Multisonic Ultracleaning and Laser-Activated Irrigation Effect Compared to Passive Ultrasonic Activation for Debridement in Minimally Invasive Instrumentation of Necrotic Oval Root Canals: An Ex Vivo Histological Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Size Calculation, Sample Selection, and Eligibility
2.2. Preparation of Root Canals
2.3. Allocation of Groups and Root Canal Debridement
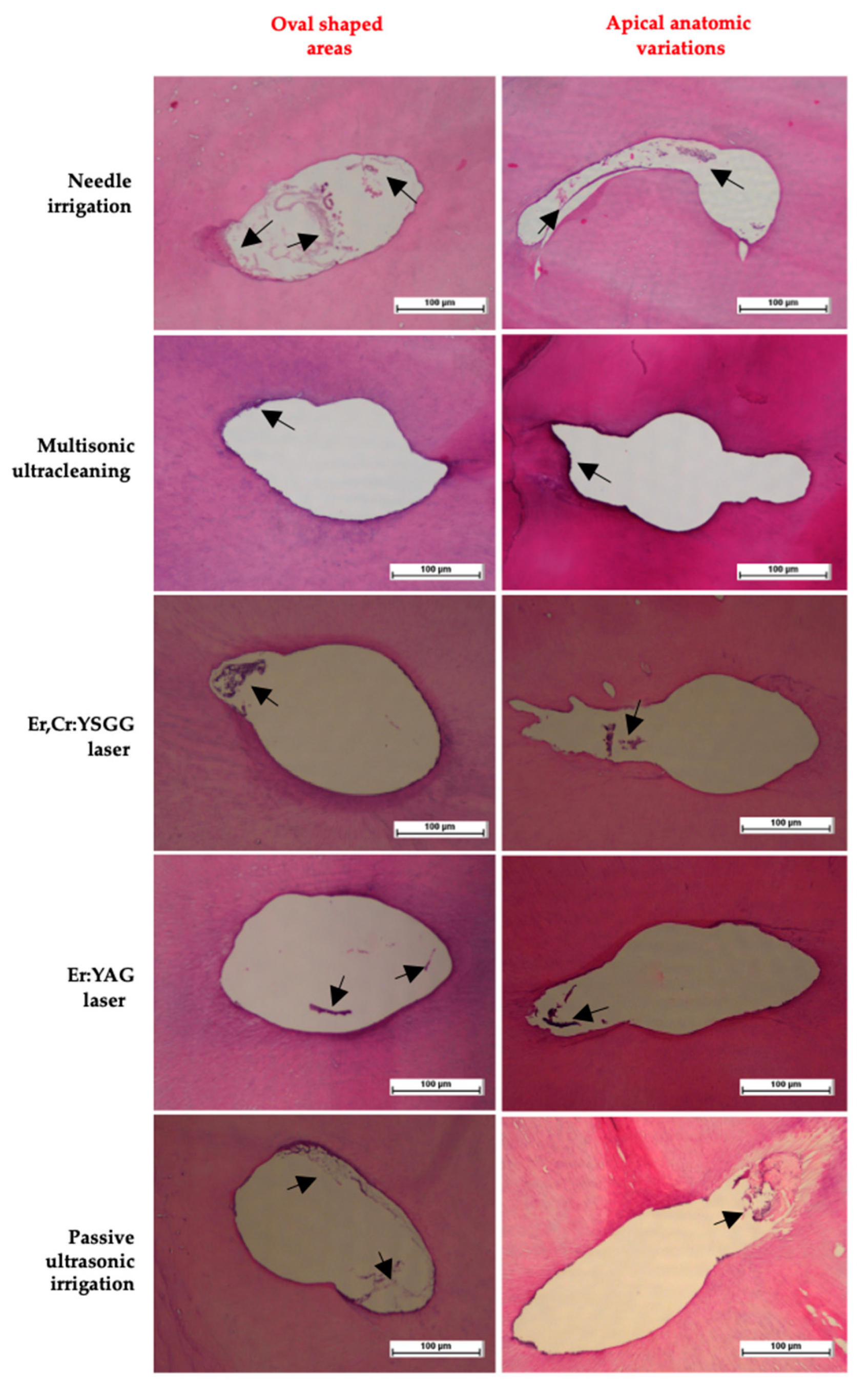
- Needle Irrigation (NI) (n:14): The root canals were irrigated via needle tip without activation in the same preparation using 3 mL of sodium hypochlorite, 2 mL of 17% EDTA (Imicryl, Imicryl Dental, Konya, Türkiye), and 3 mL of 5% sodium hypochlorite, respectively.
- Passive Ultrasonic Activation (PUI) (n:14): An ultrasonic tip (#20) at 30 kHz frequency was used to activate the solution in the root canal system. The root canals were irrigated with solutions, and the tip was placed 1 mm short of the working length. Three turns of cycles per solution using 3 mL sodium hypochlorite, 2 mL of 17% EDTA, and 3 mL of 5% sodium hypochlorite were repeated with refreshment of the solutions for 20 s, resting the solutions for 20 s inside the canal, and activation for 20 s with around 1–4 mm back-and-forth movement, respectively. All solutions were activated by the mentioned tool and protocol.
- Er:YAG (SWEEPS) (n:14): A Skypulse laser with an air–water cooling-off application was used to activate the solution in the access cavity. Three turns of cycles per solution using 3 mL sodium hypochlorite, 2 mL of 17% EDTA, and 3 mL of 5% sodium hypochlorite were repeated with refreshment of the solutions for 20 s, resting the solutions for 20 s inside the canal, and activation for 20 s, respectively. All solutions were activated by the aforementioned tool and protocol.
- Er,Cr: YSGG (n:14): A Waterlase iPlus laser was used to activate the solution in the root canal system with a fiber tip (RFT2-25). The console was set to 50 Hz, 25 mJ, and 1.25 W with an air–water cooling-off application. The tip was placed 1 mm short of the working length. Three turns of cycles per solution using 3 mL sodium hypochlorite, 2 mL of 17% EDTA, and 3 mL of 5% sodium hypochlorite were repeated with refreshment of the solutions for 20 s, resting the solutions for 20 s inside the canal, and activation for 20 s with around 1–4 mm back-and-forth movement, respectively. All solutions were activated by the aforementioned tool and protocol.
- Multisonic ultracleaning system (n:14): A GentleWave® G4 with ProControl™ software system with a CleanFlow handpiece was used for activation. The handpiece was connected to the main console following the manufacturer’s recommendations. An occlusal platform was fabricated to adapt the SoundSeal (Sonendo Inc., Laguna Hills, CA, USA) handpiece to the airtight seal access cavity. The mechanism of fluid dynamics was based on the action generated by the GentleWave console, which was set and operated. Measures of 5% sodium hypochlorite, 17% EDTA, and 5% sodium hypochlorite were used with distilled water in between for 5 min at a flow rate of 45–50 mL/min.
2.4. Histological Analysis and Morphometric Evaluation
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NaOCl | Sodium hypochlorite |
| EDTA | Ethylenediaminetetraacetic acid |
| NI | Needle irrigation |
| PUI | Passive ultrasonic activation |
| wt | Weight |
| vol | Volume |
| M | Mean |
| SD | Standard deviation |
References
- Ordinola-Zapata, R.; Noblett, W.C.; Perez-Ron, A.; Ye, Z.; Vera, J. Present Status and Future Directions of Intracanal Medicaments. Int. Endod. J. 2022, 55, 613–636. [Google Scholar] [CrossRef] [PubMed]
- De-Deus, G.; Barino, B.; Zamolyi, R.Q.; Souza, E.; Fonseca Júnior, A.; Fidel, S.; Sergio Fidel, R.A. Suboptimal Debridement Quality Produced by the Single-File F2 Protaper Technique in Oval-Shaped Canals. J. Endod. 2010, 36, 1897–1900. [Google Scholar] [CrossRef] [PubMed]
- Rae, O.; Parashos, P. Prevalence and Morphology of Different Root Canal Systems in Mandibular Premolars: A Cross-Sectional Observational Study. Aust. Dent. J. 2024, 69, 112–123. [Google Scholar] [CrossRef]
- Neelakantan, P.; Romero, M.; Vera, J.; Daood, U.; Khan, A.U.; Yan, A.; Cheung, G.S.P. Biofilms in Endodontics—Current Status and Future Directions. Int. J. Mol. Sci. 2017, 18, 1748. [Google Scholar] [CrossRef] [PubMed]
- Violich, D.R.; Chandler, N.P. The Smear Layer in Endodontics—A Review. Int. Endod. J. 2010, 43, 2–15. [Google Scholar] [CrossRef]
- Arias, A.; Peters, O.A. Present Status and Future Directions: Canal Shaping. Int. Endod. J. 2022, 55, 637–655. [Google Scholar] [CrossRef]
- Özbay, Y.; Özdemir, O. Evaluation of Laser-Activated Irrigation on Evidence-Based Endodontology: A Bibliometric and Scientometric Analysis of Recent Articles. G. Ital. Endod. 2022, 36, 10–21. [Google Scholar]
- Coşkun Başoğlu, E.; Koçak, S.; Özdemir, O.; Koçak, M.M.; Sağlam, B.C. Efficacy of Various Activation Techniques on Tubule Penetration of Resin-Based and Bioceramic Root Canal Sealers: An in Vitro Confocal Microscopy Study. Aust. End. J. 2023, 49, 381–389. [Google Scholar] [CrossRef]
- Huynh, A.; Poly, A.; Yamaguchi, M.; Jalali, P. Efficacy of Laser Activated versus Sonic Activated Irrigation for Debris Removal in Conservatively Instrumented Root Canals. J. Endod. 2024; in press. [Google Scholar] [CrossRef]
- Ozbay, Y.; Erdemir, A. Effect of several laser systems on removal of smear layer with a variety of irrigation solutions. Mic. Res. Tech. 2018, 81, 1214–1222. [Google Scholar] [CrossRef]
- Lukac, N.; Zadravec, J.; Gregorcic, P.; Lukac, M.; Jezeršek, M. Wavelength dependence of photon-induced photoacoustic streaming technique for root canal irrigation. J. Biomed. Opt. 2016, 21, 75007. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Shen, Y.; Haapasalo, M. Effectiveness of Six Irrigation Techniques with Sodium Hypochlorite in Tissue Dissolution. Cureus 2023, 15, e39208. [Google Scholar] [CrossRef] [PubMed]
- Auerbach, D.; Alaugaily, I.; Davis, S.; Azim, A.A. A Promising Approach Utilising Photothermal Energy to Disinfect the Root Canal System: An in Vitro Investigation. Aust. End. J. 2024, 50, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Lévesque, C.; Malkhassian, G.; Basrani, B. Efficacy of the GentleWave System in the Removal of Biofilm from the Mesial Roots of Mandibular Molars before and after Minimal Instrumentation: An Ex Vivo Study. Int. Endod. J. 2024, 57, 922–932. [Google Scholar] [CrossRef]
- The GentleWave G4 System with ProControl. Available online: https://gentlewave.com/doctor/gentlewave-system (accessed on 30 January 2025).
- Lertchirakarn, V.; Palamara, J.E.A.; Messer, H.H. Patterns of Vertical Root Fracture: Factors Affecting Stress Distribution in the Root Canal. J. Endod. 2003, 29, 523–528. [Google Scholar] [CrossRef]
- Kishen, A. Mechanisms and Risk Factors for Fracture Predilection in Endodontically Treated Teeth. Endod. Top. 2006, 13, 57–83. [Google Scholar] [CrossRef]
- Lin, F.; Ordinola-Zapata, R.; Fok, A.S.L.; Lee, R. Influence of Minimally Invasive Endodontic Access Cavities and Bonding Status of Resin Composites on the Mechanical Property of Endodontically-Treated Teeth: A Finite Element Study. Dent. Mater. 2022, 38, 242–250. [Google Scholar] [CrossRef]
- Rathke, A.; Frehse, H.; Bechtold, M. Ex Vivo Investigation on the Effect of Minimally Invasive Endodontic Treatment on Vertical Root Fracture Resistance and Crack Formation. Sci. Rep. 2024, 14, 13205. [Google Scholar] [CrossRef]
- Ramírez-Muñoz, A.; Escribano-Capdevila, M.; Navarrete, N.; Vieira, G.C.S.; Salamanca-Ramos, M.; Ortolani-Seltenerich, P.S.; Aranguren, J.; Pérez, A.R. Comparative Micro-CT Analysis of Minimally Invasive Endodontic Systems Using 3D-Printed Replicas and Natural Teeth. Materials 2024, 17, 5279. [Google Scholar] [CrossRef]
- Supavititpattana, N.; Suebnukarn, S.; Phumpatrakom, P.; Budsaba, K. Micro-Computed Tomography Evaluation of Minimally Invasive Root Canal Preparation in 3D-Printed C-Shaped Canal. Aust. End. J. 2024, 50, 621–628. [Google Scholar] [CrossRef]
- Varela, P.; Souza, E.; de Deus, G.; Duran-Sindreu, F.; Mercadé, M. Effectiveness of Complementary Irrigation Routines in Debriding Pulp Tissue from Root Canals Instrumented with a Single Reciprocating File. Int. Endod. J. 2019, 52, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Lee, O.Y.S.; Khan, K.; Li, K.Y.; Shetty, H.; Abiad, R.S.; Cheung, G.S.P.; Neelakantan, P. Influence of Apical Preparation Size and Irrigation Technique on Root Canal Debridement: A Histological Analysis of Round and Oval Root Canals. Int. Endod. J. 2019, 52, 1366–1376. [Google Scholar] [CrossRef] [PubMed]
- Jidewar, N.; Chandak, M. A Protocol for a Comparative Evaluation of the Fracture Resistance of Endodontically Treated Teeth Reinforced with Cention N, Resin-Modified Glass Ionomer Cement (RMGIC) and Short Fiber Reinforced Flowable Composite as an Intraorifice Barrier. F1000Res 2024, 13, 49. [Google Scholar] [CrossRef] [PubMed]
- Puleio, F.; Lo Giudice, G.; Militi, A.; Bellezza, U.; Lo Giudice, R. Does Low-Taper Root Canal Shaping Decrease the Risk of Root Fracture? A Systematic Review. Dent. J. 2022, 10, 94. [Google Scholar] [CrossRef]
- Kılıç, Y.; Karataşlıoğlu, E.; Kaval, M.E. The Effect of Root Canal Preparation Size and Taper of Middle Mesial Canals on Fracture Resistance of the Mandibular Molar Teeth: An In Vitro Study. J. Endod. 2021, 47, 1467–1471. [Google Scholar] [CrossRef]
- Nawar, N.N.; Elkholy, M.M.A.; Ha, W.N.; Saber, S.M.; Kim, H.C. Optimum Shaping Parameters of the Middle Mesial Canal in Mandibular First Molars: A Finite Element Analysis Study. J. Endod. 2023, 49, 567–574. [Google Scholar] [CrossRef]
- Park, K.H.; Ordinola-Zapata, R.; Noblett, W.C.; Lima, B.P.; Staley, C. The Effect of Ultrasonic and Multisonic Irrigation on Root Canal Microbial Communities: An Ex Vivo Study. Int. Endod. J. 2024, 57, 895–906. [Google Scholar] [CrossRef]
- Ordinola-Zapata, R.; Mansour, D.; Saavedra, F.; Staley, C.; Chen, R.; Fok, A.S. In Vitro Efficacy of a Non-Instrumentation Technique to Remove Intracanal Multispecies Biofilm. Int. Endod. J. 2022, 55, 495–504. [Google Scholar] [CrossRef]
- Wu, M.K.; van der Sluis, L.W.M.; Wesselink, P.R. The capability of two hand instrumentation techniques to remove the inner layer of dentine in oval canals. Int. Endod. J. 2003, 36, 218–224. [Google Scholar] [CrossRef]
- Gazzaneo, I.; Vieira, G.C.S.; Pérez, A.R.; Alves, F.R.F.; Gonçalves, L.S.; Mdala, I.; Siqueira, J.F., Jr.; Rôças, I.N. Root Canal Disinfection by Single- and Multiple-Instrument Systems: Effects of Sodium Hypochlorite Volume, Concentration, and Retention Time. J. Endod. 2019, 45, 736–741. [Google Scholar] [CrossRef]
- Arruda-Vasconcelos, R.; Barbosa-Ribeiro, M.; Louzada, L.M.; Lemos, B.I.N.; de-Jesus-Soares, A.; Ferraz, C.C.R.; Almeida, J.F.A.; Marciano, M.A.; Gomes, B.P.F.A. Efficacy of 6% Sodium Hypochlorite on Infectious Content of Teeth with Symptomatic Irreversible Pulpitis. J. Endod. 2022, 48, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Wu, S.; Yang, Y.; Wan, J. Efficacy of Five Irrigation Techniques in Removing Calcium Hydroxide from Simulated S-Shaped Root Canals. J. Dent. Sci. 2022, 17, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Feng, B.; Shi, S.; Sun, D.; Wu, D. The Effect of Different Activation Irrigations on Intracanal Smear Layer Removal: A Vitro Study. Front. Bioeng. Biotechnol. 2024, 12, 1507525. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, B.F.A.; Reddy, D.; Machado, R.; Soares Júunior, P.C.; Ignácio, S.A.; Fernandes Couto, D.A.; Frasquetti, K.S.; Ditzel Westphalen, V.P.; Carneiro, E.; da Silva Neto, U.X. Smear Layer Removal Comparing Conventional Irrigation, Passive Ultrasonic Irrigation, EndoActivator System, and a New Sonic Device (Perfect Clean System) by Scanning Electron Microscopy: An Ex Vivo Study. PLoS ONE 2024, 19, e0314940. [Google Scholar] [CrossRef]
- Meire, M.; De Moor, R.J.G. Principle and antimicrobial efficacy of laser-activated irrigation: A narrative review. Int. Endod. J. 2024, 57, 841–860. [Google Scholar] [CrossRef]
- Blanken, J.; Verdaasdonk, R. Cavitation as a working mechanism of the Er, Cr: YSGG laser in endodontics: A visualization study. J. Oral Laser Appl. 2007, 7, 97–106. [Google Scholar]
- Matsumoto, H.; Yoshimine, Y.; Akamine, A. Visualization of irrigant flow and cavitation induced by Er: YAG laser within a root canal model. J. Endod. 2011, 37, 839–843. [Google Scholar] [CrossRef]

| Groups | N | n | Mean ± SD |
|---|---|---|---|
| Needle irrigation | 14 | 3 | 15.64 ± 7.23 |
| Multisonic ultracleaning | 14 | 3 | 1.54 ± 1.46 a |
| Er,Cr:YSGG laser | 14 | 3 | 5.04 ± 3.63 ab |
| Er:YAG laser | 14 | 3 | 3.54 ± 2.81 a |
| Passive ultrasonic | 14 | 3 | 8.40 ± 5.92 abcd |
| Groups | Mean | SD | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|---|---|
| 1. Needle irrigation | 15.64 | 7.23 | - | ||||
| 2. Multisonic ultracleaning | 1.54 | 1.46 | <0.001 *** | - | |||
| 3. Er,Cr:YSGG laser | 5.04 | 3.63 | <0.001 *** | 0.007 ** | - | ||
| 4. Er:YAG laser | 3.54 | 2.81 | <0.001 *** | 0.298 ns | 0.599 ns | - | |
| 5. Passive ultrasonic | 8.4 | 5.92 | <0.001 *** | <0.001 *** | 0.011 * | <0.001 *** | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gündoğar, M.; Özdemir, O.; Gündoğar, Ö.; Bektaş, S.; Demir, F.N.; Bolat, N. Multisonic Ultracleaning and Laser-Activated Irrigation Effect Compared to Passive Ultrasonic Activation for Debridement in Minimally Invasive Instrumentation of Necrotic Oval Root Canals: An Ex Vivo Histological Analysis. J. Clin. Med. 2025, 14, 2597. https://doi.org/10.3390/jcm14082597
Gündoğar M, Özdemir O, Gündoğar Ö, Bektaş S, Demir FN, Bolat N. Multisonic Ultracleaning and Laser-Activated Irrigation Effect Compared to Passive Ultrasonic Activation for Debridement in Minimally Invasive Instrumentation of Necrotic Oval Root Canals: An Ex Vivo Histological Analysis. Journal of Clinical Medicine. 2025; 14(8):2597. https://doi.org/10.3390/jcm14082597
Chicago/Turabian StyleGündoğar, Mustafa, Olcay Özdemir, Özgecan Gündoğar, Sibel Bektaş, Fadile Nur Demir, and Nergiz Bolat. 2025. "Multisonic Ultracleaning and Laser-Activated Irrigation Effect Compared to Passive Ultrasonic Activation for Debridement in Minimally Invasive Instrumentation of Necrotic Oval Root Canals: An Ex Vivo Histological Analysis" Journal of Clinical Medicine 14, no. 8: 2597. https://doi.org/10.3390/jcm14082597
APA StyleGündoğar, M., Özdemir, O., Gündoğar, Ö., Bektaş, S., Demir, F. N., & Bolat, N. (2025). Multisonic Ultracleaning and Laser-Activated Irrigation Effect Compared to Passive Ultrasonic Activation for Debridement in Minimally Invasive Instrumentation of Necrotic Oval Root Canals: An Ex Vivo Histological Analysis. Journal of Clinical Medicine, 14(8), 2597. https://doi.org/10.3390/jcm14082597






