 ± 58 a | 59 ± 60 ab | 66 ± 65 abc | <0.001 |
| BMI (kg/m2) | 23.3 ± 3.5 | 22.7 ± 3.2 a | 22.1 ± 3.2 ab | 21.2 ± 3.0 abc | <0.001 |
| Diabetes (%) | 4125 (45.9%) | 4220 (47.0%) | 3875 (43.2%) | 3659 (40.8%) | <0.001 |
| CCI score | 6.85 ± 2.71 | 6.82 ± 2.67 | 6.78 ± 2.73 | 6.95 ± 2.75 bc | <0.001 |
| Arteriovenous fistula (%) | 7758 (86.4%) | 7706 (85.8%) | 7681 (85.6%) | 7468 (83.2%) | <0.001 |
| Kt/Vurea | 1.48 ± 0.26 | 1.51 ± 0.27 a | 1.54 ± 0.26 ab | 1.58 ± 0.28 abc | <0.001 |
| UFV (L/session) | 2.28 ± 1.03 | 2.25 ± 0.95 | 2.30 ± 0.93 b | 2.32 ± 0.93 ab | <0.001 |
| Hemoglobin (g/dL) | 11.0 ± 0.7 | 10.7 ± 0.6 a | 10.6 ± 0.6 ab | 10.3 ± 0.7 abc | <0.001 |
| Serum albumin (g/dL) | 4.01 ± 0.33 | 4.00 ± 0.33 | 3.99 ± 0.34 ab | 3.93 ± 0.36 abc | <0.001 |
| Serum phosphorus (mg/dL) | 4.94 ± 1.30 | 4.97 ± 1.29 | 4.98 ± 1.35 | 4.88 ± 1.38 abc | <0.001 |
| Serum calcium (mg/dL) | 8.93 ± 0.80 | 8.91 ± 0.82 | 8.93 ± 0.84 | 8.90 ± 0.86 a | 0.011 |
| Serum creatinine (mg/dL) | 9.59 ± 2.90 | 9.61 ± 2.75 | 9.60 ± 2.61 | 9.18 ± 2.46 abc | <0.001 |
| Use of RASB (%) | 5473 (61.0%) | 6329 (70.5%) | 6616 (73.7%) | 6697 (74.6%) | <0.001 |
| Use of aspirin (%) | 1234 (13.7%) | 1182 (13.2%) | 1119 (12.5%) | 1107 (12.3%) | 0.016 |
| Use of clopidogrel (%) | 717 (8.0%) | 738 (8.2%) | 679 (7.6%) | 671 (7.5%) | 0.199 |
| Use of statins (%) | 4054 (45.1%) | 3898 (43.4%) | 3658 (40.7%) | 3399 (37.9%) | <0.001 |
| MI or CHF (%) | 3485 (38.8%) | 3448 (38.4%) | 3548 (39.5%) | 3767 (42.0%) | <0.001 |
| Atrial fibrillation (%) | 672 (7.5%) | 596 (6.6%) | 648 (7.2%) | 776 (8.6%) | <0.001 |
| CVA (%) | 2398 (26.7%) | 2386 (26.6%) | 2286 (25.5%) | 2374 (26.4%) | 0.216 |
| Transferrin saturation (%) | 35.2 ± 28.7 | 35.5 ± 27.0 | 34.4 ± 25.2 b | 32.9 ± 28.1 abc | <0.001 |
| Ferritin (ng/mL) | 254 ± 257 | 266 ± 250 a | 270 ± 255 a | 281 ± 287 abc | <0.001 |
| ESA dose (IU/week) | 2465 ± 1141 | 4895 ± 1006 a | 6791 ± 1331 ab | 10,053 ± 2551 abc | <0.001 |
| Univariable | Multivariable | |||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| All-Cause Mortality | ||||
| Reference: Q1 | ||||
| Q2 | 1.03 (0.99–1.07) | 0.206 | 1.02 (0.97–1.07) | 0.405 |
| Q3 | 1.07 (1.03–1.11) | <0.001 | 1.09 (1.02–1.17) | 0.014 |
| Q4 | 1.28 (1.23–1.33) | <0.001 | 1.24 (1.15–1.35) | <0.001 |
| Reference: Q2 | ||||
| Q3 | 1.04 (1.00–1.09) | 0.041 | 1.07 (1.01–1.13) | 0.025 |
| Q4 | 1.25 (1.20–1.30) | <0.001 | 1.22 (1.14–1.30) | <0.001 |
| Reference: Q3 | ||||
| Q4 | 1.20 (1.15–1.25) | <0.001 | 1.14 (1.09–1.19) | <0.001 |
| CVE | ||||
| Reference: Q1 | ||||
| Q2 | 1.00 (0.94–1.06) | 0.931 | 0.99 (0.92–1.06) | 0.680 |
| Q3 | 0.99 (0.93–1.05) | 0.743 | 0.98 (0.88–1.08) | 0.644 |
| Q4 | 1.07 (1.01–1.14) | 0.018 | 1.03 (0.91–1.16) | 0.635 |
| Reference: Q2 | ||||
| Q3 | 0.99 (0.93–1.05) | 0.678 | 0.99 (0.91–1.08) | 0.824 |
| Q4 | 1.07 (1.01–1.14) | 0.022 | 1.05 (0.94–1.16) | 0.397 |
| Reference: Q3 | ||||
| Q4 | 1.09 (1.02–1.15) | 0.007 | 1.06 (0.98–1.13) | 0.127 |
| Univariable | Multivariable | Univariable | Multivariable | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | ||
| Males | Females | ||||||||
| Reference: Q1 | |||||||||
| Q2 | 1.07 (1.01–1.12) | 0.012 | 1.00 (0.95–1.06) | 0.931 | 0.98 (0.92–1.06) | 0.628 | 1.02 (0.94–1.10) | 0.623 | |
| Q3 | 1.13 (1.07–1.18) | <0.001 | 1.03 (0.93–1.13) | 0.601 | 1.04 (0.98–1.12) | 0.203 | 1.14 (1.03–1.26) | 0.013 | |
| Q4 | 1.45 (1.38–1.52) | <0.001 | 1.18 (1.07–1.32) | 0.002 | 1.21 (1.14–1.30) | <0.001 | 1.29 (1.14–1.47) | <0.001 | |
| Reference: Q2 | |||||||||
| Q3 | 1.06 (1.00–1.11) | 0.033 | 1.02 (0.95–1.11) | 0.565 | 1.06 (0.99–1.13) | 0.061 | 1.12 (1.02–1.22) | 0.018 | |
| Q4 | 1.36 (1.29–1.43) | <0.001 | 1.18 (1.08–1.29) | <0.001 | 1.24 (1.16–1.31) | <0.001 | 1.27 (1.13–1.42) | <0.001 | |
| Reference: Q3 | |||||||||
| Q4 | 1.29 (1.22–1.36) | <0.001 | 1.15 (1.09–1.23) | <0.001 | 1.16 (1.10–1.23) | <0.001 | 1.14 (1.06–1.22) | <0.001 | |
| <65 years old | ≥65 years old | ||||||||
| Reference: Q1 | |||||||||
| Q2 | 0.98 (0.92–1.05) | 0.556 | 0.94 (0.88–1.02) | 0.128 | 1.01 (0.96–1.06) | 0.765 | 1.00 (0.94–1.06) | 0.976 | |
| Q3 | 1.07 (1.00–1.14) | 0.042 | 1.04 (0.93–1.16) | 0.537 | 0.99 (0.95–1.05) | 0.869 | 1.01 (0.93–1.11) | 0.776 | |
| Q4 | 1.26 (1.18–1.34) | <0.001 | 1.14 (0.99–1.29) | 0.051 | 1.20 (1.14–1.26) | <0.001 | 1.14 (1.03–1.27) | 0.014 | |
| Reference: Q2 | |||||||||
| Q3 | 1.09 (1.02–1.16) | 0.009 | 1.10 (1.01–1.20) | 0.048 | 0.99 (0.94–1.04) | 0.636 | 1.01 (0.94–1.09) | 0.757 | |
| Q4 | 1.28 (1.20–1.36) | <0.001 | 1.20 (1.08–1.34) | <0.001 | 1.19 (1.13–1.25) | <0.001 | 1.14 (1.04–1.25) | 0.004 | |
| Reference: Q3 | |||||||||
| Q4 | 1.18 (1.11–1.25) | <0.001 | 1.10 (1.02–1.18) | 0.011 | 1.20 (1.14–1.26) | <0.001 | 1.13 (1.06–1.19) | <0.001 | |
| Low CCI (<7) | High CCI (≥7) | ||||||||
| Reference: Q1 | |||||||||
| Q2 | 0.98 (0.92–1.05) | 0.655 | 0.92 (0.85–0.99) | 0.040 | 1.05 (1.00–1.11) | 0.041 | 1.08 (1.01–1.14) | 0.015 | |
| Q3 | 1.07 (1.00–1.14) | 0.037 | 1.04 (0.93–1.17) | 0.464 | 1.09 (1.03–1.14) | 0.001 | 1.11 (1.02–1.22) | 0.017 | |
| Q4 | 1.29 (1.21–1.37) | <0.001 | 1.14 (0.99–1.31) | 0.051 | 1.29 (1.23–1.35) | <0.001 | 1.28 (1.16–1.42) | <0.001 | |
| Reference: Q2 | |||||||||
| Q3 | 1.09 (1.02–1.16) | 0.011 | 1.13 (1.03–1.25) | 0.013 | 1.03 (0.98–1.09) | 0.220 | 1.03 (0.96–1.11) | 0.369 | |
| Q4 | 1.02 (0.95–1.09) | <0.001 | 1.24 (1.10–1.39) | <0.001 | 1.22 (1.17–1.28) | <0.001 | 1.19 (1.10–1.30) | <0.001 | |
| Reference: Q3 | |||||||||
| Q4 | 1.20 (1.13–1.28) | <0.001 | 1.10 (1.02–1.18) | 0.017 | 1.19 (1.13–1.24) | <0.001 | 1.15 (1.09–1.22) | <0.001 | |
| Low Hb (<10) | High Hb (≥10) | ||||||||
| Reference: Q1 | |||||||||
| Q2 | 1.03 (0.88–1.20) | 0.706 | 0.97 (0.82–1.16) | 0.758 | 1.02 (0.98–1.07) | 0.270 | 1.03 (0.98–1.08) | 0.217 | |
| Q3 | 1.00 (0.87–1.14) | 0.952 | 0.94 (0.76–1.15) | 0.531 | 1.07 (1.03–1.12) | 0.001 | 1.12 (1.04–1.21) | 0.002 | |
| Q4 | 1.33 (1.18–1.51) | <0.001 | 1.12 (0.89–1.40) | 0.353 | 1.22 (1.16–1.27) | <0.001 | 1.25 (1.15–1.37) | <0.001 | |
| Reference: Q2 | |||||||||
| Q3 | 0.97 (0.85–1.10) | 0.600 | 0.96 (0.81–1.15) | 0.672 | 1.05 (1.00–1.09) | 0.033 | 1.09 (1.02–1.16) | 0.007 | |
| Q4 | 1.29 (1.16–1.44) | <0.001 | 1.15 (0.94–1.40) | 0.181 | 1.19 (1.14–1.24) | <0.001 | 1.22 (1.13–1.31) | <0.001 | |
| Reference: Q3 | |||||||||
| Q4 | 1.34 (1.23–1.46) | <0.001 | 1.19 (1.07–1.33) | 0.002 | 1.13 (1.08–1.18) | <0.001 | 1.12 (1.06–1.17) | <0.001 | |
| Low ESA dose | High ESA dose | ||||||||
| Reference: Q1 | |||||||||
| Q2 | 1.04 (0.99–1.08) | 0.108 | 1.09 (1.01–1.18) | 0.026 | 1.74 (0.83–3.66) | 0.145 | 0.91 (0.43–1.92) | 0.803 | |
| Q3 | 1.11 (1.04–1.19) | 0.003 | 1.26 (1.12–1.43) | <0.001 | 1.86 (0.89–3.91) | 0.100 | 0.92 (0.44–1.96) | 0.837 | |
| Q4 | 2.41 (1.77–3.28) | <0.001 | 2.37 (1.65–3.40) | <0.001 | 2.25 (1.07–4.71) | 0.033 | 1.05 (0.49–2.24) | 0.894 | |
| Reference: Q2 | |||||||||
| Q3 | 1.07 (0.99–1.15) | 0.057 | 1.16 (1.06–1.27) | 0.001 | 1.07 (1.00–1.15) | 0.048 | 1.02 (0.94–1.10) | 0.692 | |
| Q4 | 2.33 (1.71–3.17) | <0.001 | 2.17 (1.53–3.07) | <0.001 | 1.29 (1.21–1.38) | <0.001 | 1.16 (1.04–1.29) | 0.006 | |
| Reference: Q3 | |||||||||
| Q4 | 2.17 (1.59–2.97) | <0.001 | 1.87 (1.32–2.65) | <0.001 | 1.21 (1.16–1.26) | <0.001 | 1.14 (1.07–1.21) | <0.001 | |
| Short HDV (<40 M) | Long HDV (≥40 M) | ||||||||
| Reference: Q1 | |||||||||
| Q2 | 1.04 (0.98–1.10) | 0.181 | 1.07 (0.99–1.14) | 0.056 | 1.02 (0.96–1.08) | 0.541 | 0.98 (0.92–1.05) | 0.656 | |
| Q3 | 1.11 (1.05–1.17) | <0.001 | 1.13 (1.02–1.24) | 0.016 | 1.03 (0.98–1.09) | 0.275 | 1.06 (0.96–1.17) | 0.220 | |
| Q4 | 1.36 (1.29–1.44) | <0.001 | 1.34 (1.19–1.50) | <0.001 | 1.21 (1.14–1.28) | <0.001 | 1.15 (1.03–1.30) | 0.014 | |
| Reference: Q2 | |||||||||
| Q3 | 1.06 (1.01–1.13) | 0.027 | 1.06 (0.98–1.15) | 0.171 | 1.01 (0.96–1.07) | 0.646 | 1.08 (0.99–1.18) | 0.074 | |
| Q4 | 1.31 (1.24–1.39) | <0.001 | 1.25 (1.14–1.38) | <0.001 | 1.19 (1.12–1.25) | <0.001 | 1.17 (1.06–1.29) | 0.002 | |
| Reference: Q3 | |||||||||
| Q4 | 1.23 (1.17–1.30) | <0.001 | 1.18 (1.11–1.26) | <0.001 | 1.17 (1.11–1.23) | <0.001 | 1.09 (1.02–1.16) | 0.011 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, S.-H.; Park, S.-Y.; Lim, Y.-J.; Kim, B.-Y.; Choi, J.-Y.; Do, J.-Y.; Kim, A.-Y. The Association Between Erythropoiesis Resistance Index and Clinical Outcomes in Hemodialysis Patients: A Nationwide Study. J. Clin. Med. 2025, 14, 2812. https://doi.org/10.3390/jcm14082812
Kang S-H, Park S-Y, Lim Y-J, Kim B-Y, Choi J-Y, Do J-Y, Kim A-Y. The Association Between Erythropoiesis Resistance Index and Clinical Outcomes in Hemodialysis Patients: A Nationwide Study. Journal of Clinical Medicine. 2025; 14(8):2812. https://doi.org/10.3390/jcm14082812
Chicago/Turabian StyleKang, Seok-Hui, So-Young Park, Yu-Jeong Lim, Bo-Yeon Kim, Ji-Young Choi, Jun-Young Do, and A-Young Kim. 2025. "The Association Between Erythropoiesis Resistance Index and Clinical Outcomes in Hemodialysis Patients: A Nationwide Study" Journal of Clinical Medicine 14, no. 8: 2812. https://doi.org/10.3390/jcm14082812
APA StyleKang, S.-H., Park, S.-Y., Lim, Y.-J., Kim, B.-Y., Choi, J.-Y., Do, J.-Y., & Kim, A.-Y. (2025). The Association Between Erythropoiesis Resistance Index and Clinical Outcomes in Hemodialysis Patients: A Nationwide Study. Journal of Clinical Medicine, 14(8), 2812. https://doi.org/10.3390/jcm14082812





