Upstream and Downstream Cardiovascular Changes in Rheumatic Mitral Stenosis: An Update
Abstract
1. Introduction
2. Methods
3. Changes in Mitral Valve
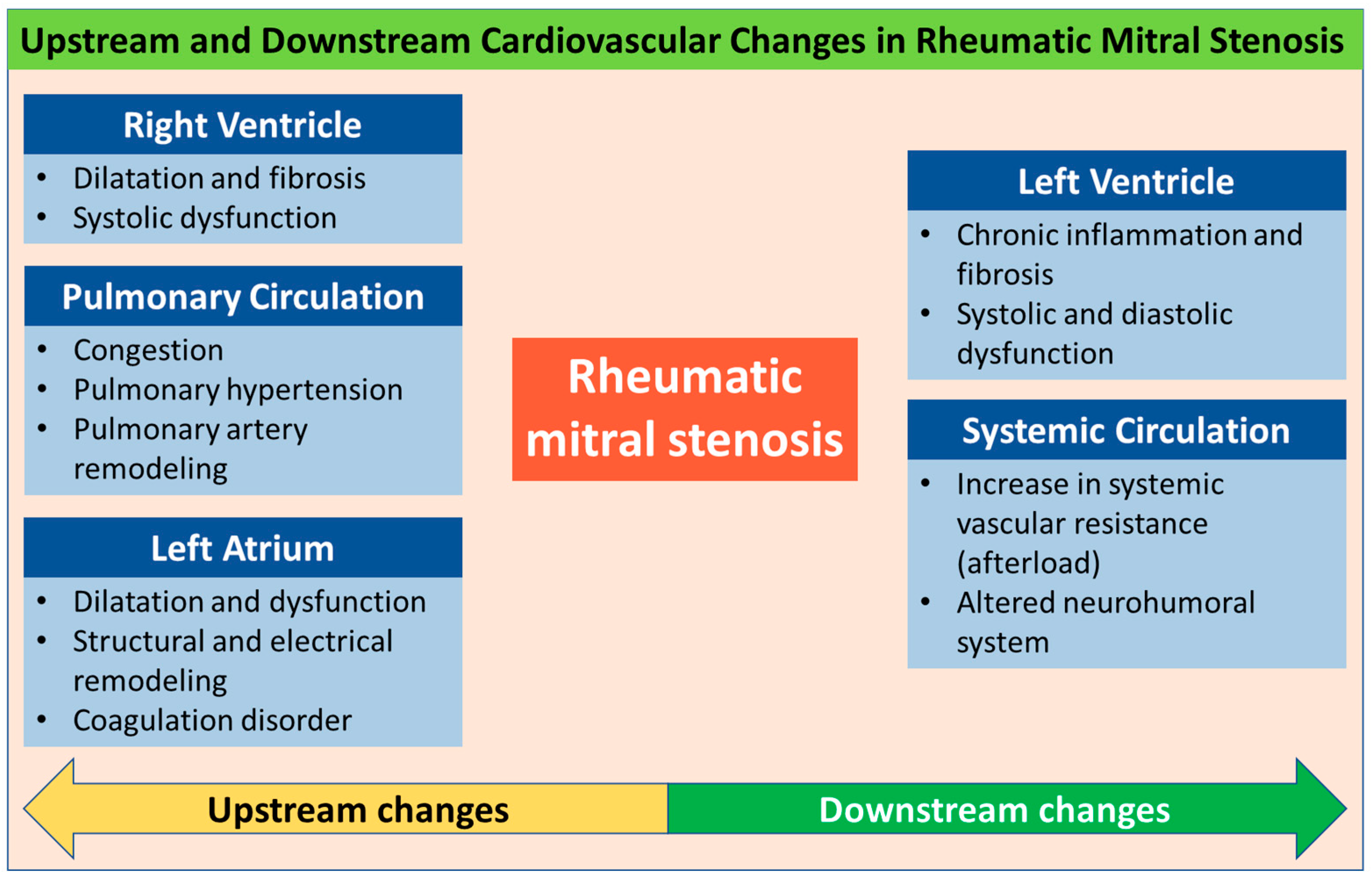
4. Upstream Changes
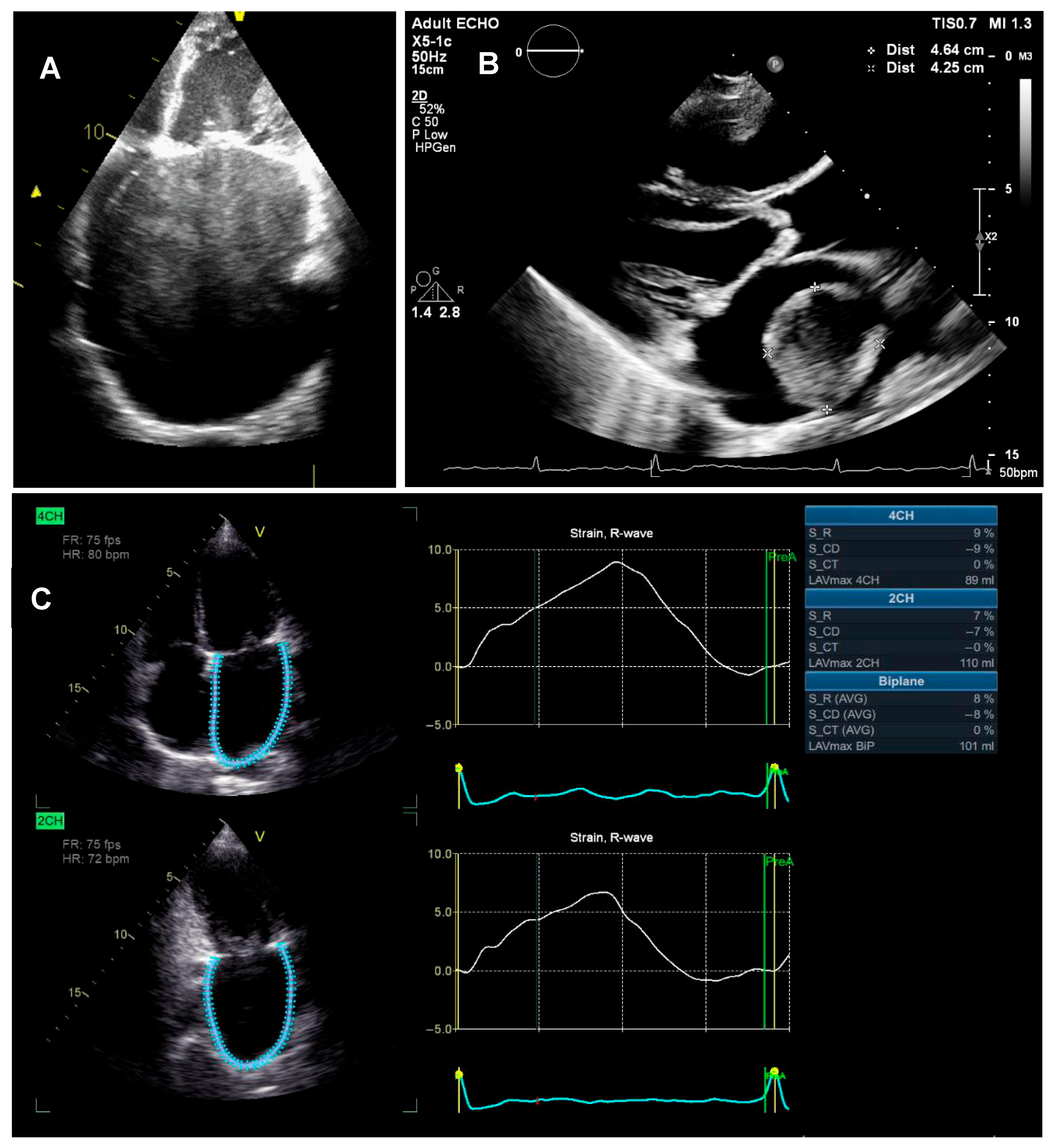
4.1. Left Atrium
4.1.1. Structural Remodeling
4.1.2. Electrical Remodeling
4.1.3. Coagulation Disorder
4.1.4. LA Dysfunction
4.2. Pulmonary Circulation
4.3. Right Ventricle
4.3.1. Structural Changes
4.3.2. Functional Changes
5. Downstream Changes
5.1. Left Ventricle
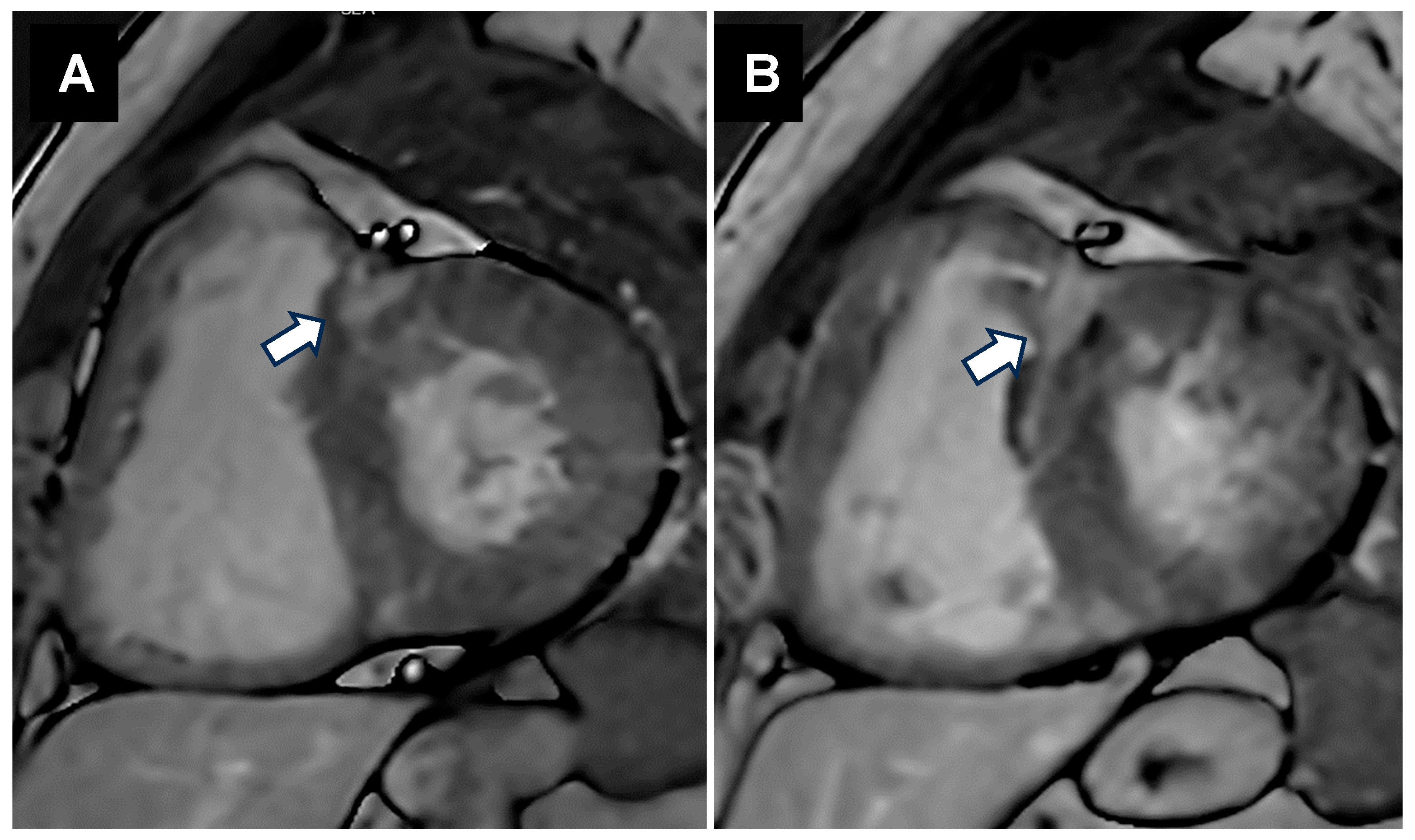
5.1.1. Structural Alterations
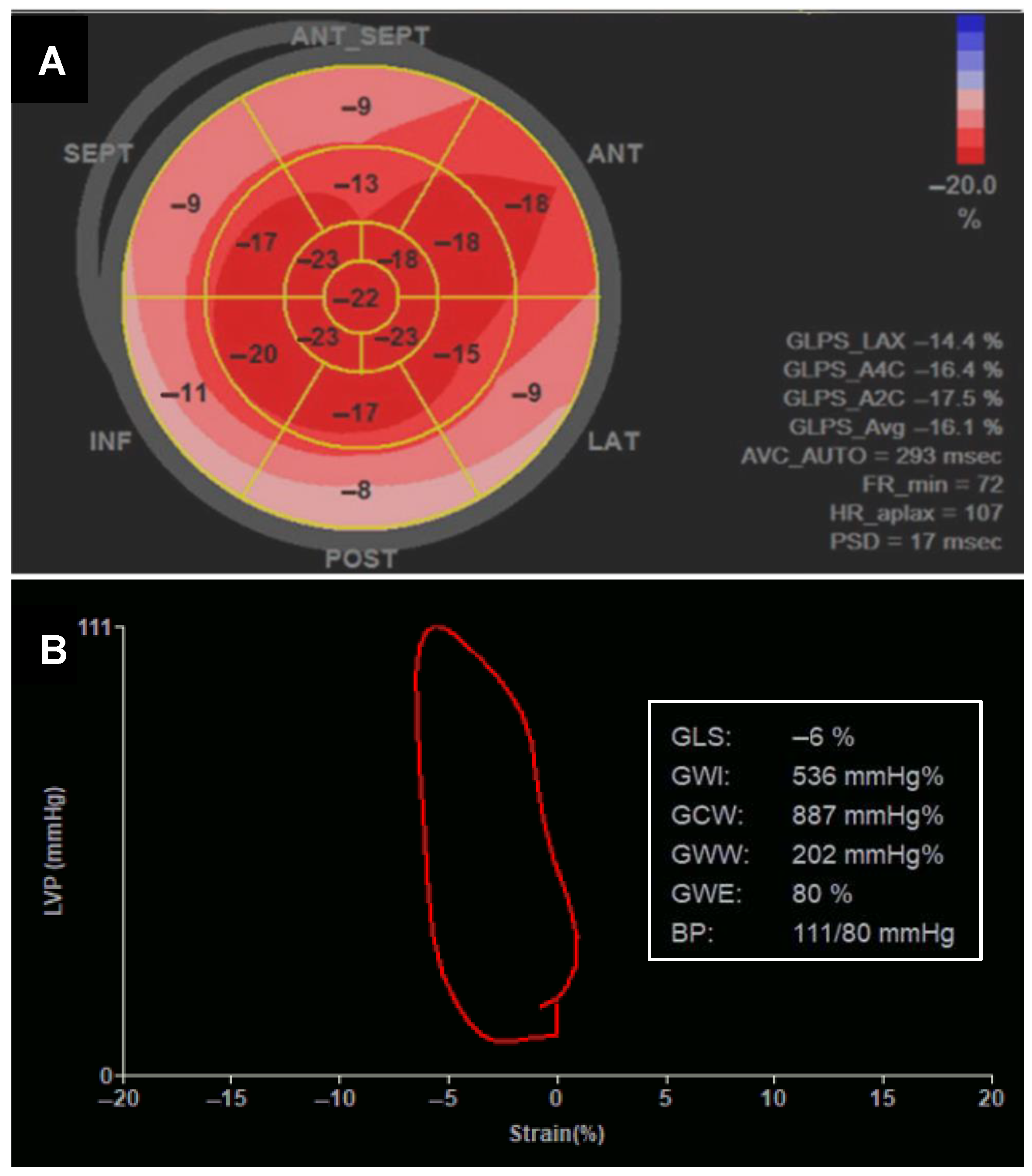
5.1.2. Changes in Systolic Function
5.1.3. Diastolic Dysfunction in MS
5.2. Systemic Circulation
6. Clinical Implications in Management Strategies
7. Conclusions
Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Ou, Z.; Yu, D.; Liang, Y.; Wu, J.; He, H.; Li, Y.; He, W.; Gao, Y.; Wu, F.; Chen, Q. Global burden of rheumatic heart disease: Trends from 1990 to 2019. Arthritis Res. Ther. 2022, 24, 138. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Tuberculosis Report 2019; WHO: Geneva, Switzerland, 2020.
- Rudiktyo, E.; Wind, A.; Doevendans, P.; Siswanto, B.B.; Cramer, M.J.; Soesanto, A.M. Characteristics of patients with rheumatic heart disease in a national referral hospital in Indonesia. Med. J. Indones. 2022, 31, 178–185. [Google Scholar] [CrossRef]
- Rwebembera, J.; Manyilirah, W.; Zhu, Z.W.; Nabbaale, J.; Namuyonga, J.; Ssinabulya, I.; Lubega, S.; Lwabi, P.; Omagino, J.; Okello, E. Prevalence and characteristics of primary left-sided valve disease in a cohort of 15,000 patients undergoing echocardiography studies in a tertiary hospital in Uganda. BMC Cardiovasc. Disord. 2018, 18, 82. [Google Scholar] [CrossRef]
- Butt, H.I.; Shahbaz, A.; Nawaz, H.; Butt, K. Comparative Clinical Characteristics of Rheumatic Heart Disease Patients Undergoing Surgical Valve Replacement. Cureus 2019, 11, e4889. [Google Scholar] [CrossRef] [PubMed]
- Meel, R.; Nethononda, R.; Libhaber, E.; Dix-Peek, T.; Peters, F.; Essop, M. Assessment of myocardial fibrosis by late gadolinium enhancement imaging and biomarkers of collagen metabolism in chronic rheumatic mitral regurgitation. Cardiovasc. J. Afr. 2018, 29, 150–154. [Google Scholar] [CrossRef]
- Ismail, A.S.; Baghdady, Y.; Salem, M.A.; Wahab, A.A. The use of MRI in quantification of the atrial fibrosis in patients with rheumatic mitral disease. Egypt. J. Radiol. Nucl. Med. 2020, 51, 199. [Google Scholar] [CrossRef]
- Rudiktyo, E.; Soesanto, A.M.; Cramer, M.J.; Yonas, E.; Teske, A.J.; Siswanto, B.B.; Doevendans, P.A. Global Left Ventricular Myocardial Work Efficiency in Patients with Severe Rheumatic Mitral Stenosis and Preserved Left Ventricular Ejection Fraction. J. Cardiovasc. Imaging 2023, 31, 191–199. [Google Scholar] [CrossRef]
- Passos, L.S.A.; Nunes, M.C.P.; Aikawa, E. Rheumatic Heart Valve Disease Pathophysiology and Underlying Mechanisms. Front. Cardiovasc. Med. 2020, 7, 612716. [Google Scholar] [CrossRef]
- Mutagaywa, R.K.; Mwakigonja, A.; Chillo, P.; Ngaiza, A.; Byomuganyizi, M.; Fundikira, L.; Cramer, M.J.; Kwesigabo, G.; Kamuhabwa, A.; Chamuleau, S. Histopathological evaluation of chronic rheumatic mitral valve stenosis: The association with clinical presentation, pathogenesis, and management at a National Cardiac Institute, Tanzania. Cardiovasc. Pathol. 2022, 60, 107434. [Google Scholar] [CrossRef]
- Elkareem, T.S.A.; Ahmed, T.A.; Mohamed, L.A. Left Atrial Remodeling in Patients with Severe Rheumatic Mitral Stenosis and Sinus Rhythm Using Two-Dimensional and Three-Dimensional Speckle Tracking Echocardiography. Cardiol. Res. 2023, 14, 142–148. [Google Scholar] [CrossRef]
- Thomas, L.; Abhayaratna, W.P. Left Atrial Reverse Remodeling: Mechanisms, Evaluation, and Clinical Significance. JACC Cardiovasc. Imaging 2017, 10, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Nattel, S.; Burstein, B.; Dobrev, D. Atrial remodeling and atrial fibrillation: Mechanisms and implications. Circ. Arrhythm. Electrophysiol. 2008, 1, 62–73. [Google Scholar] [CrossRef]
- Barger, P.M.; Kelly, D.P. Fatty acid utilization in the hypertrophied and failing heart: Molecular regulatory mechanisms. Am. J. Med. Sci. 1999, 318, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, U.; Kaleem, M.; Hanif, A.; Hanif, M. Correlation of Left Atrial Size with Mitral Valve Area & Atrial Fibrillation in The Patients of Mitral Stenosis An Echocardiographic Based Study. J. Fatima Jinnah Med. Univ. 2014, 8, 44–47. [Google Scholar]
- Bouzas-Mosquera, A.; Broullón, F.J.; Álvarez-García, N.; Méndez, E.; Peteiro, J.; Gándara-Sambade, T.; Prada, O.; Mosquera, V.X.; Castro-Beiras, A. Left atrial size and risk for all-cause mortality and ischemic stroke. Can. Med Assoc. J. 2011, 183, E657–E664. [Google Scholar] [CrossRef]
- Nattel, S.; Harada, M. Atrial remodeling and atrial fibrillation: Recent advances and translational perspectives. J. Am. Coll. Cardiol. 2014, 63, 2335–2345. [Google Scholar] [CrossRef] [PubMed]
- John, B.; Stiles, M.K.; Kuklik, P.; Chandy, S.T.; Young, G.D.; Mackenzie, L.; Szumowski, L.; Joseph, G.; Jose, J.; Worthley, S.G.; et al. Electrical remodelling of the left and right atria due to rheumatic mitral stenosis. Eur. Heart J. 2008, 29, 2234–2243. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Lee, K.L.; Chow, W.H.; Chau, E.; Lau, C.P. Internal cardioversion of chronic atrial fibrillation during percutaneous mitral commissurotomy: Insight into reversal of chronic stretch-induced atrial remodeling. Circulation 2002, 105, 2746–2752. [Google Scholar] [CrossRef]
- Watson, T.; Shantsila, E.; Lip, G.Y. Mechanisms of thrombogenesis in atrial fibrillation: Virchow’s triad revisited. Lancet 2009, 373, 155–166. [Google Scholar] [CrossRef]
- Yamamoto, K.; Ikeda, U.; Seino, Y.; Mito, H.; Fujikawa, H.; Sekiguchi, H.; Shimada, K. Coagulation activity is increased in the left atrium of patients with mitral stenosis. J. Am. Coll. Cardiol. 1995, 25, 107–112. [Google Scholar] [CrossRef]
- Peverill, R.E.; Harper, R.W.; Gelman, J.; Gan, T.E.; Harris, G.; Smolich, J.J. Determinants of Increased Regional Left Atrial Coagulation Activity in Patients With Mitral Stenosis. Circulation 1996, 94, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Haghighi, Z.O.; Naderi, N.; Amin, A.; Taghavi, S.; Sadeghi, M.; Moladoust, H.; Maleki, M.; Haghighi, H.O. Quantitative assessment of right atrial function by strain and strain rate imaging in patients with heart failure. Acta Cardiol. 2011, 66, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Amshala, R. Left Atria Function in Mitral Stenosis: Tissue Doppler Strain Imaging Before and After Balloon Mitral Valvotomy. J. Cardiovasc. Dis. Res. 2022, 13, 1299–1308. [Google Scholar]
- Kiefer, T.L.; Bashore, T.M. Pulmonary Hypertension Related to Left-Sided Cardiac Pathology. Pulm. Med. 2011, 2011, 381787. [Google Scholar] [CrossRef]
- Maeder, M.T.; Weber, L.; Buser, M.; Gerhard, M.; Haager, P.K.; Maisano, F.; Rickli, H. Pulmonary Hypertension in Aortic and Mitral Valve Disease. Front. Cardiovasc. Med. 2018, 5, 40. [Google Scholar] [CrossRef]
- Snopek, G.; Pogorzelska, H.; Rywik, T.M.; Browarek, A.; Janas, J.; Korewicki, J. Usefulness of endothelin-1 concentration in capillary blood in patients with mitral stenosis as a predictor of regression of pulmonary hypertension after mitral valve replacement or valvuloplasty. Am. J. Cardiol. 2002, 90, 188–189. [Google Scholar] [CrossRef]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e72–e227. [Google Scholar] [CrossRef]
- Baumgartner, H.; Falk, V.; Bax, J.J.; De Bonis, M.; Hamm, C.; Holm, P.J.; Iung, B.; Lancellotti, P.; Lansac, E.; Rodriguez Muñoz, D.; et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2017, 38, 2739–2791. [Google Scholar] [CrossRef]
- Ward, C.; Hancock, B.W. Extreme pulmonary hypertension caused by mitral valve disease. Natural history and results of surgery. Br. Heart J. 1975, 37, 74–78. [Google Scholar] [CrossRef]
- Collins, N.; Sugito, S.; Davies, A.; Boyle, A.; Sverdlov, A.; Attia, J.; Stewart, S.; Playford, D.; Strange, G. Prevalence and survival associated with pulmonary hypertension after mitral valve replacement: National echocardiography database of Australia study. Pulm. Circ. 2022, 12, e12140. [Google Scholar] [CrossRef]
- Pande, S.; Tewari, P.; Agarwal, S.K.; Agarwal, V.; Agrawal, V.; Chagtoo, M.; Majumdar, G.; Tewari, S. Evidence of apoptosis in right ventricular dysfunction in rheumatic mitral valve stenosis. Indian J. Med Res. 2016, 144, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Pande, S.; Agarwal, S.K.; Tewari, P.; Agrawal, V.; Srivastav, N.; Tripathi, S.; Soni, N.; Kumar, S. Right ventricular dysfunction in rheumatic heart valve disease: A clinicopathological evaluation. Natl. Med. J. India 2020, 33, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Rudiktyo, E.; Yonas, E.; Cramer, M.J.; Siswanto, B.B.; Doevendans, P.A.; Soesanto, A.M. Impact of Rheumatic Process in Left and Right Ventricular Function in Patients with Mitral Regurgitation. Glob. Heart 2023, 18, 15. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, J.M.; Grecu, L.; Nicoara, A. Right Ventricular Function in Left Heart Disease. Semin. Cardiothorac. Vasc. Anesth. 2019, 23, 88–107. [Google Scholar] [CrossRef]
- Pande, S.; Agarwal, S.K.; Dhir, U.; Chaudhary, A.; Kumar, S.; Agarwal, V. Pulmonary arterial hypertension in rheumatic mitral stenosis: Does it affect right ventricular function and outcome after mitral valve replacement? Interact. Cardiovasc. Thorac. Surg. 2009, 9, 421–425. [Google Scholar] [CrossRef]
- El Fiky, M.M.; El Nahas, Y.M.; Mourad, F.A.; Ali, I.A.; Khalifa, H.A.Z. Prognostic value of right ventricular dysfunction on clinical outcomes for patients undergoing surgical interventions for mitral valve. Egypt. J. Surg. 2024, 43, 692–699. [Google Scholar] [CrossRef]
- Gewitz, M.H.; Baltimore, R.S.; Tani, L.Y.; Sable, C.A.; Shulman, S.T.; Carapetis, J.; Remenyi, B.; Taubert, K.A.; Bolger, A.F.; Beerman, L.; et al. Revision of the Jones Criteria for the diagnosis of acute rheumatic fever in the era of Doppler echocardiography: A scientific statement from the American Heart Association. Circulation 2015, 131, 1806–1818. [Google Scholar] [CrossRef]
- Mocumbi, A.O. Rheumatic heart disease: Is continuum of care achievable in Africa? Circ. Cardiovasc. Qual. Outcomes 2017, 10, e004304. [Google Scholar] [CrossRef]
- Fraser, W.J.; Haffejee, Z.; Cooper, K. Rheumatic Aschoff nodules revisited: An immunohistological reappraisal of the cellular component. Histopathology 1995, 27, 457–461. [Google Scholar] [CrossRef]
- Roberts, W.C.; Virmani, R. Aschoff bodies at necropsy in valvular heart disease. Evidence from an analysis of 543 patients over 14 years of age that rheumatic heart disease, at least anatomically, is a disease of the mitral valve. Circulation 1978, 57, 803–807. [Google Scholar] [CrossRef]
- Putra, T.M.H.; Sukmawan, R.; Elen, E.; Atmadikoesoemah, C.A.; Desandri, D.R.; Kasim, M. Prognostic Value of Late Gadolinium Enhancement in Postoperative Morbidity following Mitral Valve Surgery in Rheumatic Mitral Stenosis. Int. J. Angiol. 2019, 28, 237–244. [Google Scholar] [CrossRef]
- Waller, B.F.; Howard, J.; Fess, S. Pathology of mitral valve stenosis and pure mitral regurgitation—Part I. Clin. Cardiol. 1994, 17, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Oran, B.; Çoban, H.; Karaaslan, S.; Atabek, E.; Gürbilek, M.; Erkul, I. Serum cardiac troponin-I in active rheumatic carditis. Indian J. Pediatr. 2001, 68, 943–944. [Google Scholar] [CrossRef] [PubMed]
- Kamblock, J.; Payot, L.; Iung, B.; Costes, P.; Gillet, T.; Le Goanvic, C.; Lionet, P.; Pagis, B.; Pasche, J.; Roy, C.; et al. Does rheumatic myocarditis really exists? Systematic study with echocardiography and cardiac troponin I blood levels. Eur. Heart J. 2003, 24, 855–862. [Google Scholar] [CrossRef]
- Narula, J.; Chopra, P.; Talwar, K.K.; Reddy, K.S.; Vasan, R.S.; Tandon, R.; Bhatia, M.L.; Southern, J.F. Does endomyocardial biopsy aid in the diagnosis of active rheumatic carditis? Circulation 1993, 88 Pt 1, 2198–2205. [Google Scholar] [CrossRef]
- Jezkova, L.; Jezek, V.; Michaljanic, A.; Dreschslerova, J. Left ventricle in mitral stenosis. Cor Vasa 1982, 24, 240–249. [Google Scholar] [PubMed]
- Heller, S.J.; Carleton, R.A. Abnormal left ventricular contraction in patients with mitral stenosis. Circulation 1970, 42, 1099–1110. [Google Scholar] [CrossRef]
- Mohan, J.C.; Khalilullah, M.; Arora, R. Left ventricular intrinsic contractility in pure rheumatic mitral stenosis. Am. J. Cardiol. 1989, 64, 240–242. [Google Scholar] [CrossRef]
- Liu, C.P.; Ting, C.T.; Yang, T.M.; Chen, J.W.; Chang, M.S.; Maughan, W.L.; Lawrence, W.; Kass, D.A. Reduced left ventricular compliance in human mitral stenosis. Role of reversible internal constraint. Circulation 1992, 85, 1447–1456. [Google Scholar] [CrossRef]
- Gash, A.K.; Carabello, B.A.; Kent, R.L.; Frazier, J.A.; Spann, J.F. Left ventricular performance in patients with coexistent mitral stenosis and aortic insufficiency. J. Am. Coll. Cardiol. 1984, 3, 703–711. [Google Scholar] [CrossRef]
- Klein, A.J.P.; Carroll, J.D. Left Ventricular Dysfunction and Mitral Stenosis. Heart Fail. Clin. 2006, 2, 443–452. [Google Scholar] [CrossRef]
- Ambari, A.M.; Setianto, B.; Santoso, A.; Radi, B.; Dwiputra, B.; Susilowati, E.; Tulrahmi, F.; Doevendans, P.A.; Cramer, M.J. Angiotensin Converting Enzyme Inhibitors (ACEIs) Decrease the Progression of Cardiac Fibrosis in Rheumatic Heart Disease Through the Inhibition of IL-33/sST2. Front. Cardiovasc. Med. 2020, 7, 115. [Google Scholar] [CrossRef] [PubMed]
- Surdacki, A.; Legutko, J.; Turek, P.; Dudek, D.; Zmudka, K.; Dubiel, J. Determinants of depressed left ventricular ejection fraction in pure mitral stenosis with preserved sinus rhythm. J. Heart Valve Dis. 1996, 5, 1–9. [Google Scholar] [PubMed]
- Elen, E.; Atmadikoesoemah, C.A.; Kasim, M.; Klinis, P. Effect of Myocardial Fibrosis on Left Ventricular Function in Rheumatic Mitral Stenosis: A Preliminary Study with Cardiac Magnetic Resonance. Indones. J. Cardiol. 2017, 38, 202–206. [Google Scholar] [CrossRef]
- Soesanto, A.M.; Desandri, D.R.; Haykal, T.M.; Kasim, M. Association between late gadolinium enhancement and global longitudinal strain in patients with rheumatic mitral stenosis. Int. J. Cardiovasc. Imaging 2019, 35, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Galli, E.; Lancellotti, P.; Sengupta, P.P.; Donal, E. LV mechanics in mitral and aortic valve diseases: Value of functional assessment beyond ejection fraction. JACC Cardiovasc. Imaging 2014, 7, 1151–1166. [Google Scholar] [CrossRef]
- Lee, Y.S.; Ching-Ping, L. Ultrastructural Pathological Study of Left Ventricular Myocardium in Patients with Isolated Rheumatic Mitral Stenosis with Normal or Abnormal Left Ventricular Function. Jpn. Heart J. 1990, 31, 435–448. [Google Scholar] [CrossRef]
- Bilen, E.; Kurt, M.; Isik, T.; Ekinci, M.; Kaya, A.; Bayram, E.; Simsek, Z.; Tanboga, I. PP-115: Severity of Mitral Stenosis and Left Ventricular Mechanics: A Speckle Tracking Study. Int. J. Cardiol. 2011, 147, S128. [Google Scholar] [CrossRef]
- Ambari, A.M.; Setianto, B.; Santoso, A.; Radi, B.; Dwiputra, B.; Susilowati, E.; Tulrahmi, F.; Wind, A.; Cramer, M.J.M.; Doevendans, P. Randomised controlled trial into the role of ramipril in fibrosis reduction in rheumatic heart disease: The RamiRHeD trial protocol. BMJ Open 2021, 11, e048016. [Google Scholar] [CrossRef]
- Wisenbaugh, T.; Essop, R.; Middlemost, S.; Skoularigis, J.; Sareli, P. Excessive vasoconstriction in rheumatic mitral stenosis with modestly reduced ejection fraction. J. Am. Coll. Cardiol. 1992, 20, 1339–1344. [Google Scholar] [CrossRef]
- Venkateshvaran, A.; Govind, S.C. Left ventricular diastolic function in mitral stenosis. Echocardiography 2020, 37, 1944–1950. [Google Scholar] [CrossRef] [PubMed]
- Sabbah, H.N.; Anbe, D.T.; Stein, P.D. Negative intraventricular diastolic pressure in patients with mitral stenosis: Evidence of left ventricular diastolic suction. Am. J. Cardiol. 1980, 45, 562–566. [Google Scholar] [CrossRef] [PubMed]
- Eleid, M.F.; Nishimura, R.A.; Lennon, R.J.; Sorajja, P. Left ventricular diastolic dysfunction in patients with mitral stenosis undergoing percutaneous mitral balloon valvotomy. Mayo Clin. Proc. 2013, 88, 337–344. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Appleton, C.P.; Gillebert, T.C.; Marino, P.N.; Oh, J.K.; Smiseth, O.A.; Waggoner, A.D.; Flachskampf, F.A.; Pellikka, P.A.; Evangelisa, A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur. J. Echocardiogr. 2009, 10, 165–193. [Google Scholar] [CrossRef]
- Sharma, V.; Stewart, R.A.H.; Zeng, I.; Raffel, C.; Kerr, A.J. Comparison of atrial and brain natriuretic peptide for the assessment of mitral stenosis. Heart Lung Circ. 2011, 20, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Kaku, K.; Hirota, Y.; Shimizu, G.; Kita, Y.; Saito, T.; Kawamura, K. Depressed myocardial contractility in mitral stenosis—An analysis by force-length and stress-shortening relationships. Jpn. Circ. J. 1988, 52, 35–43. [Google Scholar] [CrossRef]
- Ashino, K.; Gotoh, E.; Sumita, S.I.; Moriya, A.; Ishii, M. Percutaneous transluminal mitral valvuloplasty normalizes baroreflex sensitivity and sympathetic activity in patients with mitral stenosis. Circulation 1997, 96, 3443–3449. [Google Scholar] [CrossRef]
- Kumar, R.K.; Antunes, M.J.; Beaton, A.; Mirabel, M.; Nkomo, V.T.; Okello, E.; Regmi, P.R.; Reményi, B.; Sliwa-Hähnle, K.; Zühlke, L.J.; et al. Contemporary Diagnosis and Management of Rheumatic Heart Disease: Implications for Closing the Gap: A Scientific Statement From the American Heart Association. Circulation 2020, 142, E337–E357. [Google Scholar] [CrossRef]
- de Dassel, J.L.; de Klerk, N.; Carapetis, J.R.; Ralph, A.P. How many doses make a difference? An analysis of secondary prevention of rheumatic fever and rheumatic heart disease. J. Am. Heart Assoc. 2018, 7, e010223. [Google Scholar] [CrossRef]
- Selvam, P.; Moorthy, K.; Sivalingam, S.; Dillon, J.; Kong, P.K.; Yakub, M.A. Is it worth repairing rheumatic mitral valve disease in children? Long-term outcomes of an aggressive approach to rheumatic mitral valve repair compared to replacement in young patients. Interact. Cardiovasc. Thorac. Surg. 2019, 28, 191–198. [Google Scholar] [CrossRef]
- Kim, W.K.; Kim, H.J.; Kim, J.B.; Jung, S.-H.; Choo, S.J.; Chung, C.H.; Lee, J.W. Clinical outcomes in 1731 patients undergoing mitral valve surgery for rheumatic valve disease. Heart 2018, 104, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Gillinov, A.M.; Suri, R.; Mick, S.; Mihaljevic, T. Robotic mitral valve surgery: Current limitations and future directions. Ann. Cardiothorac. Surg. 2016, 5, 573–576. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease: Developed by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rudiktyo, E.; Teske, A.J.; Yonas, E.; Ambari, A.M.; Cramer, M.J.; Guglielmo, M.; Semino, T.; Siswanto, B.B.; Doevendans, P.A.; Soesanto, A.M. Upstream and Downstream Cardiovascular Changes in Rheumatic Mitral Stenosis: An Update. J. Clin. Med. 2025, 14, 2639. https://doi.org/10.3390/jcm14082639
Rudiktyo E, Teske AJ, Yonas E, Ambari AM, Cramer MJ, Guglielmo M, Semino T, Siswanto BB, Doevendans PA, Soesanto AM. Upstream and Downstream Cardiovascular Changes in Rheumatic Mitral Stenosis: An Update. Journal of Clinical Medicine. 2025; 14(8):2639. https://doi.org/10.3390/jcm14082639
Chicago/Turabian StyleRudiktyo, Estu, Arco J. Teske, Emir Yonas, Ade M. Ambari, Maarten J. Cramer, Marco Guglielmo, Tommaso Semino, Bambang Budi Siswanto, Pieter A. Doevendans, and Amiliana M. Soesanto. 2025. "Upstream and Downstream Cardiovascular Changes in Rheumatic Mitral Stenosis: An Update" Journal of Clinical Medicine 14, no. 8: 2639. https://doi.org/10.3390/jcm14082639
APA StyleRudiktyo, E., Teske, A. J., Yonas, E., Ambari, A. M., Cramer, M. J., Guglielmo, M., Semino, T., Siswanto, B. B., Doevendans, P. A., & Soesanto, A. M. (2025). Upstream and Downstream Cardiovascular Changes in Rheumatic Mitral Stenosis: An Update. Journal of Clinical Medicine, 14(8), 2639. https://doi.org/10.3390/jcm14082639






