Intra-Individual Differences of the Femoral Cortical Thickness Index in Elderly Patients with a Proximal Femoral Fracture
Abstract
1. Introduction
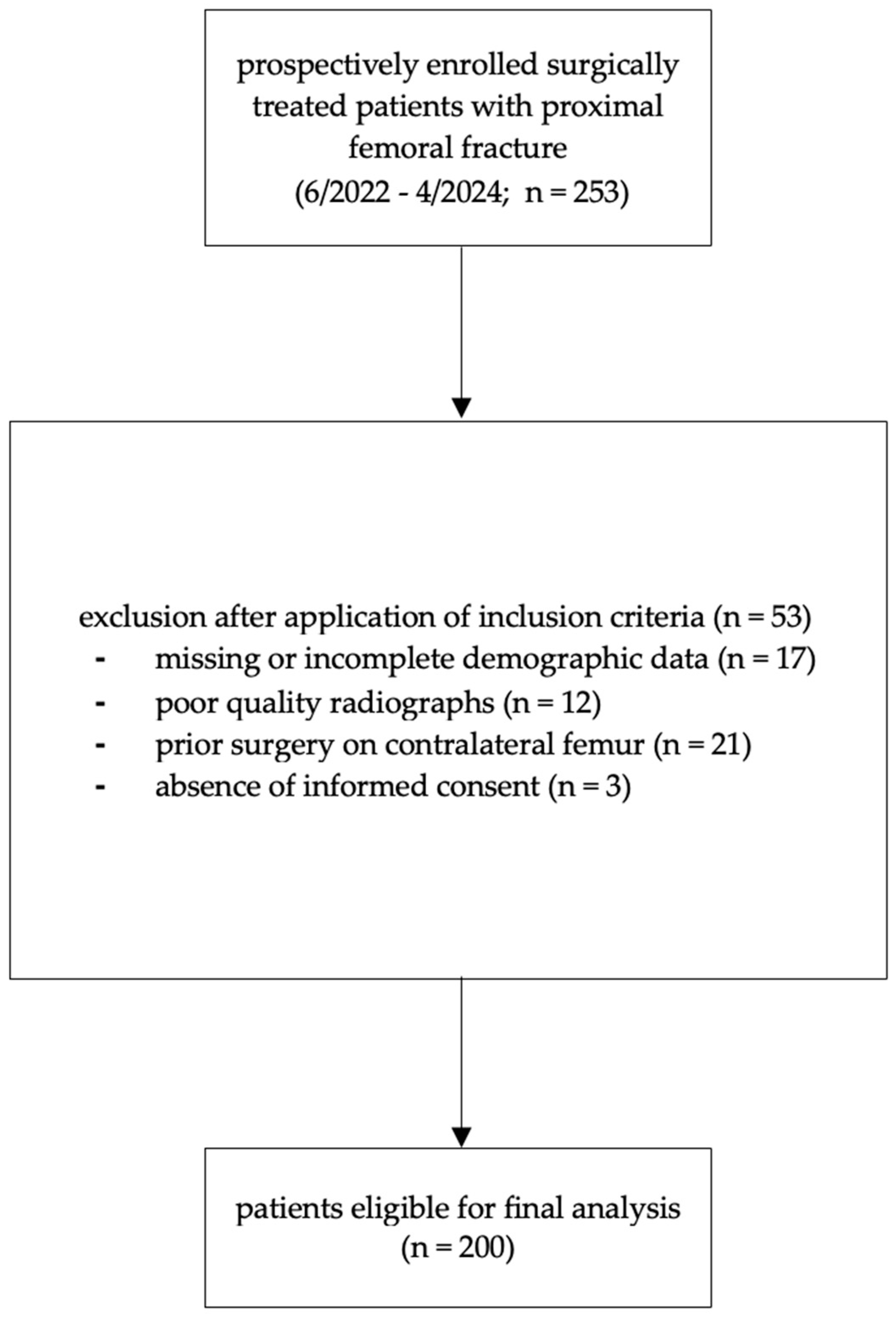
2. Materials and Methods
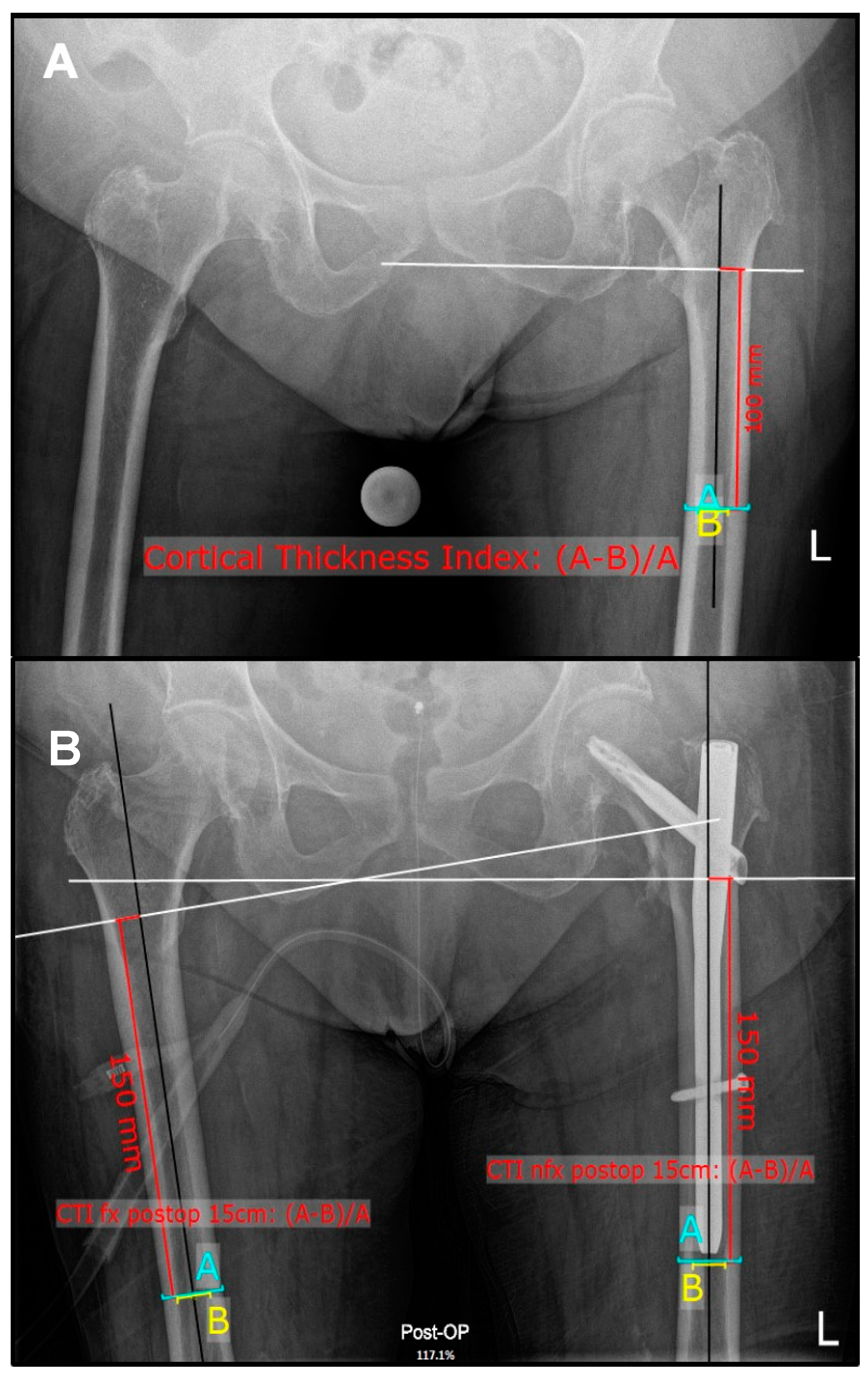
2.1. Radiographic Measurements
2.2. Statistical Analysis
3. Results
3.1. Patient Demographics
3.2. Inter-Rater Reliability
3.3. Comparison of CTI Within the Subgroups
3.4. Correlations Between CTI and Age at Surgery and BMI
3.5. Sex-Specific Differences Within the Subgroups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Consensus development conference: Diagnosis, prophylaxis, and treatment of osteoporosis. Am. J. Med. 1993, 94, 646–650. [Google Scholar] [CrossRef]
- NIH. Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. JAMA 2001, 285, 785–795. [Google Scholar] [CrossRef]
- Reginster, J.-Y.; Burlet, N. Osteoporosis: A still increasing prevalence. Bone 2006, 38, 4–9. [Google Scholar] [CrossRef]
- Xiao, P.-L.; Cui, A.-Y.; Hsu, C.-J.; Peng, R.; Jiang, N.; Xu, X.-H.; Ma, Y.-G.; Liu, D.; Lu, H.-D. Global, regional prevalence, and risk factors of osteoporosis according to the World Health Organization diagnostic criteria: A systematic review and meta-analysis. Osteoporos. Int. 2002, 33, 2137–2153. [Google Scholar] [CrossRef]
- Wright, N.C.; Looker, A.C.; Saag, K.G.; Curtis, J.R.; Delzell, E.S.; Randall, S.; Dawson-Hughes, B. The Recent Prevalence of Osteoporosis and Low Bone Mass in the United States Based on Bone Mineral Density at the Femoral Neck or Lumbar Spine. JBMR 2014, 29, 2520–2526. [Google Scholar] [CrossRef]
- Rashki Kemmak, A.; Rezapour, A.; Jahangiri, R.; Nikjoo, S.; Farabi, H.; Soleimanpour, S. Economic burden of osteoporosis in the world: A systematic review. Med. J. Islam. Repub. Iran. 2020, 34, 154. [Google Scholar] [CrossRef]
- Burge, R.; Dawson-Hughes, B.; Solomon, D.H.; Wong, J.B.; King, A.; Tosteson, A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J. Bone Miner. Res. 2007, 22, 465–475. [Google Scholar] [CrossRef]
- Johnell, O.; Kanis, J.A. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos. Int. 2006, 17, 1726–1733. [Google Scholar] [CrossRef]
- Keene, G.S.; Parker, M.J.; Pryor, G.A. Mortality and morbidity after hip fractures. BMJ 1993, 307, 1248–1250. [Google Scholar] [CrossRef]
- Poór, G.; Atkinson, E.J.; O’Fallon, W.M.; Melton, L.J., 3rd. Determinants of reduced survival following hip fractures in men. Clin. Orthop. Relat. Res. 1995, 319, 260–265. [Google Scholar] [PubMed]
- Hernlund, E.; Svedbom, A.; Ivergard, M.; Compston, J.; Cooper, C.; Stenmark, J.; McCloskey, E.V.; Jonsson, B.; Kanis, J.A. Osteoporosis in the European Union: Medical management, epidemiology and economic burden. Arch. Osteoporos. 2013, 8, 136. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A.; Johnell, O. Requirements for DXA for the management of osteoporosis in Europe. Osteoporos. Int. 2005, 16, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Cummings, S.R.; Melton, L.J. Epidemiology and outcomes of osteoporotic fractures. Lancet 2002, 359, 1761–1767. [Google Scholar] [CrossRef]
- Abrahamsen, B.; van Staa, T.; Ariely, R.; Olson, M.; Cooper, C. Excess mortality following hip fracture: A systematic epidemiological review. Osteoporos. Int. 2009, 20, 1633–1650. [Google Scholar] [CrossRef]
- Gullberg, B.; Johnell, O.; Kanis, J.A. World-wide Projections for Hip Fracture. Osteoporos. Int. 1997, 7, 407–413. [Google Scholar] [CrossRef]
- Cooper, C.; Campion, G.; Melton, L.J. Hip fractures in the elderly: A world-wide projection. Osteoporos. Int. 1992, 2, 285–289. [Google Scholar] [CrossRef]
- Deshpande, N.; Hadi, M.S.; Lillard, J.C.; Passias, P.G.; Linzey, J.R.; Saadeh, Y.S.; LaBagnara, M.; Park, P. Alternatives to DEXA for the assessment of bone density: A systematic review of the literature and future recommendations. J. Neurosurg. Spine 2023, 38, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Choksi, P.; Jepsen, K.J.; Clines, G.A. The challenges of diagnosing osteoporosis and the limitations of currently available tools. Clin. Diabetes Endocrinol. 2018, 4, 12. [Google Scholar] [CrossRef]
- Dorr, L.D.; Faugere, M.-C.; Mackel, A.M.; Gruen, T.A.; Bognar, B.; Malluche, H.H. Structural and cellular assessment of bone quality of proximal femur. Bone 1993, 14, 231–242. [Google Scholar] [CrossRef]
- Hadidi, F.; Khalil, O.A.; Alhadidy, A.; Alqawaba, R.; Hammad, Y.; Samarah, O.Q.; Hamdan, M.; Yasin, M.S.; Hawa, A.F.; AlRyalat, A. Correlation between plain X-ray measurements and DXA scans, and its possible use as a screening tool for performing DXA scans. Int. J. Clin. Pract. 2021, 75, e14784. [Google Scholar] [CrossRef]
- Sah, A.P.; Thornhill, T.S.; LeBoff, M.S.; Glowacki, J. Correlation of plain radiographic indices of the hip with quantitative bone mineral density. Osteoporos. Int. 2007, 18, 1119–1126. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, G.; Ipci, F.B. Evaluation of the Relationship between Osteoporosis Parameters in Plain Hip Radiography and DXA Results in 156 Patients at a Single Center in Turkey. Diagnostics 2023, 13, 2519. [Google Scholar] [CrossRef]
- Nguyen, B.N.; Hoshino, H.; Togawa, D.; Matsuyama, Y. Cortical Thickness Index of the Proximal Femur: A Radiographic Parameter for Preliminary Assessment of Bone Mineral Density and Osteoporosis Status in the Age 50 Years and Over Population. Clin. Orthop. Surg. 2018, 10, 149. [Google Scholar] [CrossRef]
- Im, G.-I.; Park, P.-G.; Moon, S.-W. The relationship between radiological parameters from plain hip radiographs and bone mineral density in a Korean population. J. Bone Miner. Metab. 2012, 30, 504–508. [Google Scholar] [CrossRef]
- Faundez, J.; Carmona, M.; Klaber, I.; Zamora, T.; Botello, E.; Schweitzer, D. Radiographic Assessment of Bone Quality Using 4 Radiographic Indexes: Canal Diaphysis Ratio Is Superior. J. Arthroplast. 2024, 39, 427–432. [Google Scholar] [CrossRef]
- Baumgärtner, R.; Heeren, N.; Quast, D.; Babst, R.; Brunner, A. Is the cortical thickness index a valid parameter to assess bone mineral density in geriatric patients with hip fractures? Arch. Orthop. Trauma. Surg. 2015, 135, 805–810. [Google Scholar] [CrossRef]
- Rudloff, M.I.; Smith, W.R. Intramedullary Nailing of the Femur: Current Concepts Concerning Reaming. J. Orthop. Trauma. 2009, 23, S12–S17. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef]
- Pulkkinen, P.; Partanen, J.; Jalovaara, P.; Jämsä, T. Combination of bone mineral density and upper femur geometry improves the prediction of hip fracture. Osteoporos. Int. 2004, 15, 274–280. [Google Scholar] [CrossRef]
- Kanis, J.A.; Johnell, O.; Oden, A.; Johansson, H.; McCloskey, E. FRAXTM and the assessment of fracture probability in men and women from the UK. Osteoporos. Int. 2008, 19, 385–397. [Google Scholar] [CrossRef]
- Pothong, W.; Adulkasem, N. Comparative evaluation of radiographic morphologic parameters for predicting subsequent contralateral fragility hip fracture. Int. Orthop. 2023, 47, 1837–1843. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, A.; Tacci, F.; Leigheb, M.; Costantino, C.; Pedrazzini, A.; Pedrazzi, G.; Vaienti, E.; Ceccarelli, F.; Pogliacomi, F. Injuries of the trochanteric region: Can analysis of radiographic indices help in prediction of recurrent osteoporotic hip fractures? Acta Biomed. 2017, 88, 43–49. [Google Scholar] [PubMed]
- Nash, W.; Harris, A. The Dorr Type and Cortical Thickness Index of the Proximal Femur for Predicting Peri-Operative Complications during Hemiarthroplasty. J. Orthop. Surg. 2014, 22, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Courtney, A.C.; Hayes, W.C.; Gibson, L.J. Age-related differences in post-yield damage in human cortical bone. Experiment and model. J. Biomech. 1996, 29, 1463–1471. [Google Scholar] [CrossRef]
- Pistoia, W.; van Rietbergen, B.; Lochmüller, E.-M.; Lill, C.; Eckstein, F.; Rüegsegger, P. Estimation of distal radius failure load with micro-finite element analysis models based on three-dimensional peripheral quantitative computed tomography images. Bone 2002, 30, 842–848. [Google Scholar] [CrossRef]
- Nishiyama, K.K.; Macdonald, H.M.; Buie, H.R.; A Hanley, D.; Boyd, S.K. Postmenopausal women with osteopenia have higher cortical porosity and thinner cortices at the distal radius and tibia than women with normal aBMD: An in vivo HR-pQCT study. J. Bone Miner. Res. 2010, 25, 882–890. [Google Scholar] [CrossRef]
- Guyan, F.; Gianduzzo, E.; Waltenspül, M.; Dietrich, M.; Kabelitz, M. Cortical Thickness Index and Canal Calcar Ratio: A Comparison of Proximal Femoral Fractures and Non-Fractured Femora in Octogenarians to Centenarians. J. Clin. Med. 2024, 13, 981. [Google Scholar] [CrossRef]
- Kanis, J.A. Diagnosis of osteoporosis and assessment of fracture risk. Lancet 2002, 359, 1929–1936. [Google Scholar] [CrossRef]
- Yu, E.W.; Thomas, B.J.; Brown, J.K.; Finkelstein, J.S. Simulated increases in body fat and errors in bone mineral density measurements by DXA and QCT. J. Bone Miner. Res. 2012, 27, 119–124. [Google Scholar] [CrossRef]
- Someya, K.; Mochizuki, T.; Hokari, S.; Tanifuji, O.; Katsumi, R.; Koga, H.; Takahashi, Y.; Kobayashi, K.; Morise, Y.; Sakamoto, M.; et al. Age- and sex-related characteristics in cortical thickness of femoral diaphysis for young and elderly subjects. J. Bone Miner. Metab. 2020, 38, 533–543. [Google Scholar] [CrossRef]
- Huppke, C.M.; Fahlbusch, H.; Frosch, K.-H.; Krause, M.; von Rehlingen-Prinz, F. Cortical bone thickness on preoperative CT scans as predictor of bone quality in distal femur fractures: A retrospective study in Caucasians. Arch. Orthop. Trauma. Surg. 2023, 144, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Beck, T.J.; Wang, X.-F.; Seeman, E. Structural and Biomechanical Basis of Sexual Dimorphism in Femoral Neck Fragility Has Its Origins in Growth and Aging. J. Bone Miner. Res. 2003, 18, 1766–1774. [Google Scholar] [CrossRef] [PubMed]
- Cui, P.; Wang, W.; Wang, Z.; Hu, X.; Liu, X.; Kong, C.; Lu, S. The association between body mass index and bone mineral density in older adults: A cross-sectional study of community population in Beijing. BMC Musculoskelet. Disord. 2024, 25, 655. [Google Scholar] [CrossRef] [PubMed]
| Variable | Femoral Neck Fracture | Trochanteric Fracture |
|---|---|---|
| Number of patients | 100 | 100 |
| Sex, n (%) | ||
| female | 70 (70) | 74 (74) |
| male | 30 (30) | 26 (26) |
| Age at time of surgery, years ± SD | 82 ± 9 | 85 ± 8.45 |
| BMI (kg/m2) ± SD | 23.3 ± 4.29 | 22.9 ± 4.15 |
| Body weight (kg) ± SD | 64.3 ± 12.46 | 61.7 ± 12.33 |
| Affected side, n | ||
| left | 41 | 50 |
| right | 59 | 50 |
| CTI ± SD NFx at 10 cm | CTI ± SD NFx at 15 cm | r Value | p Value | |
|---|---|---|---|---|
| Femoral neck fracture | 0.544 ± 0.061 | 0.502 ± 0.06 | r = 0.664 | p < 0.001 |
| Trochanteric fracture | 0.512 ± 0.078 | 0.481 ± 0.072 | r = 0.766 | p < 0.001 |
| CTI ± SD NFx at 15 cm | CTI ± SD Fx at 15 cm | p Value | |
|---|---|---|---|
| Femoral neck fracture | 0.502 ± 0.06 | 0.478 ± 0.065 | p < 0.001 |
| Trochanteric fracture | 0.481 ± 0.072 | 0.457 ± 0.069 | p < 0.001 |
| Variable | Femoral Neck Fracture Females | Femoral Neck Fracture Males | Trochanteric Fracture Females | Trochanteric Fracture Males |
|---|---|---|---|---|
| CTI ± SD NFx at 10 cm | 0.540 ± 0.062 | 0.552 ± 0.057 | 0.514 ± 0.079 | 0.506 ± 0.077 |
| CTI ± SD NFx at 15 cm | 0.488 ± 0.064 | 0.534 ± 0.045 | 0.473 ± 0.075 | 0.504 ± 0.069 |
| Pearson’s correlation | r = 0.716 | r = 0.538 | r = 0.807 | r = 0.742 |
| p value | p < 0.001 | p = 0.046 | p < 0.001 | p = 0.841 |
| Variable | Femoral Neck Fracture Females | Femoral Neck Fracture Males | Trochanteric Fracture Females | Trochanteric Fracture Males |
|---|---|---|---|---|
| CTI ± SD Fx at 15 cm | 0.461 ± 0.06 | 0.517 ± 0.062 | 0.451 ± 0.072 | 0.474 ± 0.058 |
| CTI ± SD NFx at 15 cm | 0.488 ± 0.064 | 0.534 ± 0.045 | 0.473 ± 0.075 | 0.504 ± 0.069 |
| p value | p < 0.001 | p = 0.083 | p < 0.001 | p = 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guyan, F.; Waltenspül, M.; Dietrich, M.; Kabelitz, M. Intra-Individual Differences of the Femoral Cortical Thickness Index in Elderly Patients with a Proximal Femoral Fracture. J. Clin. Med. 2025, 14, 2654. https://doi.org/10.3390/jcm14082654
Guyan F, Waltenspül M, Dietrich M, Kabelitz M. Intra-Individual Differences of the Femoral Cortical Thickness Index in Elderly Patients with a Proximal Femoral Fracture. Journal of Clinical Medicine. 2025; 14(8):2654. https://doi.org/10.3390/jcm14082654
Chicago/Turabian StyleGuyan, Flurina, Manuel Waltenspül, Michael Dietrich, and Method Kabelitz. 2025. "Intra-Individual Differences of the Femoral Cortical Thickness Index in Elderly Patients with a Proximal Femoral Fracture" Journal of Clinical Medicine 14, no. 8: 2654. https://doi.org/10.3390/jcm14082654
APA StyleGuyan, F., Waltenspül, M., Dietrich, M., & Kabelitz, M. (2025). Intra-Individual Differences of the Femoral Cortical Thickness Index in Elderly Patients with a Proximal Femoral Fracture. Journal of Clinical Medicine, 14(8), 2654. https://doi.org/10.3390/jcm14082654






