The Naples Prognostic Score as a Predictor of High-Risk Coronary Plaques Detected on Coronary CT Angiography in Chronic Coronary Syndrome
Abstract
1. Introduction
2. Materials and Methods
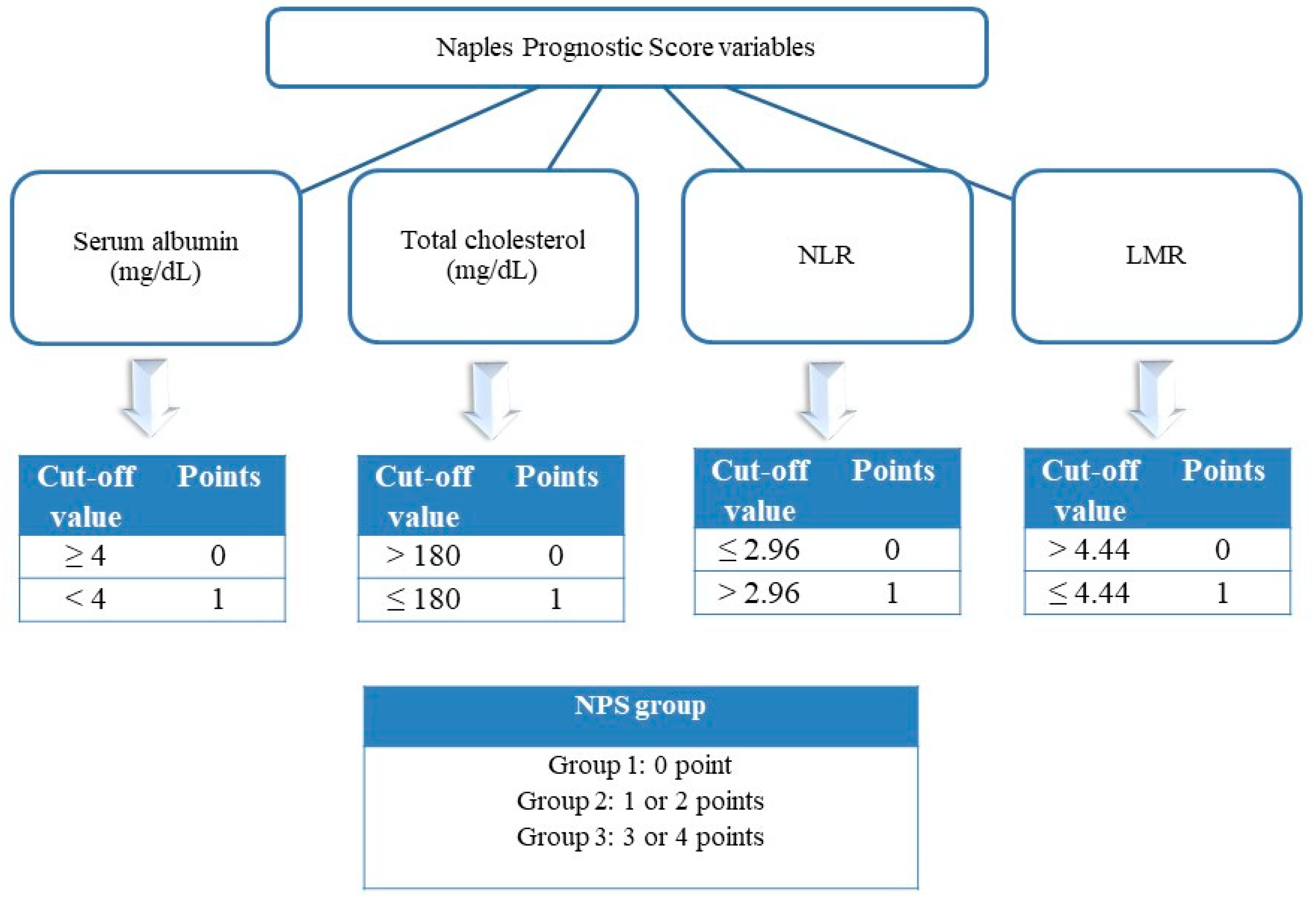
2.1. Study Population
2.2. Coronary Computed Tomography Angiography and Invasive Coronary Angiography
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CAC | Coronary artery calcification |
| CAD | Coronary artery disease |
| CCS | Chronic coronary syndrome |
| CCTA | Coronary CT angiography |
| HRP | High-risk coronary plaques |
| HU | Hounsfield units |
| LMR | Lymphocyte-to-monocyte ratio |
| NLR | Neutrophil-to-lymphocyte ratio |
| NPS | Naples Prognostic Score |
| NRS | Napkin-ring sign |
| SC | Spotty calcification |
References
- Motoyama, S.; Ito, H.; Sarai, M.; Kondo, T.; Kawai, H.; Nagahara, Y.; Harigaya, H.; Kan, S.; Anno, H.; Takahashi, H.; et al. Plaque Characterization by Coronary Computed Tomography Angiography and the Likelihood of Acute Coronary Events in Mid-Term Follow-Up. J. Am. Coll. Cardiol. 2015, 66, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Waksman, R.; Di Mario, C.; Torguson, R.; Ali, Z.A.; Singh, V.; Skinner, W.H.; Artis, A.K.; Cate, T.T.; Powers, E.; Kim, C.; et al. Identification of patients and plaques vulnerable to future coronary events with near-infrared spectroscopy intravascular ultrasound imaging: A prospective, cohort study. Lancet 2019, 394, 1629–1637. [Google Scholar] [CrossRef] [PubMed]
- Prati, F.; Romagnoli, E.; Gatto, L.; La Manna, A.; Burzotta, F.; Ozaki, Y.; Marco, V.; Boi, A.; Fineschi, M.; Fabbiocchi, F.; et al. Relationship between coronary plaque morphology of the left anterior descending artery and 12 months clinical outcome: The CLIMA study. Eur. Heart J. 2020, 41, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Erlinge, D.; Maehara, A.; Ben-Yehuda, O.; Bøtker, H.E.; Maeng, M.; Kjøller-Hansen, L.; Engstrøm, T.; Matsumura, M.; Crowley, A.; Dressler, O.; et al. Identification of vulnerable plaques and patients by intracoronary near-infrared spectroscopy and ultrasound (PROSPECT II): A prospective natural history study. Lancet 2021, 397, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Petrie, J.R.; Guzik, T.J.; Touyz, R.M. Diabetes, Hypertension, and Cardiovascular Disease: Clinical Insights and Vascular Mechanisms. Can. J. Cardiol. 2018, 34, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Galizia, G.; Lieto, E.; Auricchio, A.; Cardella, F.; Mabilia, A.; Podzemny, V.; Castellano, P.; Orditura, M.; Napolitano, V. Naples prognostic score, based on nutritional and inflammatory status, is an independent predictor of long-term outcome in patients undergoing surgery for colorectal cancer. Dis. Colon Rectum 2017, 60, 1273–1284. [Google Scholar] [CrossRef] [PubMed]
- Kılıç, O.; Suygun, H.; Mustu, M.; Ozpamuk Karadeniz, F.; Ozer, S.F.; Senol, H.; Kaya, D.; Buber, I.; Karakurt, A. Is the Naples prognostic score useful for predicting heart failure mortality? Kardiologiia 2023, 63, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Şaylık, F.; Çınar, T.; Selçuk, M.; Akbulut, T.; Hayıroğlu, M.İ.; Tanboğa, İ.H. Evaluation of Naples score for long-term mortality in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Angiology 2023, 14, 33197231170982. [Google Scholar] [CrossRef] [PubMed]
- Vrints, C.; Andreotti, F.; Koskinas, K.C.; Rossello, X.; Adamo, M.; Ainslie, J.; Banning, A.P.; Budaj, A.; Buechel, R.R.; Chiariello, G.A.; et al. 2024 ESC Guidelines for the management of chronic coronary syndromes. Eur. Heart J. 2024, 45, 3415–3437. [Google Scholar] [CrossRef] [PubMed]
- Maurovich-Horvat, P.; Ferencik, M.; Voros, S.; Merkely, B.; Hoffmann, U. Comprehensive plaque assessment by coronary CT angiography. Nat. Rev. Cardiol. 2014, 11, 390–402. [Google Scholar] [CrossRef] [PubMed]
- Attiq, A.; Afzal, S.; Ahmad, W.; Kandeel, M. Hegemony of inflammation in atherosclerosis and coronary artery disease. Eur. J. Pharmacol. 2024, 966, 176338. [Google Scholar] [CrossRef] [PubMed]
- Scott, C.; Lateef, S.S.; Hong, C.G.; Dey, A.K.; Manyak, G.A.; Patel, N.H.; Zhou, W.; Sorokin, A.V.; Abdelrahman, K.; Uceda, D.; et al. Inflammation, coronary plaque progression, and statin use: A secondary analysis of the Risk Stratification with Image Guidance of HMG CoA Reductase Inhibitor Therapy (RIGHT) study. Clin. Cardiol. 2022, 45, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Tudurachi, B.S.; Anghel, L.; Tudurachi, A.; Sascău, R.A.; Stătescu, C. Assessment of inflammatory hematological ratios (NLR, PLR, MLR, LMR and monocyte/HDL-cholesterol ratio) in acute myocardial infarction and particularities in young patients. Int. J. Mol. Sci. 2023, 24, 14378. [Google Scholar] [CrossRef] [PubMed]
- Kuppa, A.; Tripathi, H.; Al-Darraji, A.; Tarhuni, W.M.; Abdel-Latif, A. C-reactive protein levels and risk of cardiovascular diseases: A two-sample bidirectional Mendelian randomization study. Int. J. Mol. Sci. 2023, 24, 9129. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Yu, Y.; Chen, L.; Chen, S.; Tang, R.; Li, Q.; Wei, W.; Bao, K.; Huang, Z.; Lai, W.; et al. Independent and joint effects of high-sensitivity C-reactive protein and hypoalbuminemia on long-term all-cause mortality among coronary artery disease: A prospective and multicenter cohort study. BMC Cardiovasc. Disord. 2021, 21, 613. [Google Scholar] [CrossRef] [PubMed]
- Manolis, A.A.; Manolis, T.A.; Melita, H.; Mikhailidis, D.P.; Manolis, A.S. Low serum albumin: A neglected predictor in patients with cardiovascular disease. Eur. J. Intern. Med. 2022, 102, 24–39. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, A.; Minhas, A.S.; Kazzi, B.; Varma, B.; Choi, E.; Thakkar, A.; Michos, E.D. Sex-specific differences in cardiovascular risk factors and implications for cardiovascular disease prevention in women. Atherosclerosis 2023, 384, 117269. [Google Scholar] [CrossRef] [PubMed]
- Nurmohamed, N.S.; van Rosendael, A.R.; Danad, I.; Ngo-Metzger, Q.; Taub, P.R.; Ray, K.K.; Figtree, G.; Bonaca, M.P.; Hsia, J.; Rodriguez, F.; et al. Atherosclerosis evaluation and cardiovascular risk estimation using coronary computed tomography angiography. Eur. Heart J. 2024, 45, 1783–1800. [Google Scholar] [CrossRef] [PubMed]
- Goldsborough, E., 3rd; Osuji, N.; Blaha, M.J. Assessment of cardiovascular disease risk: A 2022 update. Endocrinol. Metab. Clin. N. Am. 2022, 51, 483–509. [Google Scholar] [CrossRef] [PubMed]


| High-Risk Plaque Present (n = 220) | High-Risk Plaque Absent (n = 533) | Total (n = 753) | p Value | ||
|---|---|---|---|---|---|
| Age | 61.7 ± 9.9 | 54.9 ± 10.3 | 56.9 ± 10.7 | <0.001 | |
| Gender (Female), n (%) | 55 (25) | 246 (46.2) | 301 (40) | <0.001 | |
| Smoking, n (%) | 66 (30) | 173 (32.5) | 239 (31.7) | 0.510 | |
| Diabetes mellitus, n (%) | 48 (21.8) | 95 (17.8) | 143 (19) | 0.204 | |
| Hypertension, n (%) | 90 (40.9) | 154 (28.9) | 244 (32.4) | 0.001 | |
| Dyslipidemia, n (%) | 32 (14.5) | 46 (8.6) | 78 (10.4) | 0.015 | |
| Chronic obstructive pulmonary disease, n (%) | 38 (17.3) | 90 (16.9) | 128 (17) | 0.898 | |
| Left ventricular ejection fraction, % | 62 (45–67) | 63 (50–69) | 62 (45–69) | 0.001 | |
| Fasting blood glucose, mg/dL | 102 (74–372) | 98 (67–396) | 99 (67–396) | 0.002 | |
| Creatinine, mg/dL | 0.9 (0.5–1.8) | 0.8 (0.5–1.3) | 0.9 (0.5–1.8) | <0.001 | |
| ALT, units/L | 18 (8–87) | 19 (3–118) | 19 (3–118) | 0.072 | |
| AST, units/L | 20 (4–66) | 20 (6–131) | 20 (4–131) | 0.930 | |
| Total cholesterol, mg/dL | 187.2 ± 46 | 190 ± 43 | 189.5 ± 43.8 | 0.357 | |
| LDL-C, mg/dL | 117.1 ± 33.5 | 120 ± 30.7 | 119.1 ± 31.5 | 0.252 | |
| HDL-C, mg/dL | 42 (17–86) | 44 (22–95) | 44 (17–95) | <0.001 | |
| Triglyceride, mg/dL | 124.5 (46–1344) | 130 (31–625) | 130 (31–1344) | 0.938 | |
| Hemoglobin, g/dL | 13.8 ± 1.7 | 13.8 ± 1.8 | 13.8 ± 1.6 | 0.994 | |
| White blood cell count, 103/µL | 7.6 ± 1.8 | 7.5 ± 1.8 | 7.6 ± 1.8 | 0.813 | |
| Platelet count, 103/µL | 238 (107–509) | 248 (53–632) | 246 (53–632) | 0.037 | |
| CRP, mg/dL | 1.9 (0.1–16) | 1.8 (0.1–26.5) | 1.9 (0.1–26.5) | 0.568 | |
| Albumin, g/dL | 4.1 ± 0.3 | 4.2 ± 0.2 | 4.1 ± 0.3 | <0.001 | |
| ACEI/ARBs, n (%) | 81 (36.8) | 140 (26.3) | 221 (29.3) | 0.004 | |
| Calcium channel blockers, n (%) | 38 (17.3) | 69 (12.9) | 107 (14.2) | 0.122 | |
| Diuretics, n (%) | 52 (23.6) | 92 (17.3) | 144 (19.1) | 0.043 | |
| Beta-blockers, n (%) | 25 (11.4) | 38 (7.1) | 63 (8.4) | 0.056 | |
| Antiplatelet, n (%) | 52 (23.6) | 82 (15.4) | 134 (17.8) | 0.007 | |
| Anticoagulant, n (%) | 8 (3.6) | 14 (2.6) | 22 (2.9) | 0.454 | |
| CACS | 99.5 (0–2521) | 0 (0–1576) | 1 (0–2521) | <0.001 | |
| Revascularization, n (%) | 109 (49.5) | 10 (1.9) | 119 (12.9) | <0.001 | |
| NPS | 1.5 ± 0.9 | 1.2 ± 0.9 | 1.3 ± 0.9 | 0.001 | |
| NPS group, n (%) | 1 | 23 (10.5) | 105 (19.7) | 128 (17) | |
| 2 | 169 (76.8) | 381 (71.5) | 550 (73) | 0.004 | |
| 3 | 28 (12.7) | 47 (8.8) | 75 (10) | ||
| NPS Group 1 (n = 128) | NPS Group 2 (n = 550) | NPS Group 3 (n = 75) | Total (n = 753) | p Value | |
|---|---|---|---|---|---|
| Age | 56.4 ± 8.6 | 56.3 ± 10.9 | 61.4 ± 11 | 56.9 ± 10.7 | 0.001 |
| Gender (Female), n (%) | 72 (56.3) | 203 (36.9) | 26 (34.7) | 301 (40) | <0.001 |
| Smoking, n (%) | 34 (26.6) | 187 (34) | 18 (24) | 239 (31.7) | 0.084 |
| Diabetes mellitus, n (%) | 31 (24.2) | 98 (17.8) | 14 (18.7) | 143 (19) | 0.250 |
| Hypertension, n (%) | 35 (27.3) | 174 (31.6) | 35 (46.7) | 244 (32.4) | 0.014 |
| Dyslipidemia, n (%) | 17 (13.3) | 53 (9.6) | 8 (10.7) | 78 (10.4) | 0.474 |
| Chronic obstructive pulmonary disease, n (%) | 25 (19.5) | 88 (16) | 15 (20) | 128 (17) | 0.484 |
| Left ventricular ejection fraction, % | 62 (55–69) | 63 (45–68) | 61 (50–66) | 62 (45–69) | 0.059 |
| Fasting blood glucose, mg/dL | 98.5 (72–396) | 99 (67–359) | 103 (72–372) | 99 (67–396) | 0.406 |
| Creatinine, mg/dL | 0.8 (0.5–1.3) | 0.9 (0.5–1.5) | 0.9 (0.5–1.8) | 0.9 (0.5–1.8) | 0.027 |
| ALT, units/L | 21.5 (8–59) | 18 (3–118) | 17 (7–76) | 19 (3–118) | 0.046 |
| AST, units/L | 21 (10–51) | 20 (6–131) | 20 (4–53) | 20 (4–131) | 0.153 |
| Total cholesterol, mg/dL | 230.2 ± 37.1 | 184 ± 40.8 | 160.5 ± 28.4 | 189.5 ± 43.8 | <0.001 |
| LDL-C, mg/dL | 142.4 ± 28.2 | 116 ± 30.2 | 99.1 ± 24.8 | 119.1 ± 31.5 | <0.001 |
| HDL-C, mg/dL | 45 (25–93) | 44 (17–95) | 40 (22–75) | 44 (17–95) | 0.001 |
| Triglyceride, mg/dL | 147 (55–1344) | 127 (31–625) | 112 (38–411) | 130 (31–1344) | <0.001 |
| Hemoglobin, g/dL | 14 ± 1.3 | 13.8 ± 1.6 | 13.2 ± 1.7 | 13.8 ± 1.6 | 0.004 |
| White blood cell count, 103/µL | 7.2 ± 1.6 | 7.6 ± 1.9 | 8 ± 1.6 | 7.6 ± 1.8 | 0.021 |
| Platelet count, 103/µL | 254 (126–593) | 243 (53–632) | 241 (72–485) | 246 (53–632) | 0.042 |
| CRP, mg/dL | 1.9 (0.1–8.7) | 1.8 (0.1–26.5) | 2.1 (0.1–17.5) | 1.9 (0.1–26.5) | 0.021 |
| Albumin, g/dL | 4.2 ± 0.2 | 4.1 ± 0.3 | 3.9 ± 0.3 | 4.1 ± 0.3 | <0.001 |
| ACEI/ARBs, n (%) | 31 (24.2) | 159 (28.9) | 31 (41.3) | 221 (29.3) | 0.032 |
| Calcium channel blockers, n (%) | 13 (10.2) | 74 (13.5) | 20 (26.7) | 107 (14.2) | 0.003 |
| Diuretics, n (%) | 24 (18.8) | 98 (17.8) | 22 (29.3) | 144 (19.1) | 0.059 |
| Beta-blockers, n (%) | 8 (6.3) | 47 (8.5) | 8 (10.7) | 63 (8.4) | 0.525 |
| Antiplatelet, n (%) | 16 (12.5) | 99 (18) | 19 (25.3) | 134 (17.8) | 0.068 |
| Anticoagulant, n (%) | 1 (0.8) | 16 (2.9) | 5 (6.7) | 22 (2.9) | 0.056 |
| CACS | 0 (0–1576) | 1 (0–2521) | 21 (0–2093) | 1 (0–2521) | <0.001 |
| Revascularization, n (%) | 16 (12.5) | 81 (14.7) | 22 (29.3) | 119 (15.8) | 0.003 |
| Univariable | p Value | OR | 95% CI | Multivariable | p Value | OR | 95% CI | ||
|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | Lower | Upper | ||||||
| Age | <0.001 | 1.067 | 1.049 | 1.084 | Age | <0.001 | 1.056 | 1.038 | 1.075 |
| Hypertension | 0.001 | 1.704 | 1.228 | 2.364 | Hypertension | - | - | - | - |
| Dyslipidemia | 0.017 | 1.802 | 1.113 | 2.917 | Dyslipidemia | 0.032 | 1.831 | 1.052 | 3.185 |
| Diabetes mellitus | 0.204 | 1.287 | 0.872 | 1.899 | Diabetes mellitus | 0.032 | 1.788 | 1.051 | 3.040 |
| Glucose | 0.002 | 1.006 | 1.002 | 1.009 | Glucose | 0.001 | 1.008 | 1.003 | 1.013 |
| Creatinine | <0.001 | 21.690 | 7.911 | 59.467 | Creatinine | <0.001 | 11.005 | 3.734 | 32.432 |
| NPS | 0.001 | 1.350 | 1.133 | 1.609 | NPS | 0.036 | 1.228 | 1.013 | 1.489 |
| Univariable | p Value | OR | 95% CI | Multivariable | p Value | OR | 95% CI | ||
|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | Lower | Upper | ||||||
| Age | <0.001 | 1.067 | 1.046 | 1.089 | Age | <0.001 | 1.050 | 1.026 | 1.074 |
| Hypertension | 0.001 | 2.023 | 1.358 | 3.014 | Hypertension | - | - | - | - |
| Dyslipidemia | <0.001 | 2.713 | 1.601 | 4.598 | Dyslipidemia | 0.001 | 2.787 | 1.505 | 5.161 |
| Diabetes mellitus | 0.018 | 1.733 | 1.101 | 2.729 | Diabetes mellitus | - | - | - | - |
| Glucose | 0.001 | 1.006 | 1.003 | 1.010 | Glucose | 0.005 | 1.007 | 1.002 | 1.012 |
| Creatinine | <0.001 | 34.788 | 10.707 | 113.030 | Creatinine | <0.001 | 20.062 | 5.564 | 72.343 |
| NPS | 0.004 | 1.366 | 1.102 | 1.694 | NPS | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bolgen, C.; Mazıcan, M. The Naples Prognostic Score as a Predictor of High-Risk Coronary Plaques Detected on Coronary CT Angiography in Chronic Coronary Syndrome. J. Clin. Med. 2025, 14, 2661. https://doi.org/10.3390/jcm14082661
Bolgen C, Mazıcan M. The Naples Prognostic Score as a Predictor of High-Risk Coronary Plaques Detected on Coronary CT Angiography in Chronic Coronary Syndrome. Journal of Clinical Medicine. 2025; 14(8):2661. https://doi.org/10.3390/jcm14082661
Chicago/Turabian StyleBolgen, Cagatay, and Mustafa Mazıcan. 2025. "The Naples Prognostic Score as a Predictor of High-Risk Coronary Plaques Detected on Coronary CT Angiography in Chronic Coronary Syndrome" Journal of Clinical Medicine 14, no. 8: 2661. https://doi.org/10.3390/jcm14082661
APA StyleBolgen, C., & Mazıcan, M. (2025). The Naples Prognostic Score as a Predictor of High-Risk Coronary Plaques Detected on Coronary CT Angiography in Chronic Coronary Syndrome. Journal of Clinical Medicine, 14(8), 2661. https://doi.org/10.3390/jcm14082661









