Ten-Year Observational Study of Patients with Lung Adenocarcinoma: Clinical Outcomes, Prognostic Factors, and Five-Year Survival Rates
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Statistical Analysis
3. Results
3.1. General Characteristics of the Study Group
3.2. History of Previous Neoplastic Diseases
3.3. Comorbidities Among Patients
3.4. Symptoms of Lung Adenocarcinoma
3.5. Tumor Characteristics in the Study Group
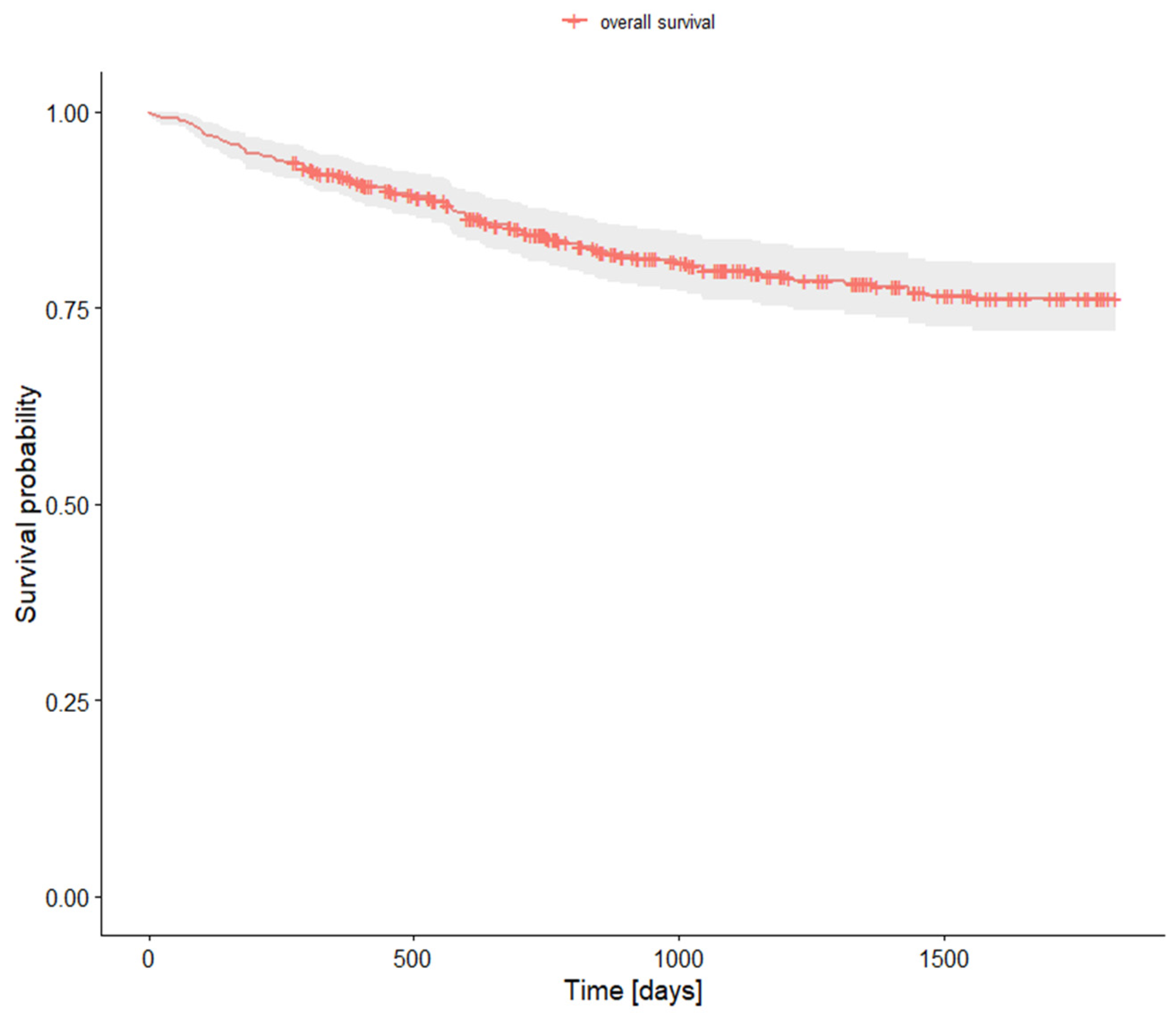
3.6. Survival Rates
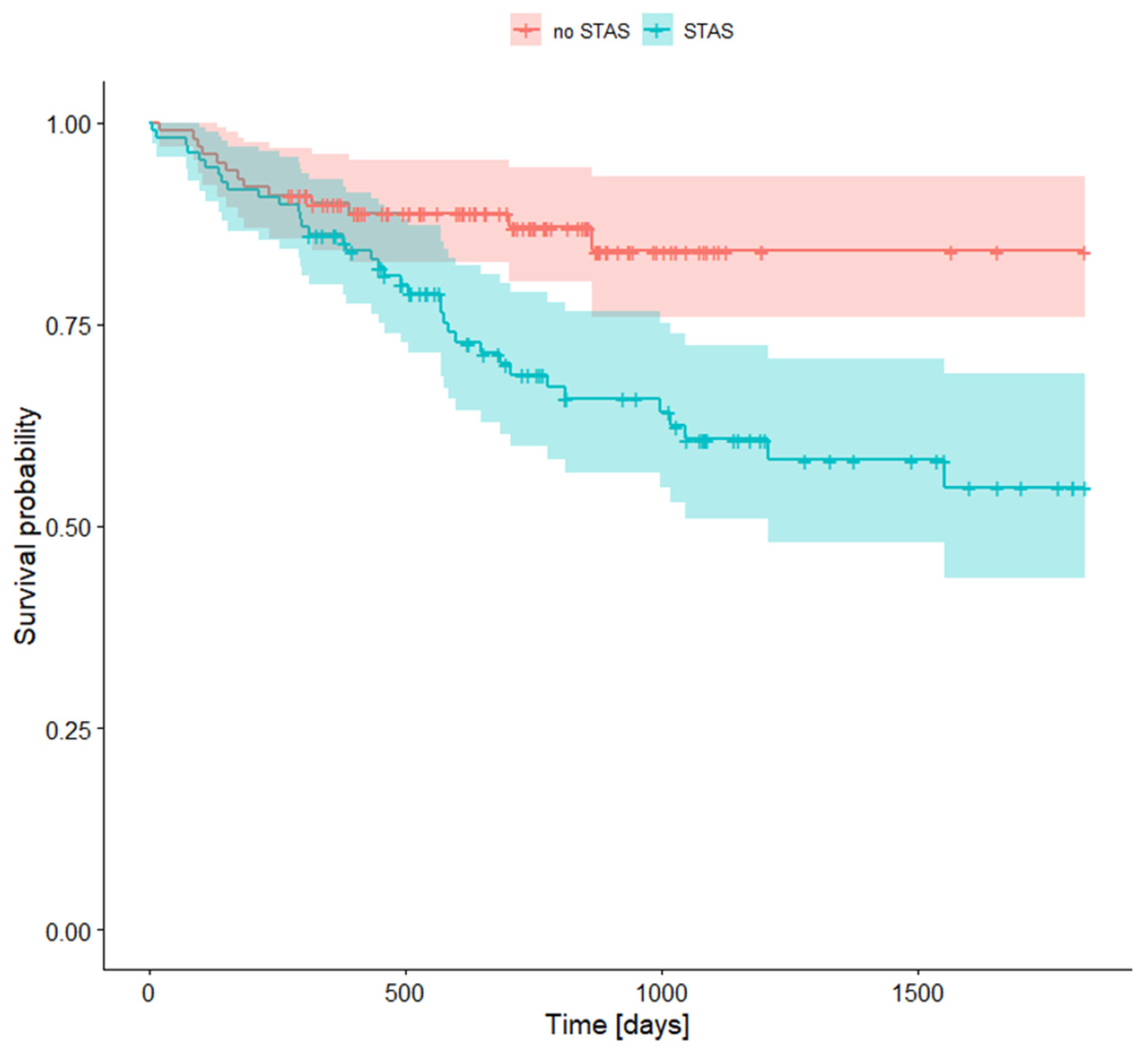
3.7. Survival Rates with STAS (Spread Through Air Spaces)
4. Discussion
4.1. Symptoms
4.2. Staging
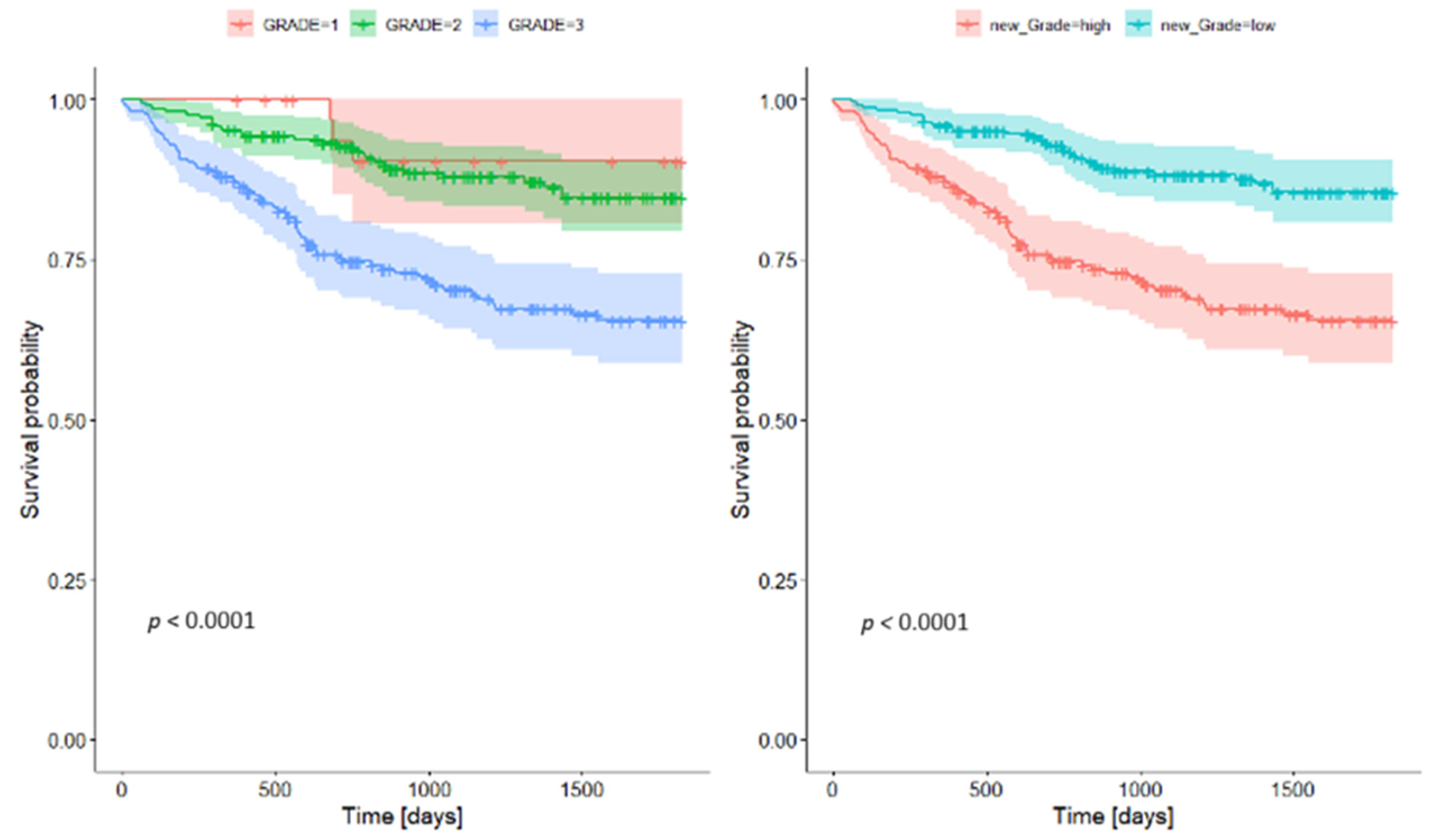
4.3. Grading
4.4. Vascular and Pleural Invasion
4.5. Spread Through Air Spaces (STAS)
4.6. Histological Type
4.7. Survival
4.8. Comorbidities
4.9. Study Limitations and Clinical Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pan, B.; Wan, L.; Li, Y.; Yang, J.; Chen, Z.; Tong, X.; Zhao, J.; Li, C. Comprehensive Pan-Cancer Analysis Identifies FHL2 Associated with Poor Prognosis in Lung Adenocarcinoma. Transl. Cancer Res. 2023, 12, 1516. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar]
- Min, S.; Zheng, Q. Clinicopathological and Prognostic Significance of NM23 Expression in Patients with Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis. Medicine 2021, 100, e27919. [Google Scholar] [CrossRef] [PubMed]
- Ran, R.; Jin, J.W.; Zhang, W.P. MALAT-1 Expression Correlates with Prognosis in Non-Small-Cell Lung Carcinoma: A Systematic Review and Meta-analysis. Dis. Markers 2021, 2021, 5424623. [Google Scholar] [PubMed]
- Fan, K.; Zhang, C.; Qi, Y.; Dai, X.; Birling, Y.; Tan, Z.; Cao, F. Prognostic Value of EZH2 in Non-Small-Cell Lung Cancers: A Meta-Analysis and Bioinformatics Analysis. BioMed Res. Int. 2020, 2020, 2380124. [Google Scholar] [CrossRef]
- Chen, J.; Liu, X.; Wu, Q.; Jiang, X.; Zeng, Z.; Li, J.; Gao, Y.; Gong, Y.; Xie, C. Systematic Analyses of a Chemokine Family-Based Risk Model Predicting Clinical Outcome and Immunotherapy Response in Lung Adenocarcinoma. Cell Transplant. 2021, 30, 09636897211055046. [Google Scholar]
- Abdeahad, H.; Salehi, M.; Yaghoubi, A.; Aalami, A.H.; Aalami, F.; Soleimanpour, S. Previous Pulmonary Tuberculosis Enhances the Risk of Lung Cancer: Systematic Reviews and Meta-Analysis. Infect. Dis. 2022, 54, 255–268. [Google Scholar] [CrossRef]
- Huang, J.; Yue, N.; Wu, J.; Shi, N.; Wang, Q.; Cui, T.; Zheng, M.; Sun, S.; Jin, H. Screening Rate and Influential Factors of Lung Cancer with Low-Dose Computed Tomography in Asian Population: A Systematic Review and Meta-Analysis. J. Public Health 2022, 44, 246–254. [Google Scholar]
- Lung Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/lung-cancer (accessed on 17 September 2024).
- Liu, X.; Huang, G.; Zhang, J.; Zhang, L.; Liang, Z. Prognostic and Clinicopathological Significance of Long Noncoding RNA MALAT-1 Expression in Patients with Non-Small Cell Lung Cancer: A Meta-Analysis. PLoS ONE 2020, 15, e0240321. [Google Scholar] [CrossRef]
- Ochman, B.; Kiczmer, P.; Ziora, P.; Rydel, M.; Borowiecki, M.; Czyżewski, D.; Drozdzowska, B. Incidence of Concomitant Neoplastic Diseases, Tumor Characteristics, and the Survival of Patients with Lung Adenocarcinoma or Squamous Cell Lung Carcinoma in Tobacco Smokers and Non-Smokers—10-Year Retrospective Single-Centre Cohort Study. Cancers 2023, 15, 1896. [Google Scholar] [CrossRef]
- Collins, L.G.; Haines, C.; Perkel, R.; Enck, R.E. Lung Cancer: Diagnosis and Management. Am. Fam. Physician 2007, 75, 56–63. [Google Scholar]
- Lung Cancer-ClinicalKey. Available online: https://www.clinicalkey.com/#!/content/playContent/1-s2.0-S0025712518301718?returnurl=https:%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS0025712518301718%3Fshowall%3Dtrue&referrer=https:%2F%2Fpubmed.ncbi.nlm.nih.gov%2F (accessed on 17 September 2024).
- Kocher, F.; Hilbe, W.; Seeber, A.; Pircher, A.; Schmid, T.; Greil, R.; Auberger, J.; Nevinny-Stickel, M.; Sterlacci, W.; Tzankov, A.; et al. Longitudinal Analysis of 2293 NSCLC Patients: A Comprehensive Study from the TYROL Registry. Lung Cancer 2015, 87, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Beckles, M.A.; Spiro, S.G.; Colice, G.L.; Rudd, R.M. Initial Evaluation of the Patient With Lung Cancer*: Symptoms, Signs, Laboratory Tests, and Paraneoplastic Syndromes. CHEST 2003, 123, 97S–104S. [Google Scholar] [CrossRef]
- Nasralla, A.; Lee, J.; Dang, J.; Turner, S. Elevated Preoperative CEA Is Associated with Subclinical Nodal Involvement and Worse Survival in Stage I Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis. J. Cardiothorac. Surg. 2020, 15, 1–8. [Google Scholar] [CrossRef]
- Fassi, E.; Mandruzzato, M.; Zamparini, M.; Bianchi, S.; Petrelli, F.; Baggi, A.; Alberti, A.; Grisanti, S.; Berruti, A. Clinical Presentation and Outcome of Patients with Enteric-Type Adenocarcinoma of the Lung: A Pooled Analysis of Published Cases. Lung Cancer 2023, 179, 107176. [Google Scholar] [CrossRef]
- WHO Classification of Tumours Editorial Board. Thoracic Tumours, 5th ed.; WHO Classification of Tumours Series, Vol. 5; International Agency for Research on Cancer: Lyon, France, 2021; ISBN 978-92-832-4506-3. [Google Scholar]
- Moreira, A.L.; Ocampo, P.S.S.; Xia, Y.; Zhong, H.; Russell, P.A.; Minami, Y.; Cooper, W.A.; Yoshida, A.; Bubendorf, L.; Papotti, M.; et al. A Grading System for Invasive Pulmonary Adenocarcinoma: A Proposal From the International Association for the Study of Lung Cancer Pathology Committee. J. Thorac. Oncol. 2020, 15, 1599–1610. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Zheng, B.; Zhao, T.; Fan, Y. Computed Tomography Features and Tumor Spread Through Air Spaces in Lung Adenocarcinoma: A Meta-Analysis. J. Thorac. Imaging 2023, 38, W19. [Google Scholar] [CrossRef] [PubMed]
- Seok, Y.; Jeong, J.Y.; Lee, E. Extent of Visceral Pleural Invasion and the Prognosis of Surgically Resected Node-negative Non-small Cell Lung Cancer. Thorac. Cancer 2017, 8, 197–202. [Google Scholar] [CrossRef]
- Ito, M.; Miyata, Y.; Yoshiya, T.; Tsutani, Y.; Mimura, T.; Murakami, S.; Ito, H.; Nakayama, H.; Okada, M. Second Predominant Subtype Predicts Outcomes of Intermediate-Malignant Invasive Lung Adenocarcinoma. Eur. J. Cardio-Thorac. Surg. 2017, 51, 218–222. [Google Scholar] [CrossRef]
- Usui, S.; Minami, Y.; Shiozawa, T.; Iyama, S.; Satomi, K.; Sakashita, S.; Sato, Y.; Noguchi, M. Differences in the Prognostic Implications of Vascular Invasion between Lung Adenocarcinoma and Squamous Cell Carcinoma. Lung Cancer 2013, 82, 407–412. [Google Scholar] [CrossRef]
- Yambayev, I.; Sullivan, T.B.; Rieger-Christ, K.M.; Servais, E.L.; Stock, C.T.; Quadri, S.M.; Sands, J.M.; Suzuki, K.; Burks, E.J. Vascular Invasion Identifies the Most Aggressive Histologic Subset of Stage I Lung Adenocarcinoma: Implications for Adjuvant Therapy. Lung Cancer 2022, 171, 82–89. [Google Scholar] [CrossRef]
- Poleri, C.; Morero, J.L.; Nieva, B.; Vaézquez, M.F.; Rodriéguez, C.; de Titto, E.; Rosenberg, M. Risk of Recurrence in Patients With Surgically Resected Stage I Non-Small Cell Lung Carcinomaa: Histopathologic and Immunohistochemical Analysis. CHEST 2003, 123, 1858–1867. [Google Scholar] [CrossRef] [PubMed]
- Travis, W.D.; Brambilla, E.; Nicholson, A.G.; Yatabe, Y.; Austin, J.H.M.; Beasley, M.B.; Chirieac, L.R.; Dacic, S.; Duhig, E.; Flieder, D.B.; et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J. Thorac. Oncol. 2015, 10, 1243–1260. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Tang, L.; Dai, L.; Shi, Y. The Prognostic Significance of Tumor Spread through Air Space in Stage I Lung Adenocarcinoma. J. Cardiothorac. Surg. 2020, 15, 1–8. [Google Scholar]
- Yin, Q.; Wang, H.; Cui, H.; Wang, W.; Yang, G.; Qie, P.; Xun, X.; Han, S.; Liu, H. Meta-Analysis of Association between CT-Based Features and Tumor Spread through Air Spaces in Lung Adenocarcinoma. J. Cardiothorac. Surg. 2020, 15, 243. [Google Scholar] [CrossRef]
- Kim, M.; Chung, Y.S.; Kim, K.A.; Shim, H.S. Prognostic Factors of Acinar- or Papillary-Predominant Adenocarcinoma of the Lung. Lung Cancer 2019, 137, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Hung, J.-J.; Yeh, Y.-C.; Jeng, W.-J.; Wu, K.-J.; Huang, B.-S.; Wu, Y.-C.; Chou, T.-Y.; Hsu, W.-H. Predictive Value of the International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society Classification of Lung Adenocarcinoma in Tumor Recurrence and Patient Survival. JCO 2014, 32, 2357–2364. [Google Scholar] [CrossRef]
- Fujikawa, R.; Muraoka, Y.; Kashima, J.; Yoshida, Y.; Ito, K.; Watanabe, H.; Kusumoto, M.; Watanabe, S.; Yatabe, Y. Clinicopathologic and Genotypic Features of Lung Adenocarcinoma Characterized by the International Association for the Study of Lung Cancer Grading System. J. Thorac. Oncol. 2022, 17, 700–707. [Google Scholar] [CrossRef]
- Bertoglio, P.; Querzoli, G.; Ventura, L.; Aprile, V.; Cattoni, M.A.; Nachira, D.; Lococo, F.; Perez, M.R.; Guerrera, F.; Minervini, F.; et al. Prognostic Impact of Lung Adenocarcinoma Second Predominant Pattern from a Large European Database. J. Surg. Oncol. 2020, 123, 560–569. [Google Scholar]
- Ou, S.I.; Zell, J.A.; Ziogas, A.; Anton-Culver, H. Prognostic Factors for Survival of Stage I Nonsmall Cell Lung Cancer Patients. Cancer 2007, 110, 1532–1541. [Google Scholar] [CrossRef]
- Yang, Y.; Xie, X.; Wang, Y.; Li, X.; Luo, L.; Yao, Y.; Li, J. A Systematic Review and Meta-Analysis of the Influence of STAS on the Long-Term Prognosis of Stage I Lung Adenocarcinoma. Transl. Cancer Res. 2021, 10, 2428. [Google Scholar] [PubMed]
- Garinet, S.; Wang, P.; Mansuet-Lupo, A.; Fournel, L.; Wislez, M.; Blons, H. Updated Prognostic Factors in Localized NSCLC. Cancers 2022, 14, 1400. [Google Scholar] [CrossRef] [PubMed]
- Urer, H.N.; Kocaturk, C.I.; Gunluoglu, M.Z.; Arda, N.; Bedirhan, M.A.; Fener, N.; Dincer, S.I. Relationship between Lung Adenocarcinoma Histological Subtype and Patient Prognosis. Ann. Thorac. Cardiovasc. Surg. 2014, 20, 12–18. [Google Scholar]
- Dutkowska, A.E.; Antczak, A. Comorbidities in Lung Cancer. Adv. Respir. Med. 2016, 84, 186–192. [Google Scholar] [CrossRef]
- Oliveros, E.; Patel, H.; Kyung, S.; Fugar, S.; Goldberg, A.; Madan, N.; Williams, K.A. Hypertension in Older Adults: Assessment, Management, and Challenges. Clin. Cardiol. 2019, 43, 99–107. [Google Scholar] [PubMed]
- Tammemagi, C.M.; Neslund-Dudas, C.; Simoff, M.; Kvale, P. Smoking and Lung Cancer Survival: The Role of Comorbidity and Treatment. CHEST 2004, 125, 27–37. [Google Scholar] [CrossRef]
- Tammemagi, C.M.; Neslund-Dudas, C.; Simoff, M.; Kvale, P. In Lung Cancer Patients, Age, Race-Ethnicity, Gender and Smoking Predict Adverse Comorbidity, Which in Turn Predicts Treatment and Survival. J. Clin. Epidemiol. 2004, 57, 597–609. [Google Scholar] [CrossRef]
- Durham, A.L.; Adcock, I.M. The Relationship between COPD and Lung Cancer. Lung Cancer 2015, 90, 121–127. [Google Scholar] [CrossRef]
- Islam, K.M.M.; Jiang, X.; Anggondowati, T.; Lin, G.; Ganti, A.K. Comorbidity and Survival in Lung Cancer Patients. Cancer Epidemiol. Biomark. Prev. 2015, 24, 1079–1085. [Google Scholar] [CrossRef]
- Aguiló, R.; Macià, F.; Porta, M.; Casamitjana, M.; Minguella, J.; Novoa, A.M. Multiple Independent Primary Cancers Do Not Adversely Affect Survival of the Lung Cancer Patient. Eur. J. Cardio-Thorac. Surg. 2008, 34, 1075–1080. [Google Scholar] [CrossRef]
| Mean | SD | 95% CI | 95% CI | |
|---|---|---|---|---|
| Age | 65.9 | 7.81 | 65.19 | 66.61 |
| Gender | n | % | 95% CI | 95% CI |
| Female | 252.00 | 53.50% | 49.00% | 58.01% |
| Male | 219.00 | 46.50% | 41.99% | 51.00% |
| Smoking Status | n | % | 95% CI | 95% CI |
| Smoker | 356 | 75.58% | 71.70% | 79.46% |
| Non-smoker | 115 | 24.42% | 20.54% | 28.30% |
| Former Neoplastic Diseases | n | % | 95% CI | 95% CI |
|---|---|---|---|---|
| Any former neoplastic diseases | 75 | 15.89% | 12.59% | 19.19% |
| Breast | 16 | 3.39% | 1.76% | 5.02% |
| Prostate | 12 | 2.54% | 1.12% | 3.96% |
| Urinary bladder | 10 | 2.12% | 0.82% | 3.42% |
| Uterus | 7 | 1.48% | 0.39% | 2.57% |
| Lung | 7 | 1.48% | 0.39% | 2.57% |
| Large intestine | 5 | 1.06% | 0.13% | 1.98% |
| Kidney | 4 | 0.85% | 0.02% | 1.68% |
| Larynx | 2 | 0.42% | 0.00% | 1.01% |
| Ovary | 2 | 0.42% | 0.00% | 1.01% |
| Stomach | 2 | 0.42% | 0.00% | 1.01% |
| Other | 12 | 2.54% | 1.12% | 3.96% |
| Neoplasms in family | 102 | 21.61% | 17.89% | 25.33% |
| Comorbidities | n | % | 95% CI | 95% CI |
|---|---|---|---|---|
| Insulin-dependent diabetes mellitus, IDDM | 4.00 | 0.85% | 0.02% | 1.68% |
| Non-insulin-dependent diabetes mellitus, NIDDM | 80.00 | 16.99% | 13.59% | 20.38% |
| Myocardial infarction in the past | 37.00 | 7.86% | 5.43% | 10.29% |
| Heart failure | 7.00 | 1.49% | 0.39% | 2.58% |
| Renal failure | 3.00 | 0.64% | 0.00% | 1.36% |
| COPD | 73.00 | 15.50% | 12.23% | 18.77% |
| Bronchial asthma | 22.00 | 4.67% | 2.77% | 6.58% |
| Epilepsy | 0.00 | 0.00% | 0.00% | 0.00% |
| Stroke in the past | 1.00 | 0.21% | 0.00% | 0.63% |
| Hypertension | 259.00 | 54.99% | 50.50% | 59.48% |
| Coronary disease | 87.00 | 18.47% | 14.97% | 21.98% |
| Blood coagulation disorders | 1.00 | 0.21% | −0.20% | 0.63% |
| Chronic venous disease | 7.00 | 1.49% | 0.39% | 2.58% |
| Cancer Symptoms | n | % | 95% CI | 95% CI |
|---|---|---|---|---|
| Any cancer symptoms | 284.00 | 60.30% | 55.88% | 64.72% |
| Cough | 133 | 28.24% | 24.17% | 32.30% |
| Pain | 27.00 | 5.73% | 3.63% | 7.83% |
| Hemoptysis | 21.00 | 4.46% | 2.59% | 6.32% |
| Dyspnea | 5.00 | 1.06% | 0.14% | 1.99% |
| n | % | 95% CI | 95% CI | |
|---|---|---|---|---|
| pT Parameter | ||||
| pT1a | 17 | 3.61% | 1.92% | 5.29% |
| pT1b | 107 | 22.72% | 18.93% | 26.50% |
| pT1c | 105 | 22.29% | 18.53% | 26.05% |
| pT2a | 118 | 25.05% | 21.14% | 28.97% |
| pT2b | 45 | 9.55% | 6.90% | 12.21% |
| pT3 | 51 | 10.83% | 8.02% | 13.63% |
| pT4 | 28 | 5.94% | 3.81% | 8.08% |
| pN Parameter | ||||
| pN0 | 399 | 84.71% | 81.46% | 87.96% |
| pN1 | 41 | 8.70% | 6.16% | 11.25% |
| pN2 | 31 | 6.58% | 4.34% | 8.82% |
| pM Parameter | ||||
| pM0 | 471 | 100.00% | 100.00% | 100.00% |
| Stage | ||||
| IA1 | 17 | 3.61% | 1.92% | 5.29% |
| IA2 | 97 | 20.59% | 16.94% | 24.25% |
| IA3 | 90 | 19.11% | 15.56% | 22.66% |
| IB | 101 | 21.44% | 17.74% | 25.15% |
| IIA | 38 | 8.07% | 5.61% | 10.53% |
| IIB | 73 | 15.50% | 12.23% | 18.77% |
| IIIA | 38 | 8.07% | 5.61% | 10.53% |
| IIIB | 16 | 3.40% | 1.76% | 5.03% |
| IVA | 1 | 0.21% | 0.00% | 0.63% |
| Grading | ||||
| G1 | 35 | 7.43% | 5.06% | 9.80% |
| G2 | 211 | 44.80% | 40.31% | 49.29% |
| G3 | 225 | 47.77% | 43.26% | 52.28% |
| Vascular invasion | ||||
| Lymphatic invasion | 88 | 18.68% | 15.16% | 22.20% |
| Vascular invasion | 71 | 15.07% | 11.84% | 18.31% |
| Pleural invasion | ||||
| 0 | 330 | 70.06% | 65.93% | 74.20% |
| 1 | 93 | 19.75% | 16.15% | 23.34% |
| 2 | 37 | 7.86% | 5.43% | 10.29% |
| 3 | 8 | 1.70% | 0.53% | 2.87% |
| Radical resection | ||||
| R0 | 439 | 93.21% | 90.93% | 95.48% |
| R1 | 18 | 3.82% | 2.09% | 5.55% |
| Spread through air spaces | ||||
| STAS | 108 | 22.93% | 19.13% | 26.73% |
| Histologic pattern | ||||
| n | % | 95% CI | 95% CI | |
| Lepidic | 126 | 28% | 23.85% | 32.15% |
| Acinar | 348 | 77.33% | 73.46% | 81.20% |
| Papillary | 81 | 18% | 14.45% | 21.55% |
| Micropapillary | 108 | 24% | 20.05% | 27.95% |
| Solid | 208 | 46.22% | 41.61% | 50.83% |
| Research Population | |||
|---|---|---|---|
| Survival Rate | 95% CI | ||
| 1-year survival rate | 0.919000 | 0.895 | 0.944 |
| 2-year survival rate | 0.843277 | 0.81 | 0.878 |
| 5-year survival rate | 0.762390 | 0.721 | 0.806 |
| Low Grade | High Grade | |||||
|---|---|---|---|---|---|---|
| Survival Rate | 95% CI | Survival Rate | 95% CI | |||
| 1-year survival rate | 0.959 | 0.935 | 0.984 | 0.875 | 0.833 | 0.919 |
| 2-year survival rate | 0.928 | 0.896 | 0.962 | 0.747 | 0.69 | 0.809 |
| 5-year survival rate | 0.865 | 0.808 | 0.906 | 0.655 | 0.588 | 0.729 |
| STAS + | STAS − | |||||
|---|---|---|---|---|---|---|
| Survival Rate | 95% CI | Survival Rate | 95% CI | |||
| 1-year survival rate | 0.861 | 0.798 | 0.929 | 0.899 | 0.842 | 0.961 |
| 2-year survival rate | 0.688 | 0.599 | 0.790 | 0.870 | 0.803 | 0.943 |
| 5-year survival rate | 0.548 | 0.436 | 0.688 | 0.841 | 0.758 | 0.934 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziora, P.; Skiba, H.; Kiczmer, P.; Zaboklicka, N.; Wypyszyńska, J.; Stachura, M.; Sito, Z.; Rydel, M.; Czyżewski, D.; Drozdzowska, B. Ten-Year Observational Study of Patients with Lung Adenocarcinoma: Clinical Outcomes, Prognostic Factors, and Five-Year Survival Rates. J. Clin. Med. 2025, 14, 2552. https://doi.org/10.3390/jcm14082552
Ziora P, Skiba H, Kiczmer P, Zaboklicka N, Wypyszyńska J, Stachura M, Sito Z, Rydel M, Czyżewski D, Drozdzowska B. Ten-Year Observational Study of Patients with Lung Adenocarcinoma: Clinical Outcomes, Prognostic Factors, and Five-Year Survival Rates. Journal of Clinical Medicine. 2025; 14(8):2552. https://doi.org/10.3390/jcm14082552
Chicago/Turabian StyleZiora, Paweł, Hanna Skiba, Paweł Kiczmer, Natalia Zaboklicka, Julia Wypyszyńska, Maria Stachura, Zuzanna Sito, Mateusz Rydel, Damian Czyżewski, and Bogna Drozdzowska. 2025. "Ten-Year Observational Study of Patients with Lung Adenocarcinoma: Clinical Outcomes, Prognostic Factors, and Five-Year Survival Rates" Journal of Clinical Medicine 14, no. 8: 2552. https://doi.org/10.3390/jcm14082552
APA StyleZiora, P., Skiba, H., Kiczmer, P., Zaboklicka, N., Wypyszyńska, J., Stachura, M., Sito, Z., Rydel, M., Czyżewski, D., & Drozdzowska, B. (2025). Ten-Year Observational Study of Patients with Lung Adenocarcinoma: Clinical Outcomes, Prognostic Factors, and Five-Year Survival Rates. Journal of Clinical Medicine, 14(8), 2552. https://doi.org/10.3390/jcm14082552






