Pharmacokinetic Characterization of Labetalol in Pregnancy (The CLIP Study): A Prospective Observational Longitudinal Pharmacokinetic/Pharmacodynamic Cohort Study During Pregnancy and Postpartum
Abstract
1. Introduction
2. Study Aims
2.1. Primary Aims
2.2. Secondary Aims
2.3. Exploratory Aims
2.4. Demographic and Clinical Information
3. Study Procedures
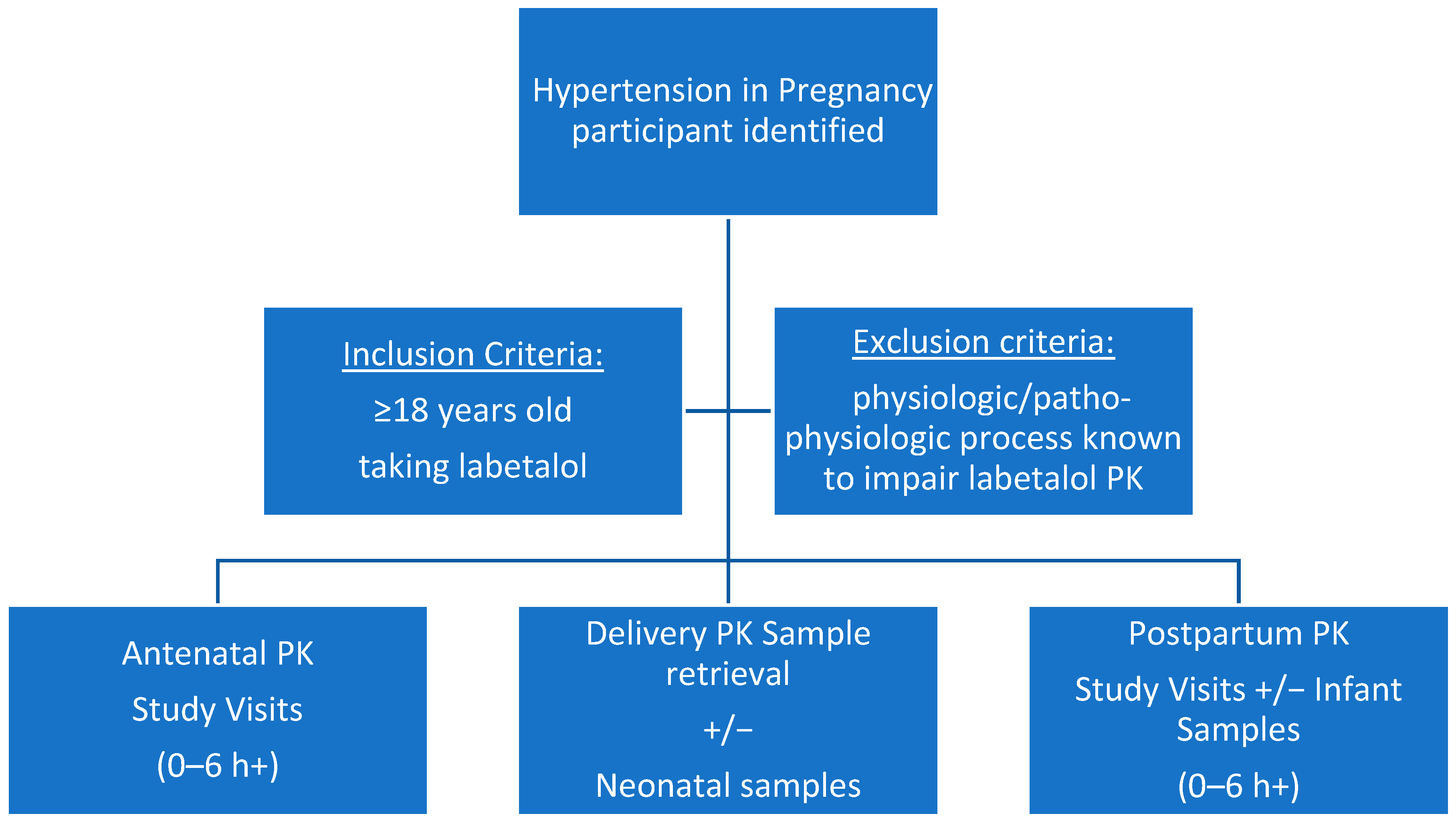
3.1. Population/Recruitment
3.2. Labetalol Dosing
3.3. PK Clinical Study Visits
3.3.1. Study Design
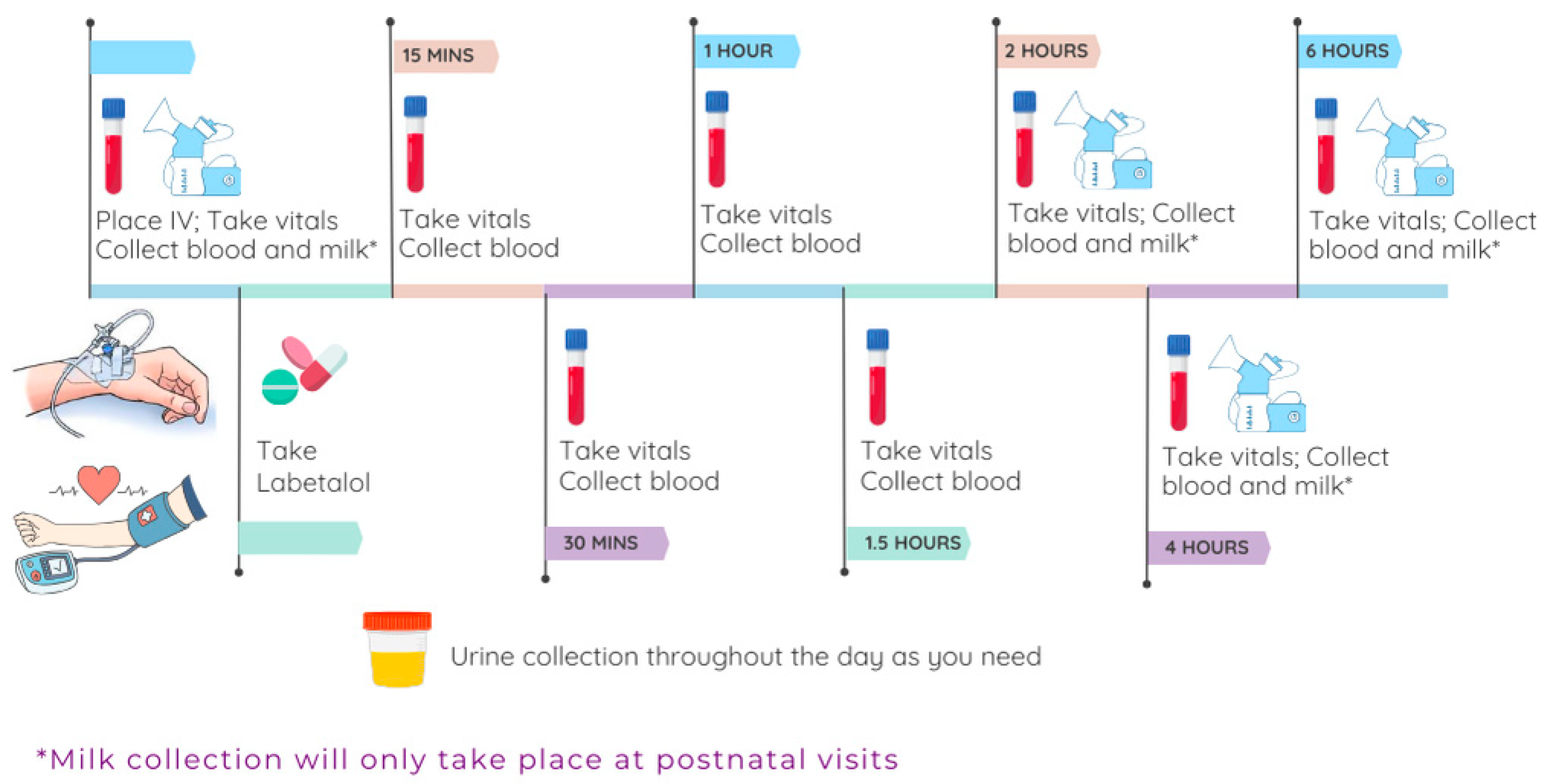
3.3.2. PK/PD Study Visit Rationale
3.3.3. PK/PD Study Visit Sample Collection
3.3.4. Pharmacogenomic Sampling
4. PK Sample Analysis Plan
4.1. Biospecimen Collection, Processing, and Storage
4.2. Chiral Separation LC-MS/MS
5. PK Analytical Plan
Population PK/PD Models
6. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Maternal Demographics | |
| Date of birth | |
| Race/ethnicity | |
| Smoking status | |
| Alcohol use | |
| Socioeconomic status (zip code, insurance) | |
| Pregnancy and Medical History | |
| Gravidity and parity | |
| Estimated due date (based on LNMP or first-trimester USS) | |
| Height | |
| Weight | |
| Past medical history (e.g., diabetes, hypertension, thyroid disease, anxiety/depression, infections) | |
| Medications during pregnancy and study period (including over-the-counter/vitamins) | |
| Pregnancy complications (pre-eclampsia, gestational diabetes mellitus, preterm labor, placenta previa, chorioamnionitis) | |
| Date of hypertension diagnosis | |
| Hypertensive complications | |
| Number of hospitalizations for management of hypertension in pregnancy | |
| Anti-hypertensive medication use and regimens (dose, frequency, dates/times) | |
| Delivery hospital | |
| Outcomes Data | |
| Type of delivery (vaginal, operative instrumental, operative cesarean section) | |
| Gestational age at delivery | |
| Race/ethnicity and sex of baby | |
| Neonatal status (NICU admission; Apgar scores; weight and height at delivery; complications, including SGA, RDS, sepsis, PDA) |
References
- Cameron, N.A.; Everitt, I.; Seegmiller, L.E.; Yee, L.M.; Grobman, W.A.; Khan, S.S. Trends in the Incidence of New-Onset Hypertensive Disorders of Pregnancy among Rural and Urban Areas in the United States, 2007 to 2019. J. Am. Heart Assoc. 2022, 11, e023791. [Google Scholar] [CrossRef] [PubMed]
- Vasconcellos, D. CDC Press Release: Hypertensive Disorders in Pregnancy Affect 1 in 7 Hospital Deliveries. Neonatology Today, 1 May 2022. [Google Scholar]
- Petersen, E.E.; Davis, N.L.; Goodman, D.; Cox, S.; Mayes, N.; Johnston, E.; Syverson, C.; Seed, K.; Shapiro-Mendoza, C.K.; Callaghan, W.M.; et al. Vital Signs: Pregnancy-Related Deaths, United States, 2011–2015, and Strategies for Prevention, 13 States, 2013–2017. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 423–429. [Google Scholar] [CrossRef]
- Say, L.; Chou, D.; Gemmill, A.; Tunçalp, Ö.; Moller, A.-B.; Daniels, J.; Gülmezoglu, A.M.; Temmerman, M.; Alkema, L. Global causes of maternal death: A WHO systematic analysis. Lancet Glob. Health 2014, 2, e323–e333. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 203: Chronic hypertension in pregnancy. Obstet. Gynecol. 2019, 133, e26–e50. [Google Scholar] [CrossRef]
- Tita, A.T.; Szychowski, J.M.; Boggess, K.; Dugoff, L.; Sibai, B.; Lawrence, K.; Hughes, B.L.; Bell, J.; Aagaard, K.; Edwards, R.K.; et al. Treatment for Mild Chronic Hypertension during Pregnancy. N. Engl. J. Med. 2022, 386, 1781–1792. [Google Scholar] [CrossRef]
- Clark, S.M.; Dunn, H.E.; Hankins, G.D. A review of oral labetalol and nifedipine in mild to moderate hypertension in pregnancy. Semin. Perinatol. 2015, 39, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.; Kerndt, C.C.; Maani, C.V. Labetalol; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- WHO Recommendations on Drug Treatment for Non-Severe Hypertension in Pregnancy: World Health Organization. 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK561247/pdf/Bookshelf_NBK561247.pdf (accessed on 15 April 2025).
- Espinoza, J.; Vidaaeff, A.; Pettker, C.M.; Simhan, H. Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin, Number 222. Obs. Gynecol 2020, 135, e237–e260. [Google Scholar]
- Hypertension and Pregnancy. Queensland Clinical Guidelines. Available online: https://www.health.qld.gov.au/__data/assets/pdf_file/0034/139948/g-hdp.pdf (accessed on 29 March 2025).
- Yang, Z.; Zhang, W.Y.; Ling, J.H.; Li, X.T.; Liu, J.T.; Qi, H. Guidelines for Diagnosis and Treatment of Hypertensive Disorder Complicating Pregnancy. Chin. J. Pract. Gynecolol. Obstet. 2020, 55, 227–238. [Google Scholar]
- MacCarthy, E.P.; Bloomfield, S.S. Labetalol: A Review of Its Pharmacology, Pharmacokinetics, Clinical Uses and Adverse Effects. Pharmacotherapy 1983, 3, 193–219. [Google Scholar] [CrossRef]
- McNeil, J.J.; Louis, W.J. Clinical Pharmacokinetics of Labetalol. Clin. Pharmacokinet. 1984, 9, 157–167. [Google Scholar] [CrossRef]
- Gold, E.H.; Chang, W.; Cohen, M.; Baum, T.; Ehrreich, S.; Johnson, G.; Prioli, N.; Sybertz, E.J. Synthesis and Comparison of Some Cardiovascular Properties of the Stereoisomers of Labetalol. J. Med. Chem. 1982, 25, 1363–1370. [Google Scholar] [CrossRef]
- Riva, E.; Mennini, T.; Latini, R. The α-and β-Adrenoceptor Blocking Activities of Labetalol and Its RR-SR (50:50) Stereoisomers. Br. J. Pharmacol. 1991, 104, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Volotinen, M.; Turpeinen, M.; Tolonen, A.; Uusitalo, J.; Mäenpää, J.; Pelkonen, O. Timolol Metabolism in Human Liver Microsomes Is Mediated Principally by CYP2D6. Drug Metab. Dispos. 2007, 35, 1135–1141. [Google Scholar] [CrossRef] [PubMed]
- Kirsten, R.; Nelson, K.; Kirsten, D.; Heintz, B. Clinical Pharmacokinetics of Vasodilators: Part II. Clin. Pharmacokinet. 1998, 35, 9–36. [Google Scholar] [CrossRef]
- Anderson, G.D.; Carr, D.B. Effect of Pregnancy on the Pharmacokinetics of Antihypertensive Drugs. Clin. Pharmacokinet. 2009, 48, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Trandate (Labetalol Hydrochloride) Product Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/018716s026lbl.pdf (accessed on 27 August 2024).
- Mehvar, R.; Brocks, D.R. Stereospecific Pharmacokinetics and Pharmacodynamics of Beta-Adrenergic Blockers in Humans. J. Pharm. Pharm. Sci. 2001, 4, 185–200. [Google Scholar]
- Carvalho, T.M.J.P.; Cavalli, R.d.C.; Cunha, S.P.; de Baraldi, C.O.; Marques, M.P.; Antunes, N.J.; Godoy, A.L.P.C.; Lanchote, V.L. Influence of gestational diabetes mellitus on the stereoselective kinetic disposition and metabolism of labetalol in hypertensive patients. Eur. J. Clin. Pharmacol. 2011, 67, 55–61. [Google Scholar] [CrossRef]
- Mulrenin, I.R.; Garcia, J.E.; Fashe, M.M.; Loop, M.S.; Daubert, M.A.; Urrutia, R.P.; Lee, C.R. The Impact of Pregnancy on Antihypertensive Drug Metabolism and Pharmacokinetics: Current Status and Future Directions. Expert Opin. Drug Metab. Toxicol. 2021, 17, 1261–1279. [Google Scholar] [CrossRef]
- Rogers, R.C.; Sibai, B.M.; Whybrew, W.D. Labetalol Pharmacokinetics in Pregnancy-Induced Hypertension. Am. J. Obstet. Gynecol. 1990, 162, 362–366. [Google Scholar] [CrossRef]
- Rubin, P.C.; Butters, L.; Kelman, A.W.; Fitzsimons, C.; Reid, J.L. Labetalol Disposition and Concentration-Effect Relationships during Pregnancy. Br. J. Clin. Pharmacol. 1983, 15, 465–470. [Google Scholar] [CrossRef]
- Saotome, T.; Minoura, S.; Terashi, K.; Sato, T.; Echizen, H.; Ishizaki, T. Labetalol in Hypertension during the Third Trimester of Pregnancy: Its Antihypertensive Effect and Pharmacokinetic-Dynamic Analysis. J. Clin. Pharmacol. 1993, 33, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Abduljalil, K.; Furness, P.; Johnson, T.N.; Rostami-Hodjegan, A.; Soltani, H. Anatomical, Physiological and Metabolic Changes with Gestational Age during Normal Pregnancy: A Database for Parameters Required in Physiologically Based Pharmacokinetic Modelling: A Database for Parameters Required in Physiologically Based Pharmacokinetic Modelling. Clin. Pharmacokinet. 2012, 51, 365–396. [Google Scholar] [CrossRef]
- Chan, S.W.; Hu, M.; Ko, S.S.W.; Tam, C.W.Y.; Fok, B.S.P.; Yin, O.Q.P.; Chow, M.S.S.; Tomlinson, B. CYP2C19 genotype has a major influence on labetalol pharmacokinetics in healthy male Chinese subjects. Eur. J. Clin. Pharmacol. 2013, 69, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Tracy, T.S.; Venkataramanan, R.; Glover, D.D.; Caritis, S.N. National Institute for Child Health and Human Development Network of Maternal-Fetal-Medicine Units. Temporal Changes in Drug Metabolism (CYP1A2, CYP2D6 and CYP3A Activity) during Pregnancy. Am. J. Obstet. Gynecol. 2005, 192, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Andrew, M.A.; Easterling, T.R.; Carr, D.B.; Shen, D.; Buchanan, M.L.; Rutherford, T.; Bennett, R.; Vicini, P.; Hebert, M.F. Amoxicillin Pharmacokinetics in Pregnant Women: Modeling and Simulations of Dosage Strategies. Clin. Pharmacol. Ther. 2007, 81, 547–556. [Google Scholar] [CrossRef]
- Caritis, S.N.; Bastian, J.R.; Zhang, H.; Kalluri, H.; English, D.; England, M.; Bobby, S.; Venkataramanan, R. An Evidence-Based Recommendation to Increase the Dosing Frequency of Buprenorphine during Pregnancy. Am. J. Obstet. Gynecol. 2017, 217, 459.e1–459.e6. [Google Scholar] [CrossRef]
- Caritis, S.N.; Hebert, M.F. A Pharmacologic Approach to the Use of Glyburide in Pregnancy. Obstet. Gynecol. 2013, 121, 1309–1312. [Google Scholar] [CrossRef]
- Hebert, M.; Ma, X.; Naraharisetti, S.; Krudys, K.; Umans, J.; Hankins, G.; Caritis, S.; Miodovnik, M.; Mattison, D.R.; Unadkat, J.; et al. Are We Optimizing Gestational Diabetes Treatment with Glyburide? The Pharmacologic Basis for Better Clinical Practice. Clin. Pharmacol. Ther. 2009, 85, 607–614. [Google Scholar] [CrossRef]
- Khatri, R.; Fallon, J.K.; Sykes, C.; Kulick, N.; Rementer, R.J.B.; Miner, T.A.; Schauer, A.P.; Kashuba, A.D.M.; Boggess, K.A.; Brouwer, K.L.R.; et al. Pregnancy-Related Hormones Increase UGT1A1-Mediated Labetalol Metabolism in Human Hepatocytes. Front. Pharmacol. 2021, 12, 655320. [Google Scholar] [CrossRef]
- Liao, M.Z.; Gao, C.; Phillips, B.R.; Neradugomma, N.K.; Han, L.W.; Bhatt, D.K.; Prasad, B.; Shen, D.D.; Mao, Q. Pregnancy Increases Norbuprenorphine Clearance in Mice by Induction of Hepatic Glucuronidation. Drug Metab. Dispos. 2018, 46, 100–108. [Google Scholar] [CrossRef]
- Center for Drug Evaluation. Research. Analytical Procedures and Methods Validation for Drugs and Biologics. U.S. Food and Drug Administration. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/analytical-procedures-and-methods-validation-drugs-and-biologics (accessed on 4 August 2024).
- ICH Q2(R2) Validation of Analytical Procedures—Scientific Guideline. European Medicines Agency (EMA). Available online: https://www.ema.europa.eu/en/ich-q2r2-validation-analytical-procedures-scientific-guideline (accessed on 4 August 2024).
- Masters, A.R.; Gufford, B.T.; Lu, J.B.L.; Metzger, I.F.; Jones, D.R.; Desta, Z. Chiral Plasma Pharmacokinetics and Urinary Excretion of Bupropion and Metabolites in Healthy Volunteers. J. Pharmacol. Exp. Ther. 2016, 358, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Beal, S.; Sheiner, L.B.; Boeckmann, R.; Bauer, R.J. NONMEM User’s Guides (1989–2009). 2009. Available online: https://www.scienceopen.com/document?vid=7bf48f4e-0bb4-4719-abe3-090ca42463be (accessed on 15 April 2025).
- Keizer, R.; Karlsson, M.; Hooker, A. Modeling and Simulation Workbench for NONMEM: Tutorial on Pirana, PsN, and Xpose. CPT Pharmacomet. Syst. Pharmacol. 2013, 2, e50. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhamidipaty-Pelosi, S.; Muralidharan, S.; Yeley, B.C.; Haas, D.M.; Quinney, S.K. Pharmacokinetic Characterization of Labetalol in Pregnancy (The CLIP Study): A Prospective Observational Longitudinal Pharmacokinetic/Pharmacodynamic Cohort Study During Pregnancy and Postpartum. J. Clin. Med. 2025, 14, 2793. https://doi.org/10.3390/jcm14082793
Bhamidipaty-Pelosi S, Muralidharan S, Yeley BC, Haas DM, Quinney SK. Pharmacokinetic Characterization of Labetalol in Pregnancy (The CLIP Study): A Prospective Observational Longitudinal Pharmacokinetic/Pharmacodynamic Cohort Study During Pregnancy and Postpartum. Journal of Clinical Medicine. 2025; 14(8):2793. https://doi.org/10.3390/jcm14082793
Chicago/Turabian StyleBhamidipaty-Pelosi, Surya, Suhaas Muralidharan, Brittany C. Yeley, David M. Haas, and Sara K. Quinney. 2025. "Pharmacokinetic Characterization of Labetalol in Pregnancy (The CLIP Study): A Prospective Observational Longitudinal Pharmacokinetic/Pharmacodynamic Cohort Study During Pregnancy and Postpartum" Journal of Clinical Medicine 14, no. 8: 2793. https://doi.org/10.3390/jcm14082793
APA StyleBhamidipaty-Pelosi, S., Muralidharan, S., Yeley, B. C., Haas, D. M., & Quinney, S. K. (2025). Pharmacokinetic Characterization of Labetalol in Pregnancy (The CLIP Study): A Prospective Observational Longitudinal Pharmacokinetic/Pharmacodynamic Cohort Study During Pregnancy and Postpartum. Journal of Clinical Medicine, 14(8), 2793. https://doi.org/10.3390/jcm14082793





