Impact of the Circadian Rhythm and Seasonal Changes on the Outcome of Cardiovascular Interventions
Abstract
1. Introduction
2. Seasonal Variations
3. Circadian Rhythms
3.1. Central and Peripheral Clocks
3.2. Circadian Cardiovascular Rhythms and Pathological Processes
3.2.1. Underlying Mechanisms
3.2.2. Blood Pressure Control
3.2.3. Chronotherapy
4. Natural Rhythms and Cardiovascular Interventions
4.1. Seasonal Variations
4.2. Circadian Variations and Cardiac Interventions
4.3. Limitations of Current Scientific Knowledge
| Authors | Year Country | N | Type of Surgery | Mortality | Postoperative Outcome Other Results |
|---|---|---|---|---|---|
| Yount KW et al. [60] | 2015 Canada | 3395 Single center | Elective heart surgery | ↑ in-hospital mortality in Afternoon (5.2% vs. 3.5% Morning), OR 2.04 | Similar incidence of postoperative complications |
| Montaigne D et al. [44] | 2018 France | 596 Single center | SAVR | no difference in operative mortality between Afternoon and Morning operations | ↓ CV complications in the Afternoon group (9% vs. 18% Morning group); no difference in hospital LOS |
| Montaigne D et al. [44] | 2018 France | 88, RCT Single center | SAVR | no difference in mortality rate | ↓ cTp-T in Afternoon; no differences in CV support, AF, hospital LOS |
| Montaigne D et al. [44] | 2018 France | 30 Single center | in vitro study SAVR | - | ↑ contractility after hypoxia-reoxygenation in samples harvested in the Afternoon (vs morning) ↓ Rev-Erbalpha gene expression |
| Baik J et al. [52] | 2019 South Korea | 1690 Single center | Off-pump CABGS | No difference 1-year mortality (2.7% Morning vs. 1.5% Afternoon) | similar 30-day major CV complications (10.5% Afternoon vs. 8.9% Morning), renal dysfunction and release of cTp-T |
| Gotte J et al. [48] | 2020 Germany | 2720 Single center | SAVR isolated or with CABGS | Similar 30-day mortality (1.5% Afternoon vs. 2.7% Morning) | Similar risk of MI in afternoon vs morning groups (HR 0.88, 95% CI 0.32–2.38) and heart failure (HR 0.91, 95% CI 0.65–1.28) |
| Kenney PS et al. [51] | 2020 Danemark | 7148 Single center | SAVR isolated or with CABGS | Similar 30-day mortality (1.5% Afternoon vs. 1.5% Morning) | Similar 30-day major CV complications (3.8% Afternoon vs. 3.3% Morning), MI ((2.4% vs. 2.0%), AF (20.6% vs. 21.4%), renal failure and hospital LOS |
| Nemeth S et al. [47] | 2021 USA | 14,078 Multicenter registry | CABGS (10,863), SAVR (3215) | Similar 30-day mortality for CABGS (1.5% Afternoon vs. 1.3% Morning) and for SAVR (1.6% Afternoon vs. 1.7% Morning) | Similar risk of 30-day MI, stroke, renal failure, infections, prolonged ventilation |
| Fudulu DP et al. [50] | 2021 UK | 105,459, National registry | CABGS (78,232), SAVR (27,227) | Similar 30-day mortality (1.0% Afternoon vs. 1.0% Morning) | ↑ preoperative risk factors in the Morning group (↓ LVEF, ↑ renal dysfunction, PHT and MI) |
| Moscarelli M et al. [46] | 2021 Italy | 124 Single center | CABGS, post-hoc analysis (RCT) | - | Similar postoperative release of cTp, energetic substrate in cardiac tissue samples (ATP/ADP and ATP/AMP ratios) |
| Michaud M et al. [54] | 2012–2018-Canada | 538 Single center | SAVR with/without CABGS | Similar 30-day mortality (5.2% Afternoon vs. 2.0% Morning) | Similar postoperative cTp MI, AKI, stroke and hospital LOS |
| Immohr MB et al. [53] | 2024-Germany | 235 Single center | Heart transplantation | No difference in 30-day mortality (9.2% Afternoon vs. 11.4% Morning) | Similar rates of postoperative AKI, infections, and acute graft rejection |
| Fournier S et al. [55] | 2014 Switzerland | 1021 Single center | PCI | N.R. | ↑ Post-PCI MI in the Afternoon |
| Vincent F et al. [49] | 2014 International | 5586 Multicenter | TAVR (4457) SAVR (1129) | No difference in 30-day mortality after TAVR (8.6% Afternoon vs. 8.1% Morning) and after SAVR (8.6% Afternoon vs. 9.1% Morning) | Similar rate of AKI, new pacemaker and major bleeding in the Afternoon and Morning groups after TAVR and SAVR Similar functional recovery after TAVR and SAVR |
5. Conclusions
Funding
Conflicts of Interest
References
- Kumar, R.; Kumar, A.; Sardhara, J. Pineal Gland—A Spiritual Third Eye: An Odyssey of Antiquity to Modern Chronomedicine. Indian J. Neurosurg. 2018, 7, 1–4. [Google Scholar] [CrossRef]
- Askitopoulou, H. Sleep and Dreams: From Myth to Medicine in Ancient Greece. J. Anesth. Hist. 2015, 1, 70–75. [Google Scholar] [CrossRef]
- Loudon, A.S.I.; Semikhodskii, A.G.; Crosthwaite, S.K. A Brief History of Circadian Time. Trends Genet. 2000, 16, 477–481. [Google Scholar] [CrossRef]
- Huang, R.-C. The Discoveries of Molecular Mechanisms for the Circadian Rhythm: The 2017 Nobel Prize in Physiology or Medicine. Biomed. J. 2018, 41, 5–8. [Google Scholar] [CrossRef]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Naghavi, M.; Ong, K.L.; Aali, A.; Ababneh, H.S.; Abate, Y.H.; Abbafati, C.; Abbasgholizadeh, R.; Abbasian, M.; Abbasi-Kangevari, M.; Abbastabar, H.; et al. Global Burden of 288 Causes of Death and Life Expectancy Decomposition in 204 Countries and Territories and 811 Subnational Locations, 1990–2021: A Systematic Analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 2100–2132. [Google Scholar] [CrossRef]
- Choong, A.M.T.L.; Marjot, J.; Wee, I.J.Y.; Syn, N.; Marjot, T.; Brightwell, R.E.; Walker, P.J. Forecasting Aortic Aneurysm Rupture: A Systematic Review of Seasonal and Atmospheric Associations. J. Vasc. Surg. 2019, 69, 1615–1632.e17. [Google Scholar] [CrossRef]
- Salam, A.; Kamran, S.; Bibi, R.; Korashy, H.M.; Parray, A.; Mannai, A.A.; Ansari, A.A.; Kanikicharla, K.K.; Gashi, A.Z.; Shuaib, A. Meteorological Factors and Seasonal Stroke Rates: A Four-Year Comprehensive Study. J. Stroke Cerebrovasc. Dis. 2019, 28, 2324–2331. [Google Scholar] [CrossRef]
- Wu, H.; Wang, Z.; Li, M.; Liu, Q.; Liu, W.; Qiao, Z.; Bai, T.; Liu, Y.; Zhang, C.; Sun, P.; et al. A Systematic Review and Meta-Analysis of Seasonal and Monthly Variability in the Incidence of Acute Aortic Dissection. Ann. Vasc. Surg. 2022, 85, 383–394. [Google Scholar] [CrossRef]
- Marti-Soler, H.; Gonseth, S.; Gubelmann, C.; Stringhini, S.; Bovet, P.; Chen, P.-C.; Wojtyniak, B.; Paccaud, F.; Tsai, D.-H.; Zdrojewski, T.; et al. Seasonal Variation of Overall and Cardiovascular Mortality: A Study in 19 Countries from Different Geographic Locations. PLoS ONE 2014, 9, e113500. [Google Scholar] [CrossRef]
- Nakamura, K.; Nakamura, Y.; Kataoka, N. A Hypothalamomedullary Network for Physiological Responses to Environmental Stresses. Nat. Rev. Neurosci. 2022, 23, 35–52. [Google Scholar] [CrossRef]
- Stewart, S.; Keates, A.K.; Redfern, A.; McMurray, J.J.V. Seasonal Variations in Cardiovascular Disease. Nat. Rev. Cardiol. 2017, 14, 654–664. [Google Scholar] [CrossRef] [PubMed]
- Wimalasena, N.N.; Chang-Richards, A.; Wang, K.I.-K.; Dirks, K.N. Housing Risk Factors Associated with Respiratory Disease: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 2815. [Google Scholar] [CrossRef]
- Jani, R.; Mhaskar, K.; Tsiampalis, T.; Kassaw, N.A.; González, M.Á.M.; Panagiotakos, D.B. Circulating 25-Hydroxy-Vitamin D and the Risk of Cardiovascular Diseases. Systematic Review and Meta-Analysis of Prospective Cohort Studies. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 3282–3304. [Google Scholar] [CrossRef]
- Grant, W.B.; Boucher, B.J. An Exploration of How Solar Radiation Affects the Seasonal Variation of Human Mortality Rates and the Seasonal Variation in Some Other Common Disorders. Nutrients 2022, 14, 2519. [Google Scholar] [CrossRef]
- Abrignani, M.G.; Lombardo, A.; Braschi, A.; Renda, N.; Abrignani, V. Climatic Influences on Cardiovascular Diseases. World J. Cardiol. 2022, 14, 152–169. [Google Scholar] [CrossRef]
- Dibner, C.; Schibler, U.; Albrecht, U. The Mammalian Circadian Timing System: Organization and Coordination of Central and Peripheral Clocks. Annu. Rev. Physiol. 2010, 72, 517–549. [Google Scholar] [CrossRef]
- Allada, R.; Bass, J. Circadian Mechanisms in Medicine. N. Engl. J. Med. 2021, 384, 550–561. [Google Scholar] [CrossRef]
- Brown, S.A. Circadian Metabolism: From Mechanisms to Metabolomics and Medicine. Trends Endocrinol. Metab. 2016, 27, 415–426. [Google Scholar] [CrossRef]
- Rabinovich-Nikitin, I.; Lieberman, B.; Martino, T.A.; Kirshenbaum, L.A. Circadian-Regulated Cell Death in Cardiovascular Diseases. Circulation 2019, 139, 965–980. [Google Scholar] [CrossRef]
- Rabinovich-Nikitin, I.; Kirshenbaum, L.A. Circadian Regulated Control of Myocardial Ischemia-Reperfusion Injury. Trends Cardiovasc. Med. 2024, 34, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chatham, J.C.; Young, M.E. Circadian Regulation of Cardiac Physiology: Rhythms That Keep the Heart Beating. Annu. Rev. Physiol. 2020, 82, 79–101. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, Y.; Escames, G.; Yang, Z.; Zhao, H.; Qian, L.; Xue, C.; Xu, D.; Acuña-Castroviejo, D.; Yang, Y. Deciphering Clock Genes as Emerging Targets against Aging. Ageing Res. Rev. 2022, 81, 101725. [Google Scholar] [CrossRef]
- Acosta-Rodríguez, V.A.; Rijo-Ferreira, F.; Green, C.B.; Takahashi, J.S. Importance of Circadian Timing for Aging and Longevity. Nat. Commun. 2021, 12, 2862. [Google Scholar] [CrossRef]
- Crnko, S.; Cour, M.; Van Laake, L.W.; Lecour, S. Vasculature on the Clock: Circadian Rhythm and Vascular Dysfunction. Vasc. Pharmacol. 2018, 108, 1–7. [Google Scholar] [CrossRef]
- Young, M.E. The Cardiac Circadian Clock. JACC Basic Transl. Sci. 2023, 8, 1613–1628. [Google Scholar] [CrossRef]
- Hanif, A.; Okafor, D.K.; Katyal, G.; Kaur, G.; Ashraf, H.; Bodapati, A.; Nath, T.S. Shifting Rhythms: A Systematic Review Exploring the Multifaceted Effects of Shift Work and Circadian Disruption on Employee Cardiovascular Health. Cureus 2024, 16, e71003. [Google Scholar] [CrossRef]
- Rana, S.; Prabhu, S.D.; Young, M.E. Chronobiological Influence Over Cardiovascular Function: The Good, the Bad, and the Ugly. Circ. Res. 2020, 126, 258–279. [Google Scholar] [CrossRef]
- West, A.S.; Schønsted, M.I.; Iversen, H.K. Impact of the Circadian Clock on Fibrinolysis and Coagulation in Healthy Individuals and Cardiovascular Patients—A Systematic Review. Thromb. Res. 2021, 207, 75–84. [Google Scholar] [CrossRef]
- Ohashi, N.; Isobe, S.; Ishigaki, S.; Yasuda, H. Circadian Rhythm of Blood Pressure and the Renin–Angiotensin System in the Kidney. Hypertens. Res. 2017, 40, 413–422. [Google Scholar] [CrossRef]
- Nadeem, A.; Rais, T.; Aamir, M.; Habte, A.; Siddiqui, T.; Karamat, R.I.; Munsab, R.; Habib, A. Acetylsalicylic Acid Dosed at Bedtime vs. Dosed in the Morning for Circadian Rhythm of Blood Pressure—A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2024, 11, 1346265. [Google Scholar] [CrossRef]
- Buurma, M.; Van Diemen, J.J.K.; Thijs, A.; Numans, M.E.; Bonten, T.N. Circadian Rhythm of Cardiovascular Disease: The Potential of Chronotherapy with Aspirin. Front. Cardiovasc. Med. 2019, 6, 84. [Google Scholar] [CrossRef]
- Roush, G.C.; Fapohunda, J.; Kostis, J.B. Evening Dosing of Antihypertensive Therapy to Reduce Cardiovascular Events: A Third Type of Evidence Based on a Systematic Review and Meta-Analysis of Randomized Trials. J. Clin. Hypertens. 2014, 16, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, I.S.; Rogers, A.; Poulter, N.R.; Williams, B.; Brown, M.J.; Webb, D.J.; Ford, I.; Rorie, D.A.; Guthrie, G.; Grieve, J.W.K.; et al. Cardiovascular Outcomes in Adults with Hypertension with Evening versus Morning Dosing of Usual Antihypertensives in the UK (TIME Study): A Prospective, Randomised, Open-Label, Blinded-Endpoint Clinical Trial. Lancet 2022, 400, 1417–1425. [Google Scholar] [CrossRef]
- Ogbebor, O.; Odugbemi, B.; Maheswaran, R.; Patel, K. Seasonal Variation in Mortality Secondary to Acute Myocardial Infarction in England and Wales: A Secondary Data Analysis. BMJ Open 2018, 8, e019242. [Google Scholar] [CrossRef]
- Bai, L.; Li, Q.; Wang, J.; Lavigne, E.; Gasparrini, A.; Copes, R.; Yagouti, A.; Burnett, R.T.; Goldberg, M.S.; Cakmak, S.; et al. Increased Coronary Heart Disease and Stroke Hospitalisations from Ambient Temperatures in Ontario. Heart 2018, 104, 673–679. [Google Scholar] [CrossRef]
- Peták, F.; Kovács, B.N.; Agócs, S.; Virág, K.; Nyári, T.; Molnár, A.; Südy, R.; Lengyel, C.; Babik, B. Seasonal Changes in Proportion of Cardiac Surgeries Associated with Diabetes, Smoking and Elderly Age. PLoS ONE 2022, 17, e0274105. [Google Scholar] [CrossRef]
- Martin, T.J.; Eltorai, A.E.M.; Kennedy, K.; Sellke, F.; Ehsan, A. Seasonality of Postoperative Pneumonia after Coronary Artery Bypass Grafting: A National Inpatient Sample Study. J. Card. Surg. 2020, 35, 1258–1266. [Google Scholar] [CrossRef]
- Mori, M.; Wang, Y.; Mahajan, S.; Geirsson, A.; Krumholz, H.M. Associations Between the Severity of Influenza Seasons and Mortality and Readmission Risks After Elective Surgical Aortic Valve Replacement and Coronary Artery Bypass Graft Surgery in Older Adults. JAMA Netw. Open 2020, 3, e2031078. [Google Scholar] [CrossRef]
- Shuhaiber, J.H.; Goldsmith, K.; Nashef, S.A.M. The Influence of Seasonal Variation on Cardiac Surgery: A Time-Related Clinical Outcome Predictor. J. Thorac. Cardiovasc. Surg. 2008, 136, 894–899. [Google Scholar] [CrossRef]
- Luo, Z.-R.; Lin, Z.-Q.; Chen, L.; Qiu, H.-F. Effects of Seasonal and Climate Variations on In-Hospital Mortality and Length of Stay in Patients with Type A Aortic Dissection. J. Cardiothorac. Surg. 2021, 16, 252. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Wu, Q.; Chen, X.; Chen, X.; Xie, L.; Chen, L. Seasonal and Daily Variations in the Occurence and Outcomes of Acute Stanford Type A Dissections: A Retrospective Single-Center Study. J. Cardiothorac. Surg. 2023, 18, 108. [Google Scholar] [CrossRef] [PubMed]
- Swets, M.C.; Termorshuizen, F.; De Keizer, N.F.; Van Paassen, J.; Palmen, M.; Visser, L.G.; Arbous, M.S.; Groeneveld, G.H. Influenza Season and Outcome After Elective Cardiac Surgery: An Observational Cohort Study. Ann. Thorac. Surg. 2023, 116, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Montaigne, D.; Marechal, X.; Modine, T.; Coisne, A.; Mouton, S.; Fayad, G.; Ninni, S.; Klein, C.; Ortmans, S.; Seunes, C.; et al. Daytime Variation of Perioperative Myocardial Injury in Cardiac Surgery and Its Prevention by Rev-Erbα Antagonism: A Single-Centre Propensity-Matched Cohort Study and a Randomised Study. Lancet 2018, 391, 59–69. [Google Scholar] [CrossRef]
- Ninni, S.; Seunes, C.; Ortmans, S.; Mouton, S.; Modine, T.; Koussa, M.; Jegou, B.; Edme, J.-L.; Staels, B.; Montaigne, D.; et al. Peri-Operative Acute Kidney Injury upon Cardiac Surgery Time-of-Day. Int. J. Cardiol. 2018, 272, 54–59. [Google Scholar] [CrossRef]
- Moscarelli, M.; Angelini, G.D.; Emanueli, C.; Suleiman, S.; Pepe, M.; Contegiacomo, G.; Punjabi, P.P. Remote Ischemic Preconditioning in Isolated Valve Intervention. A Pooled Meta-Analysis. Int. J. Cardiol. 2021, 324, 146–151. [Google Scholar] [CrossRef]
- Nemeth, S.; Schnell, S.; Argenziano, M.; Ning, Y.; Kurlansky, P. Daytime Variation Does Not Impact Outcome of Cardiac Surgery: Results from a Diverse, Multi-Institutional Cardiac Surgery Network. J. Thorac. Cardiovasc. Surg. 2021, 162, 56–67.e44. [Google Scholar] [CrossRef]
- Götte, J.; Zittermann, A.; Deutsch, M.A.; Schramm, R.; Bleiziffer, S.; Hata, M.; Gummert, J.F. Daytime Variation in Aortic Valve Surgery and Clinical Outcome: A Propensity Score–Matched Analysis. Ann. Thorac. Surg. 2020, 110, 558–566. [Google Scholar] [CrossRef]
- Vincent, F.; Thourani, V.H.; Ternacle, J.; Redfors, B.; Cohen, D.J.; Hahn, R.T.; Li, D.; Crowley, A.; Webb, J.G.; Mack, M.J.; et al. Time-of-Day and Clinical Outcomes After Surgical or Transcatheter Aortic Valve Replacement: Insights from the Partner Trials. Circ. Cardiovasc. Qual. Outcomes 2022, 15, e007948. [Google Scholar] [CrossRef]
- Fudulu, D.P.; Dimagli, A.; Dixon, L.; Sandhu, M.; Cocomello, L.; Angelini, G.D.; Benedetto, U. Daytime and Outcomes after Cardiac Surgery: Systematic Review and Metaanalysis, Insights from a Large UK Database Review and Post-Hoc Trial Analysis. Lancet Reg. Health Eur. 2021, 7, 100140. [Google Scholar] [CrossRef]
- Kenney, P.S.; Nielsen, P.H.; Modrau, I.S. Daytime-Dependent Cardioprotection in Cardiac Surgery: A Large Propensity-Matched Cohort Study. Ann. Thorac. Surg. 2020, 110, 1629–1636. [Google Scholar] [CrossRef] [PubMed]
- Baik, J.; Nam, J.; Oh, J.; Kim, G.W.; Lee, E.; Lee, Y.; Chung, C.H.; Choi, I. Effect of Operative Time on the Outcome of Patients Undergoing Off-pump Coronary Artery Bypass Surgery. J. Card. Surg. 2019, 34, 1220–1227. [Google Scholar] [CrossRef] [PubMed]
- Immohr, M.B.; Mehdiani, A.; Oehler, D.; Hettlich, V.H.; Jenkins, F.S.; Westenfeld, R.; Aubin, H.; Tudorache, I.; Boeken, U.; Lichtenberg, A.; et al. Impact of Circadian Rhythm and Daytime Variation on Outcome after Heart Transplantation. Clin. Transplant. 2023, 37, e14939. [Google Scholar] [CrossRef]
- Michaud, M.; Béland, V.; Noiseux, N.; Forcillo, J.; Stevens, L.-M. Daytime Variation of Clinical Outcome in Cardiac Surgery: A Propensity-Matched Cohort Study. J. Cardiothorac. Vasc. Anesth. 2021, 35, 3167–3175. [Google Scholar] [CrossRef] [PubMed]
- Fournier, S.; Puricel, S.; Morawiec, B.; Eeckhout, E.; Mangiacapra, F.; Trana, C.; Tapponnier, M.; Iglesias, J.F.; Michiels, V.; Stauffer, J.-C.; et al. Relationship between Time of Day and Periprocedural Myocardial Infarction after Elective Angioplasty. Chronobiol. Int. 2014, 31, 206–213. [Google Scholar] [CrossRef]
- Wright, M.C. Time of Day Effects on the Incidence of Anesthetic Adverse Events. Qual. Saf. Health Care 2006, 15, 258–263. [Google Scholar] [CrossRef]
- Meewisse, A.J.G.; Gribnau, A.; Thiessen, S.E.; Stenvers, D.J.; Hermanides, J.; Van Zuylen, M.L. Effect of Time of Day on Outcomes in Elective Surgery: A Systematic Review. Anaesthesia 2024, 79, 1325–1334. [Google Scholar] [CrossRef]
- Lecacheur, M.; Ammerlaan, D.J.M.; Dierickx, P. Circadian Rhythms in Cardiovascular (Dys)Function: Approaches for Future Therapeutics. npj Cardiovasc. Health 2024, 1, 21. [Google Scholar] [CrossRef]
- Talamanca, L.; Gobet, C.; Naef, F. Sex-Dimorphic and Age-Dependent Organization of 24-Hour Gene Expression Rhythms in Humans. Science 2023, 379, 478–483. [Google Scholar] [CrossRef]
- Yount, K.W.; Lau, C.L.; Yarboro, L.T.; Ghanta, R.K.; Kron, I.L.; Kern, J.A.; Ailawadi, G. Late Operating Room Start Times Impact Mortality and Cost for Nonemergent Cardiac Surgery. Ann. Thorac. Surg. 2015, 100, 1653–1659. [Google Scholar] [CrossRef]
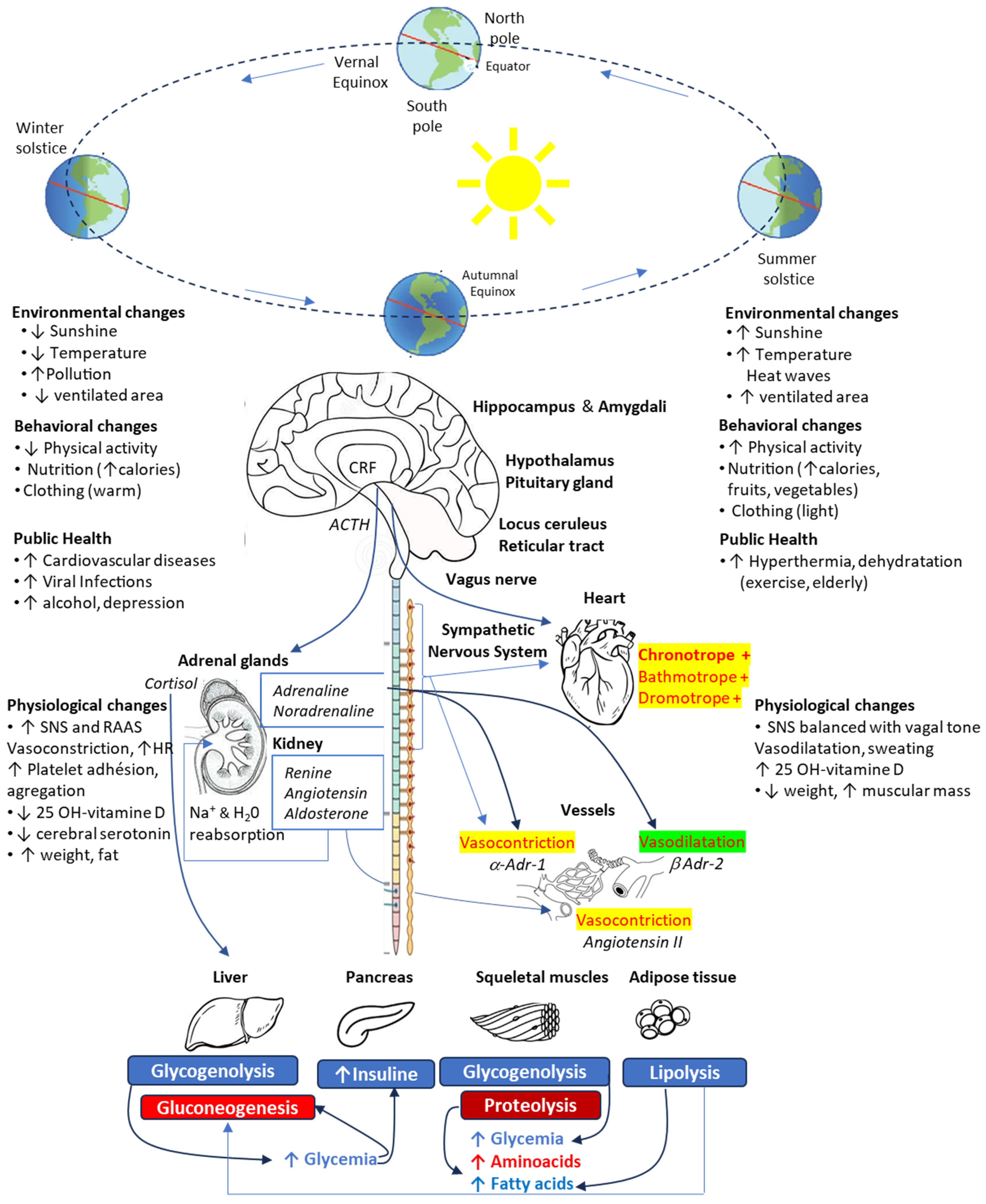

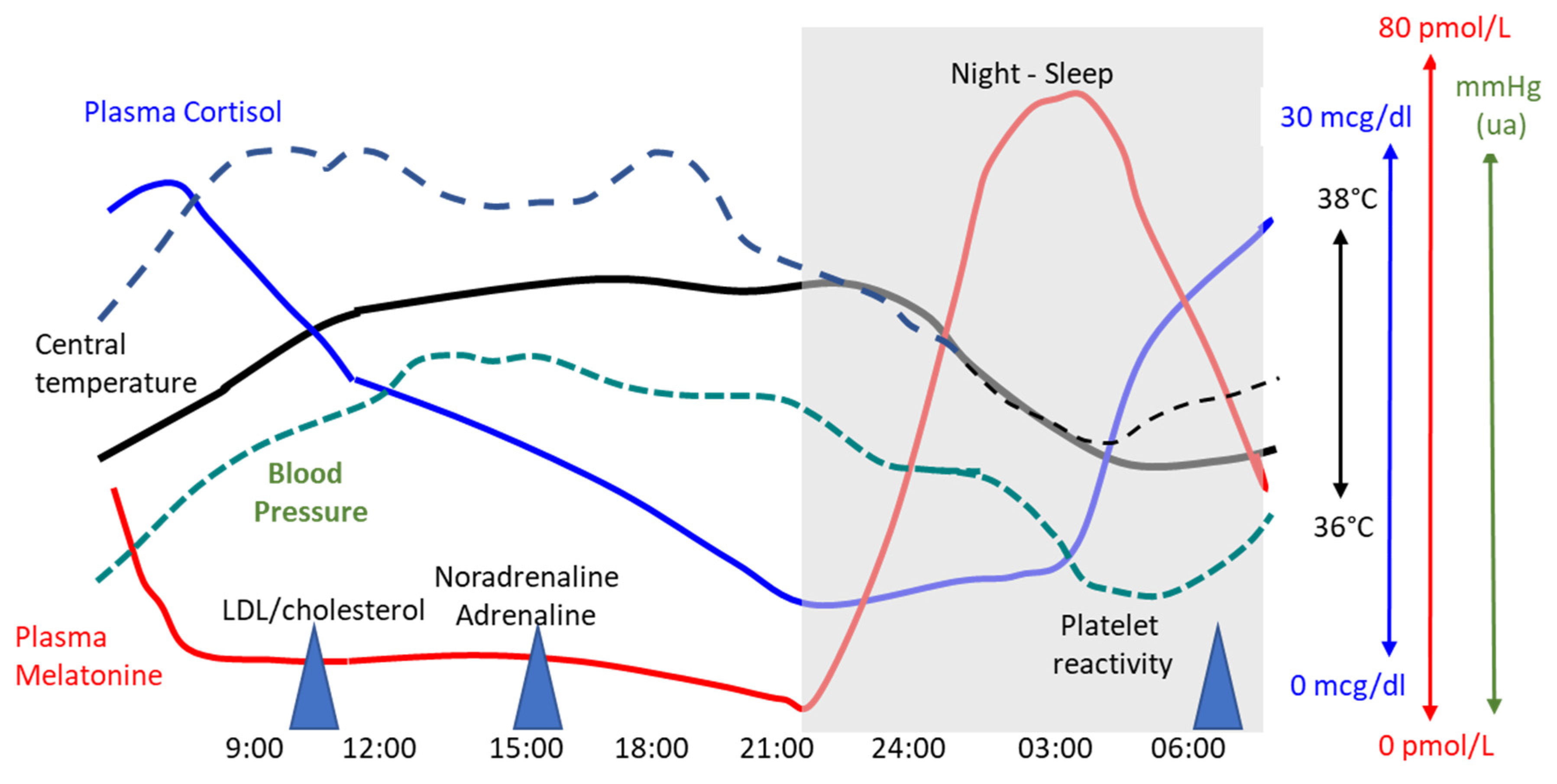
| Authors | Year-Country | N | Type of Surgery | Mortality | Postoperative Outcome Other Results |
|---|---|---|---|---|---|
| Shuhaider JH et al. [40] | 2008-UK | 16,290 Single center | isolated or combined CABGS | ↑ in-hospital mortality after isolated CABGS (OR 1.29, 95%CI 1.01–1.63) in winter | Prolonged ICU stay after isolated CABGS in winter |
| Mori et al. [39] | 2020-USA | 448,709 National database | CABGS, SAVR | Trend for increased risk of operative mortality during severe influenza epidemics (OR 1.03, 95%CI 0.96–1.10) | N.R. |
| Martin TJ et al. [38] | 2020-USA | 516,698 National database | CABGS | ↑ Risk of postoperative pneumonia (OR 1.15, 95%CI 1.07–3.12) and viral infection (OR 4.1, 95%CI 2.0–7.9) during winter | Preoperative vaccination against seasonal influenza, Hemophilus influenzae, and S. pneumoniae improve postoperative outcome |
| Luo Z-L et al. [41] | 2021-China | 404 Single center | Aortic dissection | ↑ In-hospital mortality in autumn (OR 4.0, 95%CI 1.0–17.3) and patients with CAD (OR 9.0, 95%CI 2.0–29.6) | Prolonged ICU stay in patients operated on during autumn (OR 6.0, 95% CI 2.7–7.9) |
| Petak F et al. [37] | 2022-Hungary | 9838 Single center | CABGS, SAVR | N.R. | ↑ younger patients with diabetes and smokers operated in winter No seasonality variation regarding type of surgery ↑ BP and ↑ plasma triglyceride levels in winter |
| Lin Q et al. [42] | 2023-China | 485 single center | Aortic dissection | Similar in-hospital mortality during winter (12.3%), spring (10.4%), summer (9.6%) and autumn (9.8%) | Prolonged hospital LOS during winter (median 20 days, [IQR] 2–31) vs. summer (17, IQR 4–24) |
| Swets MC et al. [43] | 2023-Netherlands | 42,277 National database | CABGS | ↑ in-hospital mortality (OR 1.67, 95% CI 1.14–2.46) during autumn and winter (influenza- like illness) | Worse outcome due to ILI epidemics, in October (vs. April) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Licker, M.; Ellenberger, C. Impact of the Circadian Rhythm and Seasonal Changes on the Outcome of Cardiovascular Interventions. J. Clin. Med. 2025, 14, 2570. https://doi.org/10.3390/jcm14082570
Licker M, Ellenberger C. Impact of the Circadian Rhythm and Seasonal Changes on the Outcome of Cardiovascular Interventions. Journal of Clinical Medicine. 2025; 14(8):2570. https://doi.org/10.3390/jcm14082570
Chicago/Turabian StyleLicker, Marc, and Christoph Ellenberger. 2025. "Impact of the Circadian Rhythm and Seasonal Changes on the Outcome of Cardiovascular Interventions" Journal of Clinical Medicine 14, no. 8: 2570. https://doi.org/10.3390/jcm14082570
APA StyleLicker, M., & Ellenberger, C. (2025). Impact of the Circadian Rhythm and Seasonal Changes on the Outcome of Cardiovascular Interventions. Journal of Clinical Medicine, 14(8), 2570. https://doi.org/10.3390/jcm14082570







