Neutrophil-to-Lymphocyte Ratio as a Prognostic Biomarker for Long-Term Survival in Older Adults at a Mental Health Care Center: A Historical Cohort Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Statistical Methods
3. Results
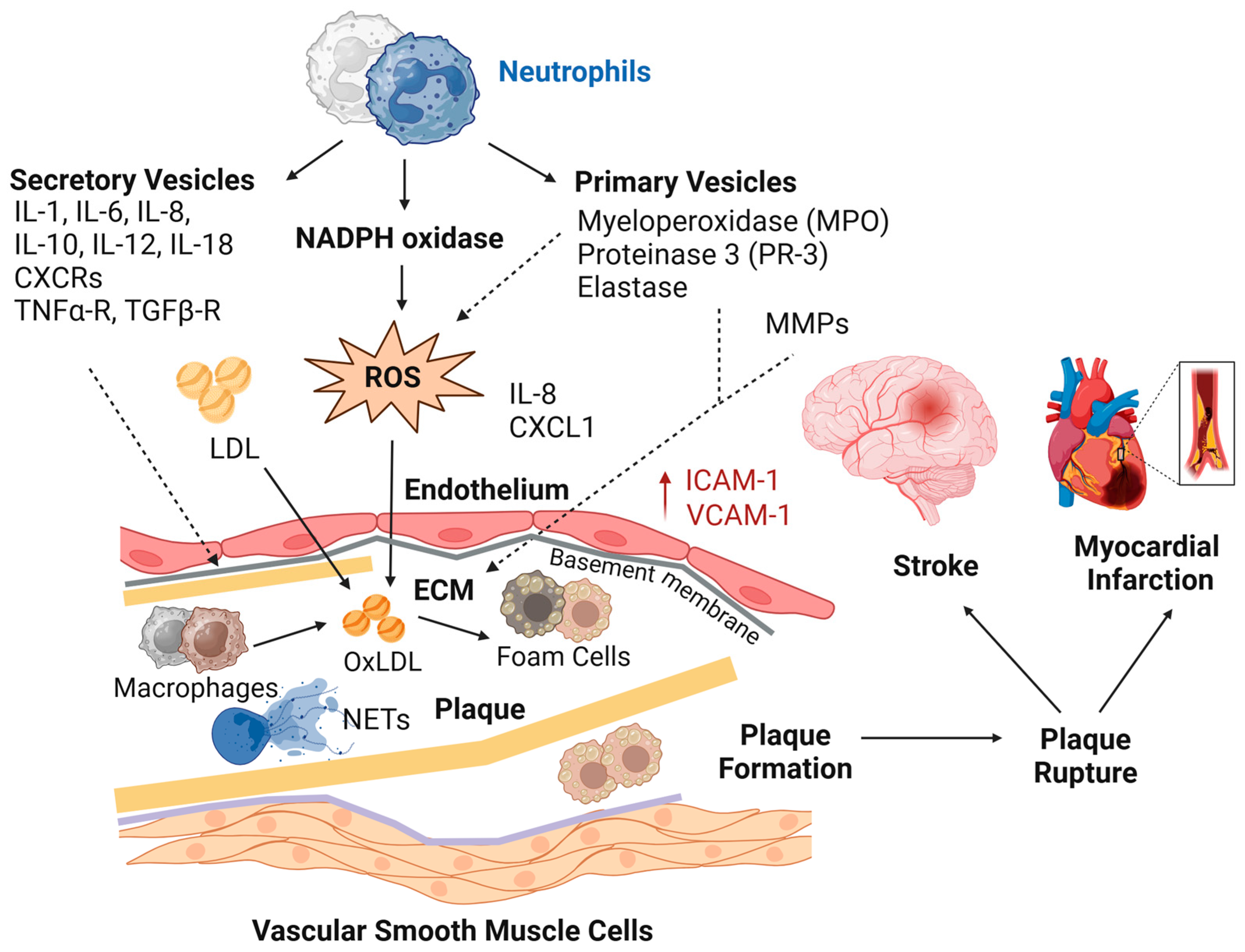
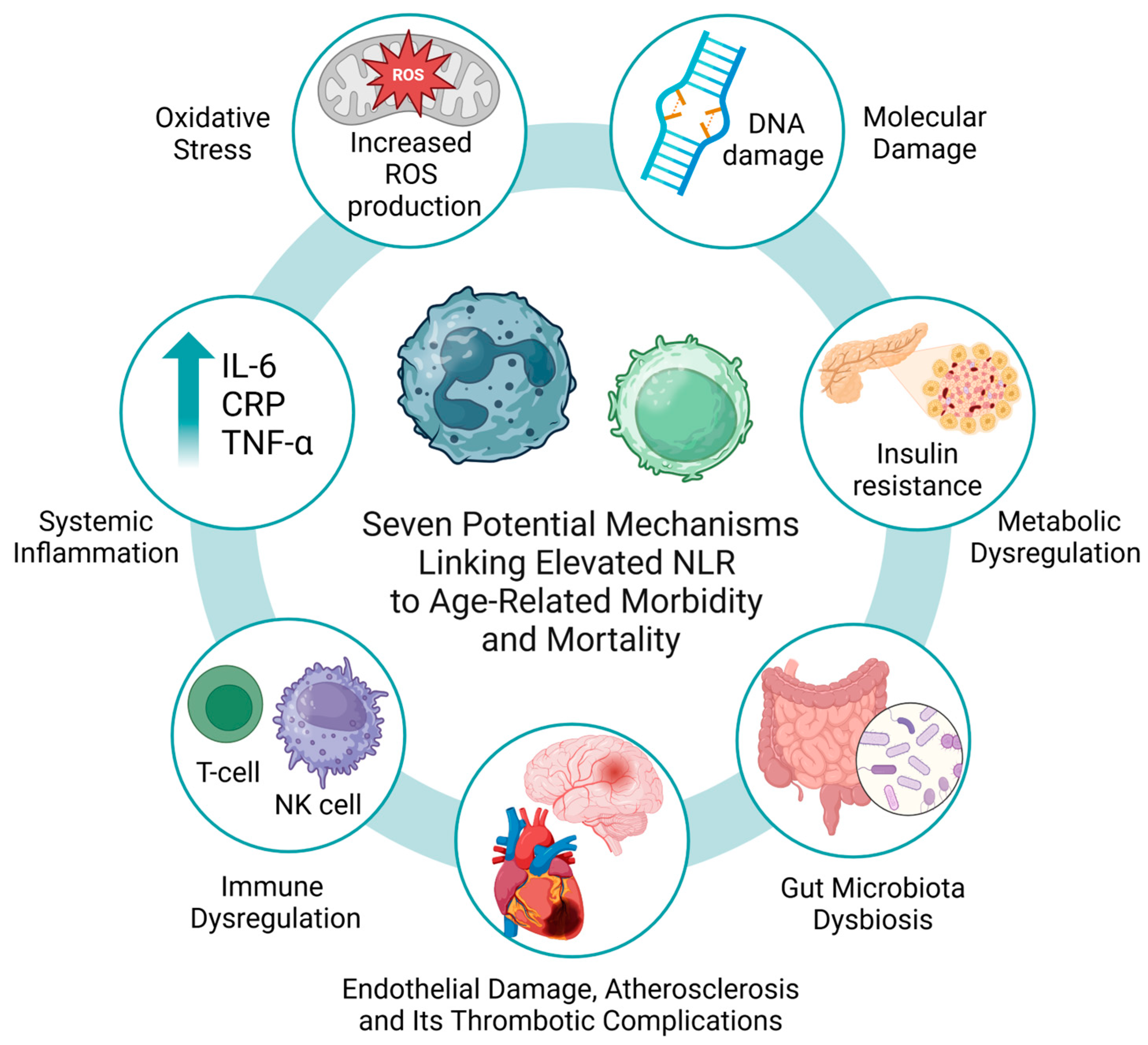
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chmielewski, P.P.; Data, K.; Strzelec, B.; Farzaneh, M.; Anbiyaiee, A.; Zaheer, U.; Uddin, S.; Sheykhi-Sabzehpoush, M.; Mozdziak, P.; Zabel, M.; et al. Human Aging and Age-Related Diseases: From Underlying Mechanisms to Pro-Longevity Interventions. Aging Dis. 2024, 16, 2. [Google Scholar] [CrossRef]
- Fülöp, T.; Dupuis, G.; Witkowski, J.M.; Larbi, A. The Role of Immunosenescence in the Development of Age-Related Diseases. Rev. Investig. Clin. 2016, 68, 84–91. [Google Scholar] [PubMed]
- Santoro, A.; Bientinesi, E.; Monti, D. Immunosenescence and inflammaging in the aging process: Age-related diseases or longevity? Ageing Res. Rev. 2021, 71, 101422. [Google Scholar] [PubMed]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar]
- Ley, K.; Hoffman, H.M.; Kubes, P.; Cassatella, M.A.; Zychlinsky, A.; Hedrick, C.C.; Catz, S.D. Neutrophils: New insights and open questions. Sci. Immunol. 2018, 3, eaat4579. [Google Scholar]
- Franceschi, C.; Campisi, J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69 (Suppl. S1), S4–S9. [Google Scholar] [CrossRef]
- Buonacera, A.; Stancanelli, B.; Colaci, M.; Malatino, L. Neutrophil to Lymphocyte Ratio: An Emerging Marker of the Relationships between the Immune System and Diseases. Int. J. Mol. Sci. 2022, 23, 3636. [Google Scholar] [CrossRef]
- Yoshida, Y.; Iwasa, H.; Kim, H.; Suzuki, T. Association between Neutrophil-to-Lymphocyte Ratio and Physical Function in Older Adults: A Community-Based Cross-Sectional Study in Japan. Int. J. Environ. Res. Public Health 2022, 19, 8996. [Google Scholar] [CrossRef]
- van der Willik, K.D.; Fani, L.; Rizopoulos, D.; Licher, S.; Fest, J.; Schagen, S.B.; Ikram, M.K.; Ikram, M.A. Balance between innate versus adaptive immune system and the risk of dementia: A population-based cohort study. J. Neuroinflammation 2019, 16, 68. [Google Scholar]
- Azab, B.; Zaher, M.; Weiserbs, K.F.; Torbey, E.; Lacossiere, K.; Gaddam, S.; Gobunsuy, R.; Jadonath, S.; Baldari, D.; McCord, D.; et al. Usefulness of neutrophil to lymphocyte ratio in predicting short- and long-term mortality after non-ST-elevation myocardial infarction. Am. J. Cardiol. 2010, 106, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Bhat, T.; Teli, S.; Rijal, J.; Bhat, H.; Raza, M.; Khoueiry, G.; Meghani, M.; Akhtar, M.; Costantino, T. Neutrophil to lymphocyte ratio and cardiovascular diseases: A review. Expert Rev. Cardiovasc. Ther. 2013, 11, 55–59. [Google Scholar]
- Li, W.; Hou, M.; Ding, Z.; Liu, X.; Shao, Y.; Li, X. Prognostic Value of Neutrophil-to-Lymphocyte Ratio in Stroke: A Systematic Review and Meta-Analysis. Front. Neurol. 2021, 12, 686983. [Google Scholar]
- Ethier, J.L.; Desautels, D.; Templeton, A.; Shah, P.S.; Amir, E. Prognostic role of neutrophil-to-lymphocyte ratio in breast cancer: A systematic review and meta-analysis. Breast Cancer Res. 2017, 19, 2. [Google Scholar] [CrossRef] [PubMed]
- Malietzis, G.; Giacometti, M.; Kennedy, R.H.; Athanasiou, T.; Aziz, O.; Jenkins, J.T. The emerging role of neutrophil to lymphocyte ratio in determining colorectal cancer treatment outcomes: A systematic review and meta-analysis. Ann. Surg. Oncol. 2014, 21, 3938–3946. [Google Scholar] [PubMed]
- Romano, F.J.; Ronga, R.; Ambrosio, F.; Arundine, D.; Longo, V.; Galetta, D.; Gridelli, C.; Maione, P.; Palma, V.; Damiano, V.; et al. Neutrophil-to-Lymphocyte Ratio Is a Major Prognostic Factor in Non-small Cell Lung Carcinoma Patients Undergoing First Line Immunotherapy with Pembrolizumab. Cancer Diagn. Progn. 2023, 3, 44–52. [Google Scholar]
- Iwai, N.; Okuda, T.; Sakagami, J.; Harada, T.; Ohara, T.; Taniguchi, M.; Sakai, H.; Oka, K.; Hara, T.; Tsuji, T.; et al. Neutrophil to lymphocyte ratio predicts prognosis in unresectable pancreatic cancer. Sci. Rep. 2020, 10, 18758. [Google Scholar]
- Pirozzolo, G.; Gisbertz, S.S.; Castoro, C.; van Berge Henegouwen, M.I.; Scarpa, M. Neutrophil-to-lymphocyte ratio as prognostic marker in esophageal cancer: A systematic review and meta-analysis. J. Thorac. Dis. 2019, 11, 3136–3145. [Google Scholar]
- Vartolomei, M.D.; Porav-Hodade, D.; Ferro, M.; Mathieu, R.; Abufaraj, M.; Foerster, B.; Kimura, S.; Shariat, S.F. Prognostic role of pretreatment neutrophil-to-lymphocyte ratio (NLR) in patients with non-muscle-invasive bladder cancer (NMIBC): A systematic review and meta-analysis. Urol. Oncol. 2018, 36, 389–399. [Google Scholar]
- Kawahara, T.; Fukui, S.; Sakamaki, K.; Ito, Y.; Ito, H.; Kobayashi, N.; Izumi, K.; Yokomizo, Y.; Miyoshi, Y.; Makiyama, K.; et al. Neutrophil-to-lymphocyte ratio predicts prostatic carcinoma in men undergoing needle biopsy. Oncotarget 2015, 6, 32169–32176. [Google Scholar]
- Lee, P.Y.; Oen, K.Q.X.; Lim, G.R.S.; Hartono, J.L.; Muthiah, M.; Huang, D.Q.; Teo, F.S.W.; Li, A.Y.; Mak, A.; Chandran, N.S.; et al. Neutrophil-to-Lymphocyte Ratio Predicts Development of Immune-Related Adverse Events and Outcomes from Immune Checkpoint Blockade: A Case-Control Study. Cancers 2021, 13, 1308. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Chen, X.; Gao, P.; Song, Y.; Huang, X.; Yang, Y.; Zhao, J.; Ma, B.; Gao, X.; Wang, Z. Can the Neutrophil to Lymphocyte Ratio Be Used to Determine Gastric Cancer Treatment Outcomes? A Systematic Review and Meta-Analysis. Dis. Markers 2016, 2016, 7862469. [Google Scholar] [CrossRef] [PubMed]
- Adamstein, N.H.; MacFadyen, J.G.; Rose, L.M.; Glynn, R.J.; Dey, A.K.; Libby, P.; Tabas, I.A.; Mehta, N.N.; Ridker, P.M. The neutrophil-lymphocyte ratio and incident atherosclerotic events: Analyses from five contemporary randomized trials. Eur. Heart J. 2021, 42, 896–903. [Google Scholar] [CrossRef]
- Dilektasli, E.; Inaba, K.; Haltmeier, T.; Wong, M.D.; Clark, D.; Benjamin, E.R.; Lam, L.; Demetriades, D. The prognostic value of neutrophil-to-lymphocyte ratio on mortality in critically ill trauma patients. J. Trauma Acute Care Surg. 2016, 81, 882–888. [Google Scholar] [CrossRef]
- Jiang, J.; Liu, R.; Yu, X.; Yang, R.; Xu, H.; Mao, Z.; Wang, Y. The neutrophil-lymphocyte count ratio as a diagnostic marker for bacteraemia: A systematic review and meta-analysis. Am. J. Emerg. Med. 2019, 37, 1482–1489. [Google Scholar] [CrossRef] [PubMed]
- Drăgoescu, A.N.; Pădureanu, V.; Stănculescu, A.D.; Chiuțu, L.C.; Tomescu, P.; Geormăneanu, C.; Pădureanu, R.; Iovănescu, V.F.; Ungureanu, B.S.; Pănuș, A.; et al. Neutrophil to Lymphocyte Ratio (NLR)-A Useful Tool for the Prognosis of Sepsis in the ICU. Biomedicines 2021, 10, 75. [Google Scholar] [CrossRef]
- Han, C.; Zeng, J.; Lin, R.; Liu, J.; Qian, W.; Ding, Z.; Hou, X. The utility of neutrophil to lymphocyte ratio and fluid sequestration as an early predictor of severe acute pancreatitis. Sci. Rep. 2017, 7, 10704. [Google Scholar] [CrossRef]
- Jeon, T.J.; Park, J.Y. Clinical significance of the neutrophil-lymphocyte ratio as an early predictive marker for adverse outcomes in patients with acute pancreatitis. World J. Gastroenterol. 2017, 23, 3883–3889. [Google Scholar] [CrossRef]
- Hajibandeh, S.; Hajibandeh, S.; Hobbs, N.; Mansour, M. Neutrophil-to-lymphocyte ratio predicts acute appendicitis and distinguishes between complicated and uncomplicated appendicitis: A systematic review and meta-analysis. Am. J. Surg. 2020, 219, 154–163. [Google Scholar] [CrossRef]
- Proctor, M.J.; McMillan, D.C.; Horgan, P.G.; Fletcher, C.D.; Talwar, D.; Morrison, D.S. Systemic inflammation predicts all-cause mortality: A Glasgow inflammation outcome study. PLoS ONE 2015, 10, e0116206. [Google Scholar] [CrossRef]
- Davis, J.L.; Moutinho, V., Jr.; Panageas, K.S.; Coit, D.G. A peripheral blood biomarker estimates probability of survival: The neutrophil-lymphocyte ratio in noncancer patients. Biomark. Med. 2016, 10, 953–957. [Google Scholar] [PubMed]
- Rembach, A.; Watt, A.D.; Wilson, W.J.; Rainey-Smith, S.; Ellis, K.A.; Rowe, C.C.; Villemagne, V.L.; Macaulay, S.L.; Bush, A.I.; Martins, R.N.; et al. An increased neutrophil-lymphocyte ratio in Alzheimer’s disease is a function of age and is weakly correlated with neocortical amyloid accumulation. J. Neuroimmunol. 2014, 273, 65–71. [Google Scholar] [CrossRef]
- Kinney, J.W.; Bemiller, S.M.; Murtishaw, A.S.; Leisgang, A.M.; Salazar, A.M.; Lamb, B.T. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2018, 4, 575–590. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Graubard, B.I.; Rabkin, C.S.; Engels, E.A. Neutrophil-to-lymphocyte ratio and mortality in the United States general population. Sci. Rep. 2021, 11, 464. [Google Scholar]
- Lee, J.S.; Kim, N.Y.; Na, S.H.; Youn, Y.H.; Shin, C.S. Reference values of neutrophil-lymphocyte ratio, lymphocyte-monocyte ratio, platelet-lymphocyte ratio, and mean platelet volume in healthy adults in South Korea. Medicine 2018, 97, e11138. [Google Scholar]
- Dillon, K.; Goodman, Z.T.; Kaur, S.S.; Levin, B.; McIntosh, R. Neutrophil-to-Lymphocyte Ratio Amplifies the Effects of Aging on Decrements in Grip Strength and Its Functional Neural Underpinnings. J. Gerontol. A Biol. Sci. Med. Sci. A 2023, 78, 882–889. [Google Scholar]
- Li, J.; Chen, Q.; Luo, X.; Hong, J.; Pan, K.; Lin, X.; Liu, X.; Zhou, L.; Wang, H.; Xu, Y.; et al. Neutrophil-to-Lymphocyte Ratio Positively Correlates to Age in Healthy Population. J. Clin. Lab. Anal. 2015, 29, 437–443. [Google Scholar]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef]
- Phillips, C.M.; Perry, I.J. Does inflammation determine metabolic health status in obese and nonobese adults? J. Clin. Endocrinol. Metab. 2013, 98, E1610–E1619. [Google Scholar]
- Johnson, A.M.; Olefsky, J.M. The origins and drivers of insulin resistance. Cell 2013, 152, 673–684. [Google Scholar]
- Duman, T.T.; Aktas, G.; Atak, B.M.; Kocak, M.Z.; Erkus, E.; Savli, H. Neutrophil to lymphocyte ratio as an indicative of diabetic control level in type 2 diabetes mellitus. Afr. Health Sci. 2019, 19, 1602–1606. [Google Scholar] [PubMed]
- Chmielewski, P.P.; Borysławski, K.; Chmielowiec, K.; Chmielowiec, J.; Strzelec, B. The association between total leukocyte count and longevity: Evidence from longitudinal and cross-sectional data. Ann. Anat. 2016, 204, 1–10. [Google Scholar] [PubMed]
- Chmielewski, P. Leukocyte count, systemic inflammation, and health status in older adults: A narrative review. Anthropol. Rev. 2018, 81, 81–101. [Google Scholar]
- Chmielewski, P.; Borysławski, K.; Chmielowiec, K.; Chmielowiec, J. Longitudinal and cross-sectional changes with age in selected anthropometric and physiological traits in hospitalized adults: An insight from the Polish Longitudinal Study of Aging (PLSA). Anthropol. Rev. 2015, 78, 317–336. [Google Scholar]
- Chmielewski, P.; Strzelec, B.; Chmielowiec, J.; Chmielowiec, K.; Borysławski, K. Association between body size and selected hematological parameters in men and women aged 45 and above from a hospitalized population of older adults: An insight from the Polish Longitudinal Study of Aging (1960–2000). Anthropol. Rev. 2017, 80, 171–190. [Google Scholar]
- Chmielewski, P.; Strzelec, B.; Chmielowiec, J.; Chmielowiec, K.; Borysławski, K. Association of serum bilirubin with longevity: Evidence from a retrospective longitudinal study and cross-sectional data. Anthropol. Rev. 2017, 80, 335–348. [Google Scholar]
- Chmielewski, P.; Strzelec, B.; Borysławski, K.; Chmielowiec, K.; Chmielowiec, J.; Dabrowski, P. Effects of aging on the function of the urinary system: Longitudinal changes with age in selected urine parameters in a hospitalized population of older adults. Anthropol. Rev. 2016, 79, 331–345. [Google Scholar]
- Fest, J.; Ruiter, T.R.; Groot Koerkamp, B.; Rizopoulos, D.; Ikram, M.A.; van Eijck, C.H.J.; Stricker, B.H. The neutrophil-to-lymphocyte ratio is associated with mortality in the general population: The Rotterdam Study. Eur. J. Epidemiol. 2019, 34, 463–470. [Google Scholar]
- Di Rosa, M.; Sabbatinelli, J.; Soraci, L.; Corsonello, A.; Bonfigli, A.R.; Cherubini, A.; Sarzani, R.; Antonicelli, R.; Pelliccioni, G.; Galeazzi, R.; et al. Neutrophil-to-lymphocyte ratio (NLR) predicts mortality in hospitalized geriatric patients independent of the admission diagnosis: A multicenter prospective cohort study. J. Transl. Med. 2023, 21, 835. [Google Scholar]
- Larsen, M.K.; Skov, V.; Kjær, L.; Eickhardt-Dalbøge, C.S.; Knudsen, T.A.; Kristiansen, M.H.; Sørensen, A.L.; Wienecke, T.; Andersen, M.; Ottesen, J.T.; et al. Neutrophil-to-lymphocyte ratio and all-cause mortality with and without myeloproliferative neoplasms-a Danish longitudinal study. Blood Cancer J. 2024, 14, 28. [Google Scholar]
- Zhang, X.; Wei, R.; Wang, X.; Zhang, W.; Li, M.; Ni, T.; Weng, W.; Li, Q. The neutrophil-to-lymphocyte ratio is associated with all-cause and cardiovascular mortality among individuals with hypertension. Cardiovasc. Diabetol. 2024, 23, 117. [Google Scholar]
- Williams, M.R.; Azcutia, V.; Newton, G.; Alcaide, P.; Luscinskas, F.W. Emerging mechanisms of neutrophil recruitment across endothelium. Trends Immunol. 2011, 32, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Glennon-Alty, L.; Hackett, A.P.; Chapman, E.A.; Wright, H.L. Neutrophils and redox stress in the pathogenesis of autoimmune disease. Free Radic. Biol. Med. 2018, 125, 25–35. [Google Scholar]
- Chen, X.; Li, A.; Ma, Q. Neutrophil-lymphocyte ratio and systemic immune-inflammation index as predictors of cardiovascular risk and mortality in prediabetes and diabetes: A population-based study. Inflammopharmacology 2024, 32, 3213–3227. [Google Scholar] [CrossRef] [PubMed]
- Chmielewski, P.; Borysławski, K.; Strzelec, B. Contemporary views on human aging and longevity. Anthropol. Rev. 2016, 79, 115–142. [Google Scholar] [CrossRef]
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A new immune-metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 2018, 14, 576–590. [Google Scholar] [CrossRef]
- Winterbourn, C.C.; Kettle, A.J.; Hampton, M.B. Reactive Oxygen Species and Neutrophil Function. Annu. Rev. Biochem. 2016, 85, 765–792. [Google Scholar]
- Li, Y.; Tian, X.; Luo, J.; Bao, T.; Wang, S.; Wu, X. Molecular mechanisms of aging and anti-aging strategies. Cell Commun. Signal. 2024, 22, 285. [Google Scholar] [CrossRef]
- Lee, M.J.; Park, S.D.; Kwon, S.W.; Woo, S.I.; Lee, M.D.; Shin, S.H.; Kim, D.H.; Kwan, J.; Park, K.S. Relation Between Neutrophil-to-Lymphocyte Ratio and Index of Microcirculatory Resistance in Patients With ST-Segment Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention. Am. J. Cardiol. 2016, 118, 1323–1328. [Google Scholar] [CrossRef]
- Döring, Y.; Drechsler, M.; Soehnlein, O.; Weber, C. Neutrophils in atherosclerosis: From mice to man. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 288–295. [Google Scholar] [CrossRef]
- Soehnlein, O. Multiple roles for neutrophils in atherosclerosis. Circ. Res. 2012, 110, 875–888. [Google Scholar]
- Zhang, X.; Kang, Z.; Yin, D.; Gao, J. Role of neutrophils in different stages of atherosclerosis. Innate Immun. 2023, 29, 97–109. [Google Scholar] [PubMed]
- Mostafa, M.N.; Osama, M. The implications of neutrophil extracellular traps in the pathophysiology of atherosclerosis and atherothrombosis. Exp. Biol. Med. 2020, 245, 1376–1384. [Google Scholar]
- Chistiakov, D.A.; Melnichenko, A.A.; Myasoedova, V.A.; Grechko, A.V.; Orekhov, A.N. Mechanisms of foam cell formation in atherosclerosis. J. Mol. Med. 2017, 95, 1153–1165. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.; Fischer, J.; Ley, K.; Sarembock, I.J. The role of inflammation in vascular injury and repair. J. Thromb. Haemost. 2003, 1, 1699–1709. [Google Scholar] [CrossRef]
- Zanoli, L.; Briet, M.; Empana, J.P.; Cunha, P.G.; Mäki-Petäjä, K.M.; Protogerou, A.D.; Tedgui, A.; Touyz, R.M.; Schiffrin, E.L.; Spronck, B.; et al. Association for Research into Arterial Structure, Physiology (ARTERY) Society, the European Society of Hypertension (ESH) Working Group on Vascular Structure and Function, and the European Network for Noninvasive Investigation of Large Arteries Vascular consequences of inflammation: A position statement from the ESH Working Group on Vascular Structure and Function and the ARTERY Society. J. Hypertens. 2020, 38, 1682–1698. [Google Scholar]
- Henein, M.Y.; Vancheri, S.; Longo, G.; Vancheri, F. The Role of Inflammation in Cardiovascular Disease. Int. J. Mol. Sci. 2022, 23, 12906. [Google Scholar] [CrossRef]
- Eo, W.K.; Chang, H.J.; Kwon, S.H.; Koh, S.B.; Kim, Y.O.; Ji, Y.I.; Kim, H.B.; Lee, J.Y.; Suh, D.S.; Kim, K.H.; et al. The Lymphocyte-Monocyte Ratio Predicts Patient Survival and Aggressiveness of Ovarian Cancer. J. Cancer 2016, 7, 289–296. [Google Scholar] [CrossRef]
- Metwally, A.M.; Kasem, A.A.H.M.; Youssif, M.I.; Hassan, S.M.; Abdel Wahab, A.H.A.; Refaat, L.A. Lymphocyte to monocyte ratio predicts survival and is epigenetically linked to miR-222-3p and miR-26b-5p in diffuse large B cell lymphoma. Sci. Rep. 2023, 13, 4899. [Google Scholar]
- Chmielewski, P.P.; Strzelec, B. Elevated leukocyte count as a harbinger of systemic inflammation, disease progression, and poor prognosis: A review. Folia Morphol. 2018, 77, 171–178. [Google Scholar] [CrossRef]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [PubMed]
- Templeton, A.J.; McNamara, M.G.; Šeruga, B.; Vera-Badillo, F.E.; Aneja, P.; Ocaña, A.; Leibowitz-Amit, R.; Sonpavde, G.; Knox, J.J.; Tran, B.; et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: A systematic review and meta-analysis. J. Natl. Cancer Inst. 2014, 106, dju124. [Google Scholar] [PubMed]
- Cupp, M.A.; Cariolou, M.; Tzoulaki, I.; Aune, D.; Evangelou, E.; Berlanga-Taylor, A.J. Neutrophil to lymphocyte ratio and cancer prognosis: An umbrella review of systematic reviews and meta-analyses of observational studies. BMC Medicine. 2020, 18, 360. [Google Scholar]
- Schafer, M.J.; Zhang, X.; Kumar, A.; Atkinson, E.J.; Zhu, Y.; Jachim, S.; Mazula, D.L.; Brown, A.K.; Berning, M.; Aversa, Z.; et al. The senescence-associated secretome as an indicator of age and medical risk. JCI Insight 2020, 5, e133668. [Google Scholar] [CrossRef]
- Dodds, R.M.; Syddall, H.E.; Cooper, R.; Benzeval, M.; Deary, I.J.; Dennison, E.M.; Der, G.; Gale, C.R.; Inskip, H.M.; Jagger, C.; et al. Grip strength across the life course: Normative data from twelve British studies. PLoS ONE 2014, 9, e113637. [Google Scholar] [CrossRef]
- Sayer, A.A.; Kirkwood, T.B. Grip strength and mortality: A biomarker of ageing? Lancet 2015, 386, 226–227. [Google Scholar]
- Studenski, S.; Perera, S.; Patel, K.; Rosano, C.; Faulkner, K.; Inzitari, M.; Brach, J.; Chandler, J.; Cawthon, P.; Connor, E.B.; et al. Gait speed and survival in older adults. JAMA 2011, 305, 50–58. [Google Scholar] [CrossRef]
- Rietman, M.L.; Spijkerman, A.M.W.; Wong, A.; van Steeg, H.; Bürkle, A.; Moreno-Villanueva, M.; Sindlinger, T.; Franceschi, C.; Grubeck-Loebenstein, B.; Bernhardt, J.; et al. Antioxidants linked with physical, cognitive and psychological frailty: Analysis of candidate biomarkers and markers derived from the MARK-AGE study. Mech. Ageing Dev. 2019, 177, 135–143. [Google Scholar] [CrossRef]
- Chaparro, M.P.; Hughes, A.; Kumari, M.; Benzeval, M. The association between self-rated health and underlying biomarker levels is modified by age, gender, and household income: Evidence from Understanding Society—The UK Household Longitudinal Study. SSM Popul. Health 2019, 8, 100406. [Google Scholar] [CrossRef]
- Castillo-Riquelme, M.; Yamada, G.; Diez Roux, A.V.; Alfaro, T.; Flores-Alvarado, S.; Barrientos, T.; Teixeira Vaz, C.; Trotta, A.; Sarmiento, O.L.; Lazo, M. Aging and self-reported health in 114 Latin American cities: Gender and socio-economic inequalities. BMC Public Health 2022, 22, 1499. [Google Scholar] [CrossRef] [PubMed]
- Duan, R.; Fu, Q.; Sun, Y.; Li, Q. Epigenetic clock: A promising biomarker and practical tool in aging. Ageing Res. Rev. 2022, 81, 101743. [Google Scholar] [CrossRef] [PubMed]
- Di Giorgio, A.; Mirijello, A.; De Gennaro, C.; Fontana, A.; Alboini, P.E.; Florio, L.; Inchingolo, V.; Zarrelli, M.; Miscio, G.; Raggi, P.; et al. Factors Associated with Delirium in COVID-19 Patients and Their Outcome: A Single-Center Cohort Study. Diagnostics 2022, 12, 544. [Google Scholar] [CrossRef] [PubMed]
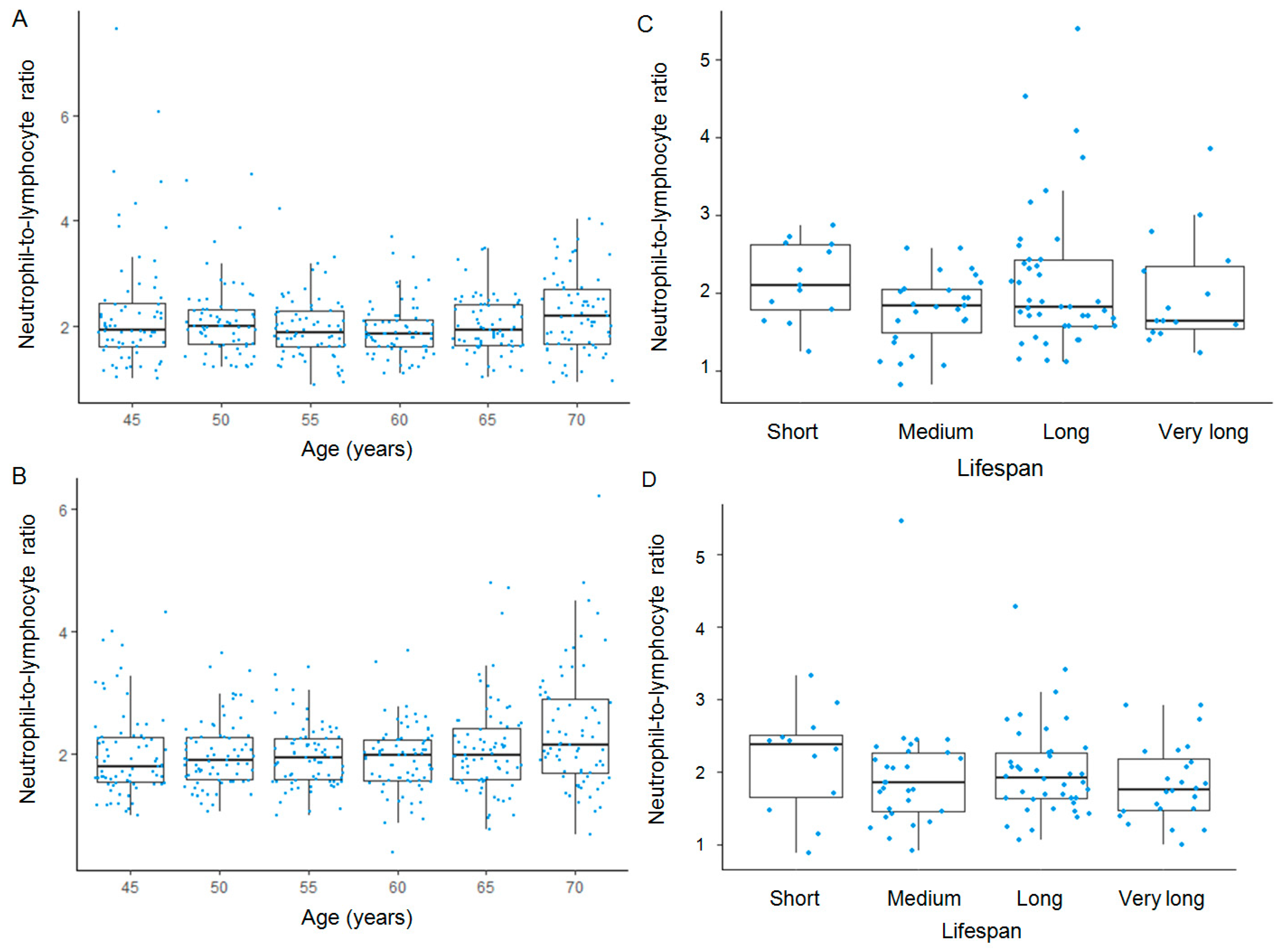

| Lifespan Category | Men | Women | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | Q1 | Median | Q3 | Max | Mean (± SD) | Min | Q1 | Median | Q3 | Max | Mean (± SD) | |
| Short | 1.3 | 1.8 | 2.3 | 2.7 | 2.9 | 2.2 (0.7) | 0.8 | 1.6 | 2.4 | 2.5 | 3.3 | 2.2 (0.7) |
| Medium | 0.8 | 1.5 | 1.8 | 2.0 | 2.6 | 1.8 (0.5) | 0.9 | 1.5 | 1.9 | 2.3 | 5.3 | 2.1 (0.8) |
| Long | 1.1 | 1.6 | 1.8 | 2.4 | 5.4 | 2.1 (1.0) | 1.1 | 1.6 | 1.9 | 2.3 | 4.3 | 2.0 (0.6) |
| Very long | 1.2 | 1.5 | 1.6 | 2.3 | 3.9 | 2.0 (0.7) | 1.0 | 1.5 | 1.7 | 2.2 | 2.9 | 1.8 (0.5) |
| Sex | Estimate | Standard Error | z-Value | Pr (>|z|) | Odds Ratio | 2.5% | 97.5% |
|---|---|---|---|---|---|---|---|
| Men | −0.187 | 0.125 | −1.493 | 0.135 | 0.829 | 0.589 | 1.015 |
| Women | −0.249 | 0.196 | −1.271 | 0.204 | 0.779 | 0.525 | 1.153 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chmielewski, P.P.; Strzelec, B.; Mozdziak, P.; Kempisty, B. Neutrophil-to-Lymphocyte Ratio as a Prognostic Biomarker for Long-Term Survival in Older Adults at a Mental Health Care Center: A Historical Cohort Analysis. J. Clin. Med. 2025, 14, 2509. https://doi.org/10.3390/jcm14072509
Chmielewski PP, Strzelec B, Mozdziak P, Kempisty B. Neutrophil-to-Lymphocyte Ratio as a Prognostic Biomarker for Long-Term Survival in Older Adults at a Mental Health Care Center: A Historical Cohort Analysis. Journal of Clinical Medicine. 2025; 14(7):2509. https://doi.org/10.3390/jcm14072509
Chicago/Turabian StyleChmielewski, Piotr Paweł, Bartłomiej Strzelec, Paul Mozdziak, and Bartosz Kempisty. 2025. "Neutrophil-to-Lymphocyte Ratio as a Prognostic Biomarker for Long-Term Survival in Older Adults at a Mental Health Care Center: A Historical Cohort Analysis" Journal of Clinical Medicine 14, no. 7: 2509. https://doi.org/10.3390/jcm14072509
APA StyleChmielewski, P. P., Strzelec, B., Mozdziak, P., & Kempisty, B. (2025). Neutrophil-to-Lymphocyte Ratio as a Prognostic Biomarker for Long-Term Survival in Older Adults at a Mental Health Care Center: A Historical Cohort Analysis. Journal of Clinical Medicine, 14(7), 2509. https://doi.org/10.3390/jcm14072509






