Minimally Invasive Management of Subclavian Artery Catheter Misplacement: The New Standard?
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
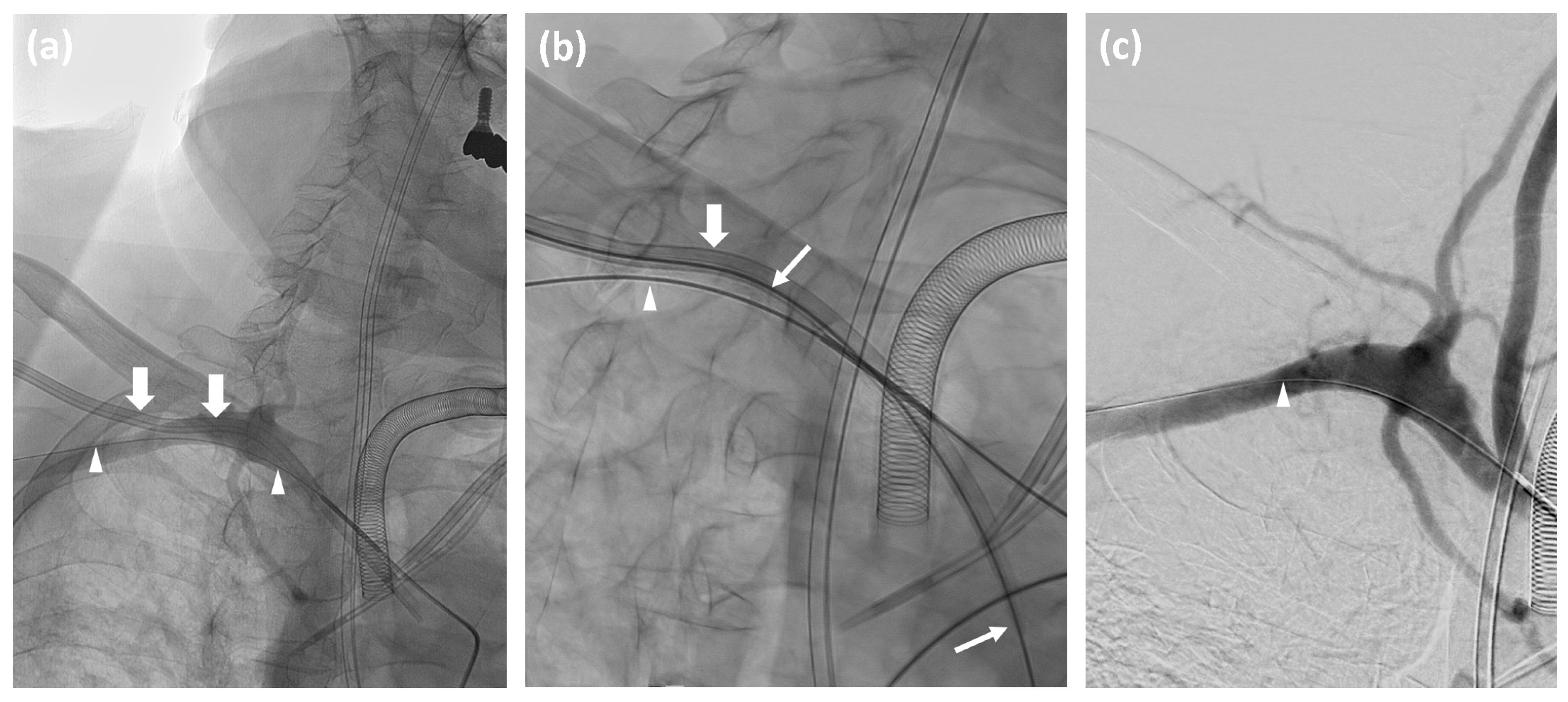
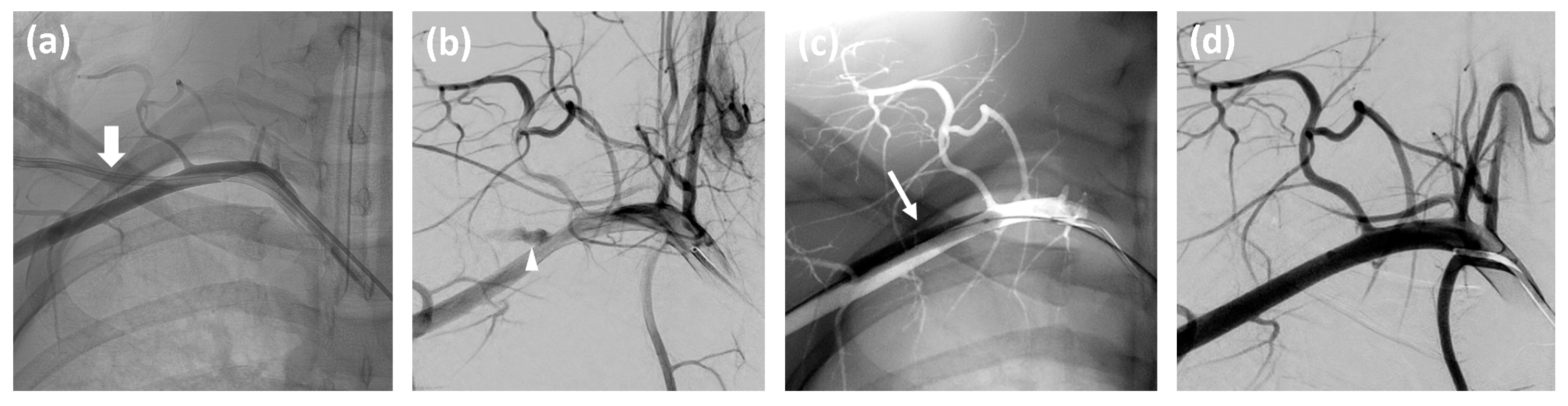
2.2. Management of Accidental Subclavian Artery Catheterization
- Establish inguinal arterial access via an ultrasound-guided puncture of the common femoral artery.
- Advance a catheter in the affected subclavian artery and perform angiographic contrast series in at least two projections to visualize the exact position of the misplaced catheter or the presence of any complications such as dissection or hemorrhage.
- Measure the vessel diameter and prepare an appropriate angioplasty balloon in the angio-suite as a precautionary measure.
- Maintain the guide wire in the affected subclavian artery throughout the procedure.
- Insert an Amplatz wire into the misplaced catheter, and then remove the catheter.
- Depending on the catheter’s diameter, deploy a 6F or 8F Angio-Seal™ vascular closure device according to the manufacturer’s instructions.
- Conduct a follow-up angiographic series to confirm successful closure.
- If hemorrhage or pseudoaneurysm is detected, inflate the prepared angioplasty balloon for at least three minutes. For persistent hemorrhage, deploy an appropriately sized stentgraft, taking care to preserve the vertebral artery origin.
- Remove the inguinal access if no complications are observed.
2.3. Angio-Seal Device
2.4. Balloon and Stentgraft Applications
2.5. Follow-Up Protocol and Definitions
3. Results
3.1. Technical Success Rates
3.2. Post-Procedure Morbidity and Mortality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jang, W.J.; Oh, J.H.; Song, Y.G. Endovascular Repair of Inadvertent Arterial Injury Induced by Central Venous Cathe-terization Using a Vascular Closure Device: A Case Report. J. Korean Soc. Radiol. 2017, 76, 282–286. [Google Scholar]
- Papastratigakis, G.; Marouli, D.; Proklou, A.; Nasis, N.; Kondili, E.; Kehagias, E. Management of Inadvertent Arterial Catheterization during Central Venous Catheter Placement: A Case Series. J. Pers. Med. 2022, 12, 1537. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, G.; White, R.D.; Bhat, R.; Chakraverty, S. Inadvertent Subclavian Artery Cannulation: Endovascular Repair Using a Collagen Closure Device-Report of Two Cases and Review of the Literature. Case Rep. Vasc. Med. 2012, 2012, 150343. [Google Scholar] [CrossRef] [PubMed]
- Chemelli, A.P.; Wiedermann, F.; Klocker, J.; Falkensammer, J.; Strasa, A.; Czermark, B.V.; Waldenberger, P.; Chemelli-Steinguber, I.E. Endovascular Management of Inadvertent Subclavian Artery Catheterization during Subcla-vian Vein Cannulation. J. Vasc. Interv. Radiol. 2010, 21, 470–476. [Google Scholar] [CrossRef]
- Cipanio, S.; Oggionio, R.; Manganio, V.; Bellandi, G.; Ercolini, L.; Michelagnoli, S. An Accidental Subclavian Artery Cannulation: Successful Catheter Removal by Percutaneous Vascular Stenting. Minerva Anestesiol. 2007, 73, 249–253. [Google Scholar]
- Lorenzo, J.F.; Rey, J.V.; Arquillo, I.L.; Encisa de Sá, J.M. Off-Label Use of Proglide Percutaneous Closure Device in Iat-rogenic Arterial Catheterizations: Our Experience. Vascular 2020, 28, 756–759. [Google Scholar] [CrossRef] [PubMed]
- Melas, N.; Saratzis, A.; Saratzis, N.; Kiskinis, D. Endovascular Repair of Inadvertent Subclavian Artery Perforation dur-ing Cannulation for Dialysis Access: Case Report and Review of the Literature. Eur. J. Emerg. Med. 2009, 16, 323–326. [Google Scholar] [CrossRef]
- van Dijk, R.B.; den Heijer, P.; de Muinck, E.; Lie, K.I. Internal Balloon Tamponade: A Non-Surgical Method for Removal of Accidentally Placed Sheaths from the Subclavian Artery. Cathet. Cardiovasc. Diagn. 1993, 29, 325–328. [Google Scholar] [CrossRef]
- Tanmit, P.; Angkasith, P.; Teeratakulpisarn, P.; Thanapaisal, C.; Wongkonkitsin, N.; Prasertcharoensuk, S.; Panich, C. Treatment Outcome of Traumatic Subclavian Artery Injuries. Vasc. Health Risk Manag. 2021, 17, 481–487. [Google Scholar] [CrossRef]
- DuBose, J.J.; Rajani, R.; Gilani, R.; Arthurs, Z.A.; Morrison, J.J.; Clouse, W.D.; Rasmussen, T.E.; Endovascular Skills for Trauma and Resuscitative Surgery Working Group. Endovascular Management of Axillo-Subclavian Arterial Injury: A Review of Published Experience. Injury 2012, 43, 1785–1792. [Google Scholar] [CrossRef]
- Cohen, J.E.; Moshe Gomori, J.; Anner, H.; Itshayek, E. Inadvertent Subclavian Artery Cannulation Treated by Percuta-neous Closure. J. Clin. Neurosci. 2014, 21, 1973–1975. [Google Scholar] [CrossRef] [PubMed]
- Devriendt, A.; Tran-Ngoc, E.; Gottignies, P.; Castro-Rodriguez, J.; Lomas, O.; Jamart, S.; Knecht, S. Ease of Using a Dedi-cated Percutaneous Closure Device after Inadvertent Cannulation of the Subclavian Artery: Case Report. Case Rep. Med. 2009, 2009, 728629. [Google Scholar] [CrossRef]
- Micha, J.P.; Goldstein, B.H.; Lindsay, S.F.; Haskell, R.; Oglevie, S.; Rennmaier, M.A.; Brown, J.B., 3rd. Subclavian Artery Puncture Repair with Angio-Seal Deployment. Gynecol. Oncol. 2007, 104, 761–763. [Google Scholar] [CrossRef]
- Redmond, C.E.; O’Donohoe, R.; Breslin, D.; Brophy, D.P. Inadvertent Subclavian Artery Cannulation with a Central Venous Catheter; Successful Retrieval Using a Minimally Invasive Technique. Ir. Med. J. 2014, 107, 292–293. [Google Scholar]
- Shetty, S.V.; Kwolek, C.J.; Garasic, J.M. Percutaneous Closure after Inadvertent Subclavian Artery Cannulation. Cathe-ter. Cardiovasc. Interv. 2007, 69, 1050–1052. [Google Scholar] [CrossRef] [PubMed]
- Szkup, P.L. A Minimally Invasive Technique for Closing an Iatrogenic Subclavian Artery Cannulation Using the An-gio-Seal Closure Device: Two Case Reports. J. Med. Case Rep. 2012, 6, 82. [Google Scholar] [CrossRef] [PubMed]
- Gąsecka, A.; Idzik, A.; Burban, A.; Pietrasik, A. Iatrogenic Subclavian Artery Puncture Repair with Angio-Seal Deploy-ment and Balloon Occlusion. Adv. Interv. Cardiol./Postępy w Kardiologii Interwencyjnej 2021, 17, 330–331. [Google Scholar]
- Thomas, J.; Joshi, D.; Dwivedi, D.; Guha, D.; Bhatia, J.S. Iatrogenic Subclavian Artery Cannulation: Implications and Management. Indian J. Vasc. Endovasc. Surg. 2021, 8, 190–191. [Google Scholar] [CrossRef]
- Kania, T.; Kimyaghalam, A.; Scarsella, J.; Guerges, M.; Breier, Y.; Deitch, J.; Malekpour, F.; Schor, J.; Singh, K. Su-pra-Aortic Arterial Injuries Following Central Venous Catheterization Managed with Percutaneous Closure Devices: A Comprehensive Literature Review of Current Evidence. Ann. Vasc. Surg. 2023, 96, 301–307. [Google Scholar] [CrossRef]
- Rey Chaves, C.E.; Orozco, C.; Posada, E.; Gómez Zuleta, M.; Fajardo, E.; Barón, V.; Hernández Rodríguez, O.G. Percuta-neous Closure of Subclavian Iatrogenic Injuries after Central Venous Catheterization: A Latin American Experience. Front. Surg. 2023, 10, 1309920. [Google Scholar] [CrossRef]
- Wang, L.; Bai, J.; Jin, J.; Zhi, K.; Nie, S.; Qu, L. Treatment of Inadvertent Cervical Arterial Catheterization: Single-Center Experience. Vascular 2022, 31, 791–798. [Google Scholar] [CrossRef]
- Dornbos, D.L., III; Nimjee, S.M.; Smith, T.P. Inadvertent Arterial Placement of Central Venous Catheters: Systematic Re-view and Guidelines for Treatment. J. Vasc. Interv. Radiol. 2019, 30, 1785–1794. [Google Scholar] [CrossRef] [PubMed]
- Eggebrecht, H.; Haude, M.; von BC Woertgen, U.; Welge, D.; Thiele, H.; Zeymer, U.; Baumgart, D.; Schmermund, A.; Bartel, T.; Wieneke, H.; et al. Early Clinical Experience with the 6 French Angio-Seal Device: Immediate Closure of Femoral Puncture Sites after Diagnostic and Interventional Coronary Procedures. Cathet. Cardiovasc. Interv. 2001, 53, 437–442. [Google Scholar] [CrossRef]
- Nash, J.E.; Evans, D.G. The Angio-Seal Hemostatic Puncture Closure Device. Concept and Experimental Results. Herz 1999, 24, 597–606. [Google Scholar] [CrossRef]
- Sarin, S.N.; Shah, R.K.; Chun, A.; Akman, A.; Arora, S.; Rahbar, R.; Neville, R.; Venbrux, A.C. Use of the 8-F Angio-Seal Vascular Closure Device in Large-Caliber Arteriotomies. J. Endovasc. Ther. 2012, 19, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Verloh, N.; Scharf, G.; Bäumler, W.; Stroszczynski, C.; Platz Batista da Silva, N.; Fellner, C.; Schleder, S. Erroneous Placement of Central Venous Catheters in Subclavian Artery: Retrieval and Successful Hemostasis with a Femoral Closure Device. J. Vasc. Access 2022, 23, 692–697. [Google Scholar] [CrossRef] [PubMed]
- Jahromi, B.S.; Tummala, R.P.; Levy, E.I. Inadvertent Subclavian Artery Catheter Placement Complicated by Stroke: Endovascular Management and Review. Catheter. Cardiovasc. Interv. 2009, 73, 706–711. [Google Scholar] [CrossRef]
- Burbridge, B.; Stoneham, G.; Szkup, P. Percutaneous subclavian artery stent-graft placement following failed ultra-sound guided subclavian venous access. BMC Med. Imaging 2016, 16, 47. [Google Scholar]
- Kufner, S.; Cassese, S.; Groha, P.; Byrne, R.A.; Schunkert, H.; Kastrati, A.; Joner, M. Covered stents for endovascular re-pair of iatrogenic injuries of iliac and femoral arteries. Cardiovasc. Revasc. Med. 2017, 18, 334–340. [Google Scholar]
- Schoder, M.; Cejna, M.; Hölzenbein, T.; Bischof, G.; Lomoschitz, F.; Funovics, M.; Lammer, J. Elective and emergent endovascular treatment of subclavian artery aneurysms and injuries. J. Endovasc. Ther. 2011, 18, 466–480. [Google Scholar]
- Berlet, M.H.; Steffen, D.; Shaughness, G.; Hanner, J. Closure using a surgical closure device of inadvertent subclavian artery punctures during central venous catheter placement. Cardiovasc. Intervent. Radiol. 2001, 24, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Wang, F.; Liu, Y.S. Brachiocephalic Artery Stenting through the Carotid Artery: A Case Report and Review of the Literature. World J. Clin. Cases 2019, 7, 2644–2651. [Google Scholar] [CrossRef]
- Alverne, F.J.A.M.; Lima, F.O.; Rocha, F.A.; Bandeira, D.A.; Lucena, A.F.; Silva, H.C.; Lee, J.S.; Nogueira, R.G. Unfavora-ble Vascular Anatomy during Endovascular Treatment of Stroke: Challenges and Bailout Strategies. J. Stroke 2020, 22, 185–202. [Google Scholar] [CrossRef]
- Ahrari, A.; Healy, G.M.; Min, A.; Alkhalifah, F.; Oreopoulos, G.; Tan, K.T.; Jaberi, A.; Rajan, D.K.; Mafeld, S. Real-World Experience With the Angio-Seal Closure Device: Insights From Manufacturer and User Facility Device Experience Da-tabase. J. Endovasc. Ther. 2023, 18, 15266028231219226. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.Q.; Liu, F.Y.; Duan, F.; Wang, Z.J.; Song, P.; Fan, Q.S. Stent-Grafts Placement for Treatment of Massive Hem-orrhage from Ruptured Hepatic Artery after Pancreaticoduodenectomy. World J. Gastroenterol. 2010, 16, 3716–3722. [Google Scholar] [CrossRef]
- Turan, N.; Butler, S.; Larson, T.C.; Mason, A. Nontraumatic, Posterior Circulation Pseudoaneurysm of the Basilar Artery Summit with Complete Spontaneous Resolution: Case Report and Literature Review. World Neurosurg. 2017, 8, 50. [Google Scholar]
- Morano, S.G.; Coppola, L.; Latagliata, R.; Berneschi, P.; Chistolini, A.; Micozzi, A.; Girmenia, C.; Breccia, M.; Brunetti, G.; Massaro, F.; et al. Early and Late Complications Related to Cen-tral Venous Catheters in Hematological Malignancies: A Retrospective Analysis of 1102 Patients. Mediterr. J. Hematol. Infect. Dis. 2014, 6, e2014011. [Google Scholar] [CrossRef]
- Bauchmuller, K.; Condliffe, R.; Southern, J.; Billings, C.; Charalampopoulos, A.; Elliot, C.A.; Hameed, A.; Kiely, D.G.; Sabroe, I.; Thompson, A.A.R.; et al. Critical Care Outcomes in Patients with Pre-Existing Pulmo-nary Hypertension: Insights from the ASPIRE Registry. ERJ Open Res. 2021, 7, 00046-2021. [Google Scholar] [CrossRef]
| Demographics | Procedure Details | Success | Closure Device | Comments | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Age | Sex | Side | Localization * | Catheter Size (F) | Angio-Seal (F) | PTS | PATS | CS | ||
| 1 | 29 | F | L | 3 | 8.5 | 8 | Y | N | Y | Y | N |
| 2 | 84 | F | R | 2 | 8.5 | 8 | Y | N | Y | Y | N |
| 3 | 59 | F | L | 3 | 8.5 | 8 | Y | N | Y | Y | N |
| 4 | 84 | F | L | 3 | N/A | 6 | N | S | Y | Y | extravasation |
| 5 | 56 | F | L | 3 | 8.5 | 6 | Y | N | Y | Y | N |
| 6 | 76 | F | R | 2 | 8.5 | 8 | N | S | Y | Y | extravasation |
| 7 | 67 | M | L | 3 | 8.5 | 8 | Y | N | Y | Y | N |
| 8 | 42 | F | R | 2 | 8.5 | 6 | Y | N | Y | Y | N |
| 9 | 74 | F | R | 2 | 8.5 | 8 | Y | N | Y | Y | N |
| 10 | 35 | F | R | 3 | 8.5 | 8 | Y | N | Y | Y | N |
| 11 | 76 | M | R | 3 | 8.5 | 8 | Y | N | Y | Y | N |
| 12 | 74 | F | L | 2 | N/A | 6 | Y | N | Y | Y | N |
| 13 | 66 | M | R | 3 | 12 | 8 | Y | N | Y | Y | N |
| 14 | 68 | M | R | 3 | 8.5 | 8 | Y | N | Y | Y | inguinal PSA |
| 15 | 68 | F | L | 3 | 8.5 | 8 | Y | N | Y | Y | N |
| 16 | 57 | M | L | 3 | 8.5 | 8 | Y | N | Y | Y | N |
| 17 | 62 | M | L | 2 | 12 | 8 | Y | N | Y | Y | N |
| 18 | 59 | F | L | 3 | 12 | 8 | Y | N | Y | Y | N |
| 19 | 42 | M | R | 3 | 12 | 8 | N | S | Y | Y | extravasation |
| 20 | 57 | M | L | 3 | N/A | 8 | Y | N | Y | Y | N |
| 21 | 72 | M | R | 3 | 8.5 | 8 | Y | N | Y | N | N |
| 22 | 70 | F | R | 2 | 12 | 8 | Y | N | Y | Y | N |
| 23 | 47 | M | R | 3 | N/A | 8 | Y | N | Y | N | subclavian PSA |
| 24 | 20 | M | R | 1 | N/A | 8 | N | B | Y | Y | extravasation |
| 25 | 48 | M | R | 3 | 8.5 | 8 | N | S | Y | N | extravasation |
| 26 | 74 | F | L | 3 | 8.5 | 8 | Y | N | Y | N | N |
| 27 | 70 | F | L | 3 | 8.5 | 8 | Y | N | Y | Y | N |
| 28 | 61 | F | L | 3 | 8.5 | 8 | Y | N | Y | N | N |
| 29 | 58 | M | L | 3 | 12 | 8 | Y | N | Y | N | N |
| 30 | 61 | M | R | 3 | N/A | 8 | Y | N | Y | Y | N |
| 31 | 65 | F | R | 3 | N/A | 8 | Y | N | Y | N | N |
| 32 | 69 | M | R | 2 | N/A | 8 | Y | N | Y | Y | N |
| 33 | 80 | F | R | 3 | 8.5 | 8 | Y | N | Y | Y | N |
| 34 | 56 | M | R | 3 | N/A | 8 | Y | N | Y | Y | N |
| 35 | 55 | F | R | 3 | 12 | 8 | Y | N | Y | Y | N |
| 36 | 70 | M | L | 3 | N/A | 8 | Y | N | Y | Y | N |
| 37 | 52 | F | L | 1 | N/A | 8 | Y | N | Y | Y | N |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lenhart, L.; Loizides, A.; Galijasevic, M.; Lutz, M.; Freund, M.; Gizewski, E.R.; Grams, A.E. Minimally Invasive Management of Subclavian Artery Catheter Misplacement: The New Standard? J. Clin. Med. 2025, 14, 2650. https://doi.org/10.3390/jcm14082650
Lenhart L, Loizides A, Galijasevic M, Lutz M, Freund M, Gizewski ER, Grams AE. Minimally Invasive Management of Subclavian Artery Catheter Misplacement: The New Standard? Journal of Clinical Medicine. 2025; 14(8):2650. https://doi.org/10.3390/jcm14082650
Chicago/Turabian StyleLenhart, Lukas, Alexander Loizides, Malik Galijasevic, Maximilian Lutz, Martin Freund, Elke R. Gizewski, and Astrid E. Grams. 2025. "Minimally Invasive Management of Subclavian Artery Catheter Misplacement: The New Standard?" Journal of Clinical Medicine 14, no. 8: 2650. https://doi.org/10.3390/jcm14082650
APA StyleLenhart, L., Loizides, A., Galijasevic, M., Lutz, M., Freund, M., Gizewski, E. R., & Grams, A. E. (2025). Minimally Invasive Management of Subclavian Artery Catheter Misplacement: The New Standard? Journal of Clinical Medicine, 14(8), 2650. https://doi.org/10.3390/jcm14082650







