Prolonged COVID-19 Pneumonia in Patients with Hematologic Malignancies: Clinical Significance and Serial CT Findings
Abstract
1. Introduction
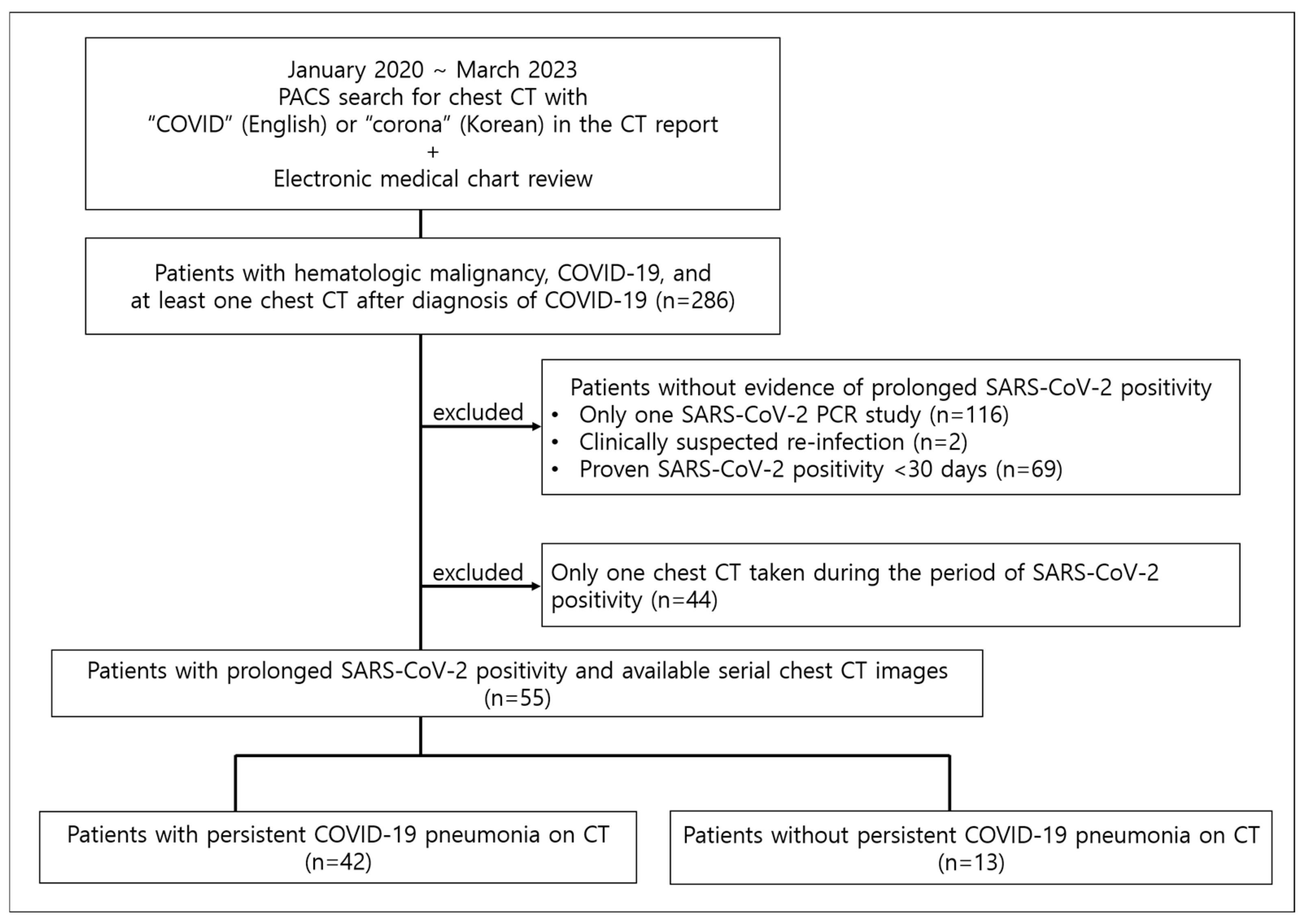
2. Materials and Methods
2.1. Patients
2.2. Image Analysis
2.3. Clinical Information Collection
2.4. Statistical Analysis
3. Results
3.1. Clinical Characteristics of Study Patients
3.2. Development of Prolonged COVID-19 Pneumonia
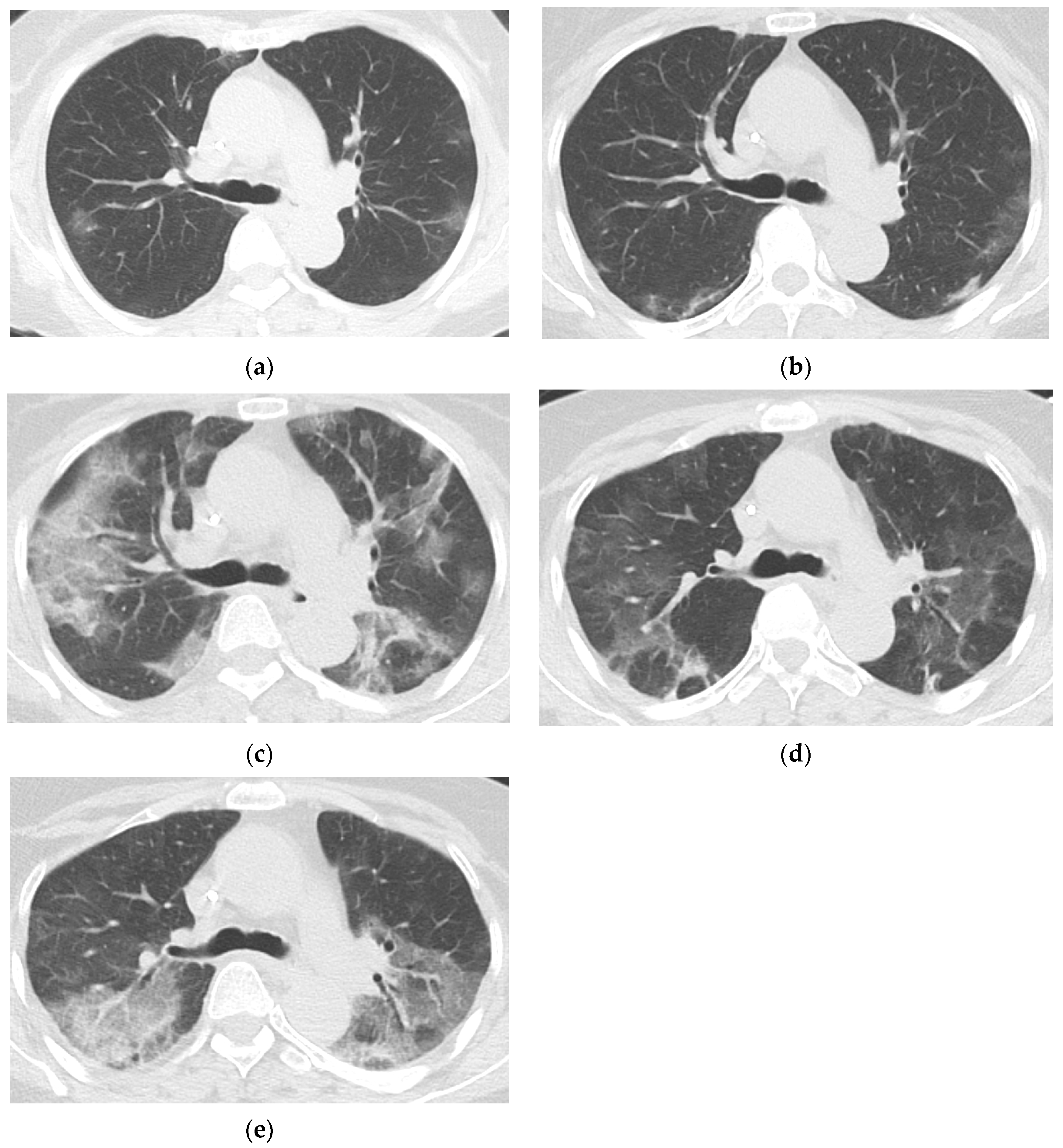
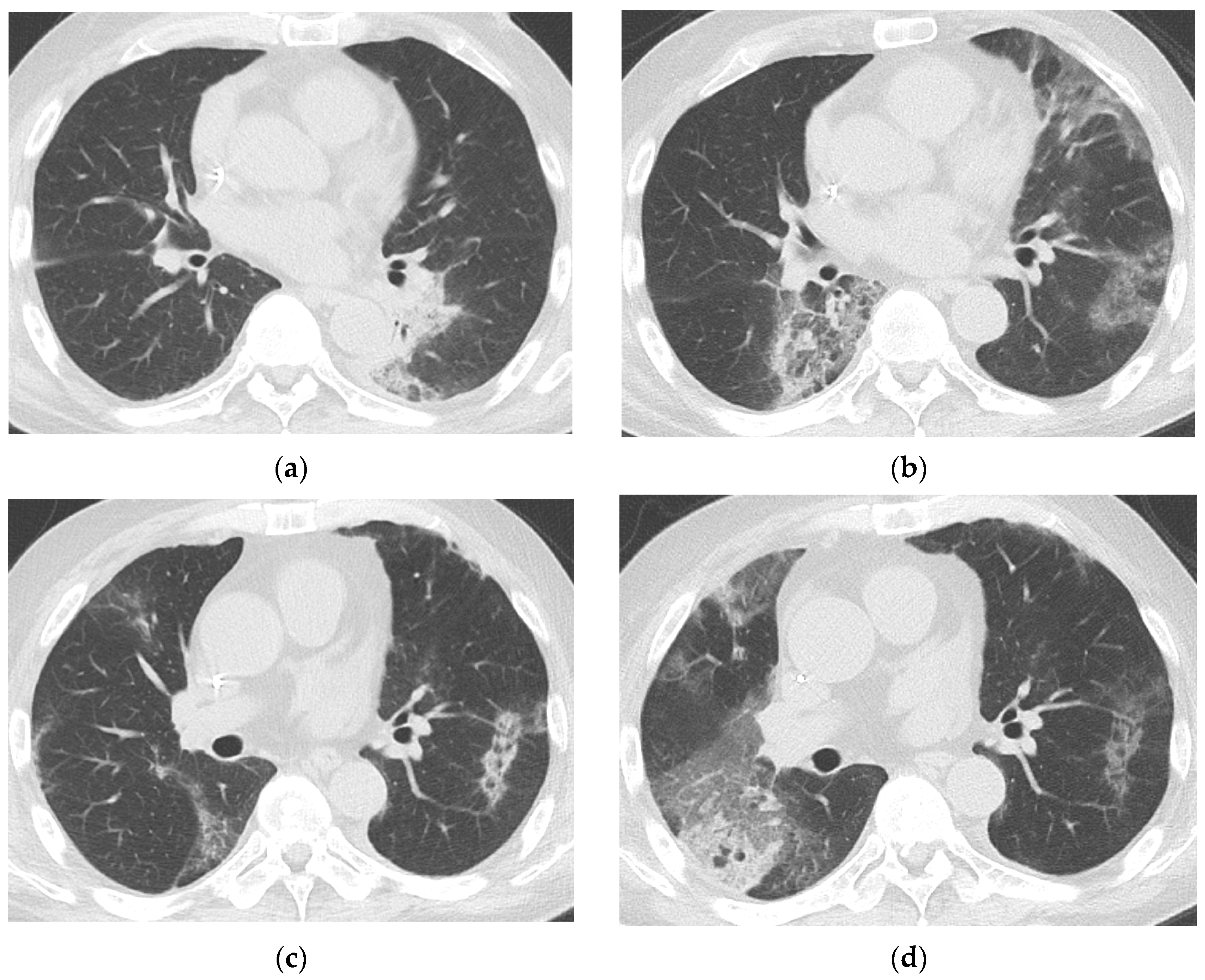
3.3. Baseline and Follow-Up Chest CT Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BCMA | B-cell-maturation antigen |
| Ct | Cycle threshold |
| CI | Confidence interval |
| GGO | Ground-glass opacity |
| IQR | Interquartile range |
| OR | Odds ratio |
| PCR | Polymerase chain reaction |
References
- Machkovech, H.M.; Hahn, A.M.; Garonzik Wang, J.; Grubaugh, N.D.; Halfmann, P.J.; Johnson, M.C.; Lemieux, J.E.; O’Connor, D.H.; Piantadosi, A.; Wei, W.; et al. Persistent SARS-CoV-2 infection: Significance and implications. Lancet Infect. Dis. 2024, 24, E453–E462. [Google Scholar] [CrossRef] [PubMed]
- Dioverti, V.; Salto-Alejandre, S.; Haidar, G. Immunocompromised Patients with Protracted COVID-19: A Review of “Long Persisters”. Curr. Transplant. Rep. 2022, 9, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.; Shah, M.K.; Hoyos, D.; Solovyov, A.; Douglas, M.; Taur, Y.; Maslak, P.; Babady, N.E.; Greenbaum, B.; Kamboj, M.; et al. Prolonged SARS-CoV-2 Infection in Patients with Lymphoid Malignancies. Cancer Discov. 2022, 12, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Beck, K.S.; Yoon, J.H.; Yoon, S.H. Radiologic Abnormalities in Prolonged SARS-CoV-2 Infection: A Systematic Review. Korean J. Radiol. 2024, 25, 473–480. [Google Scholar] [CrossRef]
- Gagelmann, N.; Passamonti, F.; Wolschke, C.; Massoud, R.; Niederwieser, C.; Adjalle, R.; Mora, B.; Ayuk, F.; Kroger, N. Antibody response after vaccination against SARS-CoV-2 in adults with hematological malignancies: A systematic review and meta-analysis. Haematologica 2022, 107, 1840–1849. [Google Scholar] [CrossRef]
- Ujjani, C.; Gooley, T.A.; Spurgeon, S.E.; Stephens, D.M.; Lai, C.; Broome, C.M.; O’Brien, S.; Zhu, H.; Laing, K.J.; Winter, A.M.; et al. Diminished humoral and cellular responses to SARS-CoV-2 vaccines in patients with chronic lymphocytic leukemia. Blood Adv. 2023, 7, 4728–4737. [Google Scholar] [CrossRef]
- Raje, N.; Anderson, K.; Einsele, H.; Efebera, Y.; Gay, F.; Hammond, S.P.; Lesokhin, A.M.; Lonial, S.; Ludwig, H.; Moreau, P.; et al. Monitoring, prophylaxis, and treatment of infections in patients with MM receiving bispecific antibody therapy: Consensus recommendations from an expert panel. Blood Cancer J. 2023, 13, 116. [Google Scholar] [CrossRef]
- Frerichs, K.A.; Verkleij, C.P.M.; Mateos, M.V.; Martin, T.G.; Rodriguez, C.; Nooka, A.; Banerjee, A.; Chastain, K.; Perales-Puchalt, A.; Stephenson, T.; et al. Teclistamab impairs humoral immunity in patients with heavily pretreated myeloma: Importance of immunoglobulin supplementation. Blood Adv. 2024, 8, 194–206. [Google Scholar] [CrossRef]
- Pan, F.; Ye, T.; Sun, P.; Gui, S.; Liang, B.; Li, L.; Zheng, D.; Wang, J.; Hesketh, R.L.; Yang, L.; et al. Time Course of Lung Changes at Chest CT during Recovery from Coronavirus Disease 2019 (COVID-19). Radiology 2020, 295, 715–721. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, C.; Hu, Y.; Li, C.; Ren, Q.; Zhang, X.; Shi, H.; Zhou, M. Temporal Changes of CT Findings in 90 Patients with COVID-19 Pneumonia: A Longitudinal Study. Radiology 2020, 296, E55–E64. [Google Scholar] [CrossRef]
- Kwee, T.C.; Kwee, R.M. Chest CT in COVID-19: What the Radiologist Needs to Know. Radiographics 2020, 40, 1848–1865. [Google Scholar] [CrossRef] [PubMed]
- Feuth, E.; Nieminen, V.; Palomaki, A.; Ranti, J.; Sucksdorff, M.; Finnila, T.; Oksi, J.; Vuorinen, T.; Feuth, T. Prolonged viral pneumonia and high mortality in COVID-19 patients on anti-CD20 monoclonal antibody therapy. Eur. J. Clin. Microbiol. Infect. Dis. 2024, 43, 723–734. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, R.; Beck, K.S.; Han, D.H.; Min, G.J.; Chang, S.; Jung, J.I.; Lee, D.G. Migratory Pneumonia in Prolonged SARS-CoV-2 Infection in Patients Treated with B-cell Depletion Therapies for B-cell Lymphoma. Korean J. Radiol. 2023, 24, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Simpson, S.; Kay, F.U.; Abbara, S.; Bhalla, S.; Chung, J.H.; Chung, M.; Henry, T.S.; Kanne, J.P.; Kligerman, S.; Ko, J.P.; et al. Radiological Society of North America Expert Consensus Document on Reporting Chest CT Findings Related to COVID-19: Endorsed by the Society of Thoracic Radiology, the American College of Radiology, and RSNA. Radiol. Cardiothorac. Imaging 2020, 2, e200152. [Google Scholar] [CrossRef]
- Mohan, M.; Nagavally, S.; Dhakal, B.; Radhakrishnan, S.V.; Chhabra, S.; D’Souza, A.; Hari, P. Risk of infections with B-cell maturation antigen-directed immunotherapy in multiple myeloma. Blood Adv. 2022, 6, 2466–2470. [Google Scholar] [CrossRef]
- Tang, L.; Huang, Z.; Mei, H.; Hu, Y. Immunotherapy in hematologic malignancies: Achievements, challenges and future prospects. Signal Transduct. Target. Ther. 2023, 8, 306. [Google Scholar] [CrossRef]
- Jeong, S.; Kim, J.S.; Lee, S.K.; Cho, E.J.; Hyun, J.; Song, W.; Kim, H.S. Tracking the Genomic Evolution of SARS-CoV-2 for 29 Months in South Korea. Viruses 2023, 15, 873. [Google Scholar] [CrossRef]
- No, J.S.; Noh, J.Y.; Lee, C.Y.; Kim, I.H.; Kim, J.A.; Ahn, Y.J.; Lee, H.; Kim, J.M.; Lee, N.J.; Lee, D.W.; et al. Dynamics of SARS-CoV-2 variants during the XBB wave in the Republic of Korea. Virus Res. 2024, 350, 199471. [Google Scholar] [CrossRef]
- Hettle, D.; Hutchings, S.; Muir, P.; Moran, E.; Consortium, C.-G.U. Persistent SARS-CoV-2 infection in immunocompromised patients facilitates rapid viral evolution: Retrospective cohort study and literature review. Clin. Infect. Pract. 2022, 16, 100210. [Google Scholar] [CrossRef]
- Freund, O.; Azolai, L.; Sror, N.; Zeeman, I.; Kozlovsky, T.; Greenberg, S.A.; Epstein Weiss, T.; Bornstein, G.; Tchebiner, J.Z.; Frydman, S. Diagnostic delays among COVID-19 patients with a second concurrent diagnosis. J. Hosp. Med. 2023, 18, 321–328. [Google Scholar] [CrossRef]
- Sochacka-Cwikla, A.; Maczynski, M.; Regiec, A. FDA-Approved Drugs for Hematological Malignancies—The Last Decade Review. Cancers 2021, 14, 87. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Meyer, K.C. Cryptogenic organising pneumonia: Current understanding of an enigmatic lung disease. Eur. Respir. Rev. 2021, 30, 210094. [Google Scholar] [CrossRef] [PubMed]
- Kory, P.; Kanne, J.P. SARS-CoV-2 organising pneumonia: ‘Has there been a widespread failure to identify and treat this prevalent condition in COVID-19?’. BMJ Open Respir Res. 2020, 7, e000724. [Google Scholar] [CrossRef] [PubMed]
- Chong, W.H.; Saha, B.K.; Chopra, A. Does COVID-19 pneumonia signify secondary organizing pneumonia?: A narrative review comparing the similarities between these two distinct entities. Heart Lung 2021, 50, 667–674. [Google Scholar] [CrossRef]
- Marquis, K.M.; Hammer, M.M.; Steinbrecher, K.; Henry, T.S.; Lin, C.Y.; Shifren, A.; Raptis, C.A. CT Approach to Lung Injury. Radiographics 2023, 43, e220176. [Google Scholar] [CrossRef]
| Clinical Characteristics | All Patients (N = 55) | COVID-19 Pneumonia (N = 42) | No COVID-19 Pneumonia (N = 13) | p Value |
|---|---|---|---|---|
| Age, years | 61 (51–65) | 61 (51–65) | 58 (52–63) | 0.317 |
| Sex, female: male | 19 (34.5%):36 (65.5%) | 15 (35.7%):27 (64.3%) | 4 (30.8%):9 (69.2%) | >0.999 |
| Hematologic malignancy | 0.011 | |||
| Leukemia | 14 (25.5%) | 7 (16.7%) | 7 (53.8%) | |
| Lymphoma | 30 (54.5%) | 25 (59.5%) | 5 (38.5%) | |
| Multiple myeloma | 9 (16.4%) | 9 (21.4%) | 0 (0.0%) | |
| Myelodysplastic syndrome | 2 (3.6%) | 1 (2.4%) | 1 (7.7%) | |
| Treatments for hematologic malignancy | ||||
| B-cell-directed antibody-based therapies b within 1 year | 36 (65.5%) | 31 (73.8%) | 5 (38.5%) | 0.042 |
| anti-CD20 agents a within 1 year | 28 (50.9%) | 24 (57.1%) | 4 (30.8%) | 0.097 |
| hematopoietic stem cell transplant | 15 (27.3%) | 10 (23.8%) | 5 (38.5%) | 0.310 |
| Vaccination against SARS-CoV-2 | 0.910 | |||
| Yes | 23 (41.8%) | 17 (40.5%) | 6 (46.2%) | |
| No | 8 (14.5%) | 6 (14.3%) | 2 (15.4%) | |
| Unknown | 24 (43.6%) | 19 (45.2%) | 5 (38.5%) | |
| Duration of SARS-CoV-2 PCR positivity, days | 69 (55–100.5) | 72 (56–108) | 65 (54–70) | 0.106 |
| Number of SARS-CoV-2 PCR tests performed | 9 (5–11.5) | 10 (6–13) | 6 (4–9) | 0.055 |
| Time interval between SARS-CoV-2 PCR tests, days | 7 (6–13) | 7 (5–13) | 7 (7–11) | 0.425 |
| Median cycle threshold value during the disease course | 0.179 | |||
| <20 | 30 (54.5%) | 25 (59.5%) | 5 (38.5%) | |
| 20–30 | 23 (41.8%) | 17 (40.5%) | 6 (46.2%) | |
| >30 | 2 (3.6%) | 0 (0.0%) | 2 (15.4%) | |
| Presumed SARS-CoV-2 subtype | 0.425 | |||
| Original strain | 3 (5.5%) | 3 (7.1%) | 0 (0.0%) | |
| Delta variant | 2 (3.6%) | 2 (4.8%) | 0 (0.0%) | |
| Omicron variant | 50 (90.9%) | 37 (88.1%) | 13 (100.0%) | |
| Delay in treatment for hematologic malignancy | 0.215 | |||
| Yes | 34 (61.8%) | 27 (64.3%) | 6 (46.2%) | |
| No | 8 (14.5%) | 4 (9.5%) | 4 (30.8%) | |
| Further treatment not planned | 13 (23.6%) | 11 (26.2%) | 3 (23.1%) | |
| Duration of treatment delay for hematologic malignancy, days | 43 (13–426) | 49 (39–104) | 29.5 (21–41) | 0.027 |
| Symptoms during persistent SARS-CoV-2 infection | 0.080 | |||
| Yes c | 50 (87.3%) | 40 (95.2%) | 10 (76.9%) | |
| No | 5 (12.7%) | 2 (4.8%) | 3 (23.1%) | |
| Total number of hospital admissions for COVID-19 | 1 (1–1) | 1 (1–1) | 1 (0–1) | 0.005 |
| Total length of hospital stay for COVID-19 | 12 (7–33) | 20 (8–41) | 6 (0–12) | 0.002 |
| Medical treatment for COVID-19 | ||||
| Systemic corticosteroids | 36 (65.5%) | 33 (78.6%) | 3 (23.1%) | <0.001 |
| Antiviral therapies | 38 (69.1%) | 28 (66.7%) | 10 (76.9%) | 0.733 |
| Remdesivir | 32 (58.2%) | 27 (64.3%) | 5 (38.5%) | |
| Nirmatrelvir/ritonavir or Molnupiravir | 13 (23.7%) | 7 (16.7%) | 6 (46.2%) | |
| ICU admission and ventilator care | 6 (10.9%) | 6 (14.3%) | 0 (0.0%) | 0.321 |
| Death | ||||
| COVID-19-specific mortality | 14 (25.5%) | 13 (31.0%) | 1 (7.7%) | 0.147 |
| 30-day all-cause mortality | 21 (38.2%) | 16 (38.1%) | 5 (38.5%) | >0.999 |
| Antibody-Based Drug | Type | Target | Indication | Number of Patients | Number of Patients with Prolonged COVID-19 Pneumonia |
|---|---|---|---|---|---|
| Rituximab | Monoclonal antibody | CD20 | B-NHL | 24 | 20 |
| Odronextabmab | Bispecific antibody | CD20/CD3 | B-NHL, DLBCL | 3 | 3 |
| Daratamumab a | Monoclonal antibody | CD38 | MM | 3 | 3 |
| Elranatamab | Bispecific antibody | BCMA/CD3 | MM | 2 | 2 |
| Inotuzumab ozogamicin | Antibody–drug conjugate | CD22 | B-NHL, B-ALL | 2 | 1 |
| Belantamab mafodotin b | Antibody–drug conjugate | BCMA | MM | 1 | 1 |
| Linvoseltamab b | Bispecific antibody | BCMA/CD3 | MM | 1 | 1 |
| Obinutuzumab | Monoclonal antibody | CD20 | DLBCL, MCL, FL, CLL | 1 | 1 |
| Teclistamab c | Bispecific antibody | BCMA/CD3 | MM | 2 | 2 |
| Total | 36 | 31 |
| Variable | Univariable Analysis | Multivariable Analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p Value | Adjusted OR (95% CI) | p Value | |
| Age | 1.03 (0.98–1.08) | 0.250 | ||
| Type of hematologic malignancy (vs. leukemia) | ||||
| Lymphoma | 5.0 (1.2–20.71) | 0.966 | ||
| Multiple myeloma | >999.99 (<0.001–>999.99) | 0.947 | ||
| Myelodysplastic syndrome | 1.0 (0.05–19.4) | 0.938 | ||
| Use of antibody-based drugs targeting B-cell lineage within 1 year | 4.51 (1.21–16.75) | 0.025 | 4.34 (1.06–17.81) | 0.041 |
| Use of Anti-CD20 agent within 1 year | 3.00 (0.80–11.31) | 0.105 | ||
| Receiving hematopoietic stem cell transplant | 0.50 (0.13–1.88) | 0.305 | ||
| Duration of SARS-CoV-2 positivity | 1.02 (1.00–1.04) | 0.119 | ||
| Presence of symptoms | 6.00 (0.88–40.87) | 0.067 | 6.10 (0.74–50.17) | 0.093 |
| Median Ct value | 0.88 (0.76–1.02) | 0.092 | 0.92 (0.79–1.08) | 0.305 |
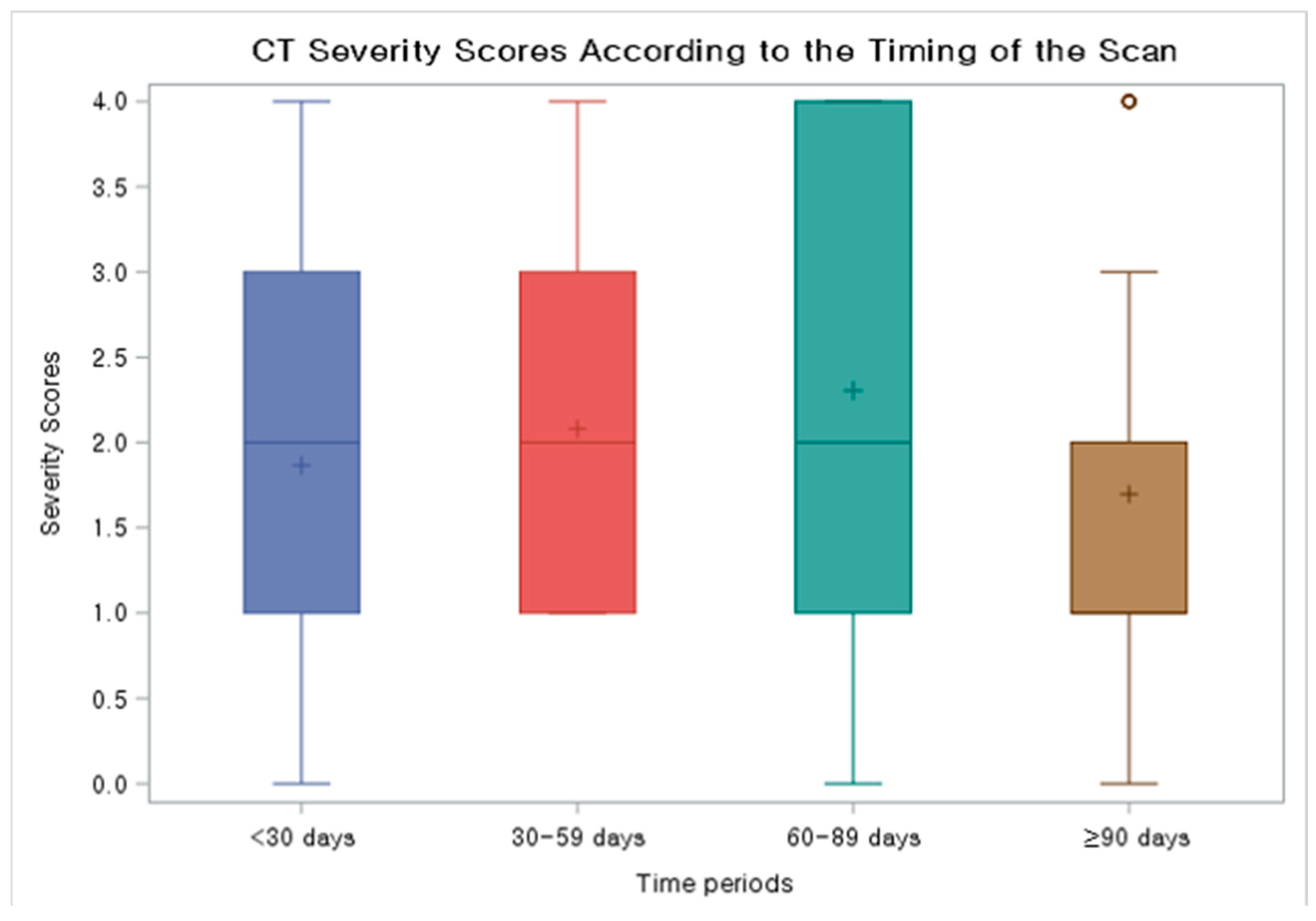
| Time of CT Exam (Days from the 1st Positive PCR) | <30 Days (N = 53) | 30–59 Days (N = 50) | 60–89 Days (N = 23) | ≥90 Days (N = 33) |
|---|---|---|---|---|
| Median number of days from the 1st positive PCR | 17 | 41.5 | 72 | 124 |
| RSNA CT categorization of COVID-19 pneumonia | ||||
| Typical | 37 (69.8%) | 39 (78.0%) | 16 (69.6%) | 18 (54.5%) |
| Indeterminate | 8 (15.1%) | 9 (18.0%) | 5 (21.7%) | 7 (21.2%) |
| Atypical | 6 (11.3%) | 2 (4.0%) | 2 (8.7%) | 4 (12.1%) |
| Negative | 2 (3.8%) | 0 (0.0%) | 0 (0.0%) | 4 (12.1%) |
| Pattern | ||||
| GGO | 23 (43.4%) | 18 (36.0%) | 11 (47.8%) | 16 (48.5%) |
| Consolidation | 4 (7.5%) | 2 (4.0%) | 3 (13.0%) | 5 (15.2%) |
| Both | 14 (26.4%) | 27 (56.0%) | 9 (39.1%) | 8 (24.2%) |
| Distribution | ||||
| Peripheral | 8 (15.1%) | 7 (14.0%) | 1 (4.3%) | 2 (6.1%) |
| Peribronchovascular | 9 (17.0%) | 4 (8.0%) | 4 (17.4%) | 3 (9.1%) |
| Both | 34 (64.2%) | 37 (74.0%) | 18 (78.3%) | 24 (72.7%) |
| Severity | ||||
| 0% involvement | 2 (3.8%) | 0 (0.0%) | 0 (0.0%) | 4 (12.1%) |
| 1–25% involvement | 24 (45.3%) | 16 (32.0%) | 8 (34.8%) | 15 (45.5%) |
| 26–50% involvement | 12 (22.6%) | 16 (32.0%) | 5 (21.7%) | 7 (21.2%) |
| 51–75% involvement | 12 (22.6%) | 15 (30.0%) | 4 (17.4%) | 1 (3.0%) |
| 76–100% involvement | 3 (5.7%) | 3 (6.0%) | 6 (26.1%) | 6 (18.2%) |
| Change in Extent | ||||
| Decreased | 5 (9.4%) | 12 (24.0%) | 7 (30.4%) | 8 (24.2%) |
| Stable | 2 (3.8%) | 9 (18.0%) | 2 (8.7%) | 14 (42.4%) |
| Increased | 10 (18.9%) | 25 (50.0%) | 12 (52.2%) | 11 (33.3%) |
| Unassessable | 36 (67.9%) | 4 (8.0%) | 2 (8.7%) | 0 (0.0%) |
| Migration | ||||
| Yes | 5 (9.4%) | 8 (16.0%) | 5 (21.7%) | 5 (15.2%) |
| No | 12 (22.6%) | 38 (76.0%) | 16 (69.6%) | 28 (84.8%) |
| Unassessable | 36 (67.9%) | 4 (8.0%) | 2 (8.7%) | 0 (0.0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, D.H.; Lee, R.; Min, G.J.; Lee, J.; Sohn, Y.; Min, E.J.; Lee, J.; Jung, J.I.; Beck, K.S. Prolonged COVID-19 Pneumonia in Patients with Hematologic Malignancies: Clinical Significance and Serial CT Findings. J. Clin. Med. 2025, 14, 2701. https://doi.org/10.3390/jcm14082701
Han DH, Lee R, Min GJ, Lee J, Sohn Y, Min EJ, Lee J, Jung JI, Beck KS. Prolonged COVID-19 Pneumonia in Patients with Hematologic Malignancies: Clinical Significance and Serial CT Findings. Journal of Clinical Medicine. 2025; 14(8):2701. https://doi.org/10.3390/jcm14082701
Chicago/Turabian StyleHan, Dae Hee, Raeseok Lee, Gi June Min, Jongmin Lee, Yejin Sohn, Eun Jeong Min, Jinyoung Lee, Jung Im Jung, and Kyongmin Sarah Beck. 2025. "Prolonged COVID-19 Pneumonia in Patients with Hematologic Malignancies: Clinical Significance and Serial CT Findings" Journal of Clinical Medicine 14, no. 8: 2701. https://doi.org/10.3390/jcm14082701
APA StyleHan, D. H., Lee, R., Min, G. J., Lee, J., Sohn, Y., Min, E. J., Lee, J., Jung, J. I., & Beck, K. S. (2025). Prolonged COVID-19 Pneumonia in Patients with Hematologic Malignancies: Clinical Significance and Serial CT Findings. Journal of Clinical Medicine, 14(8), 2701. https://doi.org/10.3390/jcm14082701





