Reconsidering Gender in Asthma: Is It All About Sex? A Perspective Review
Abstract
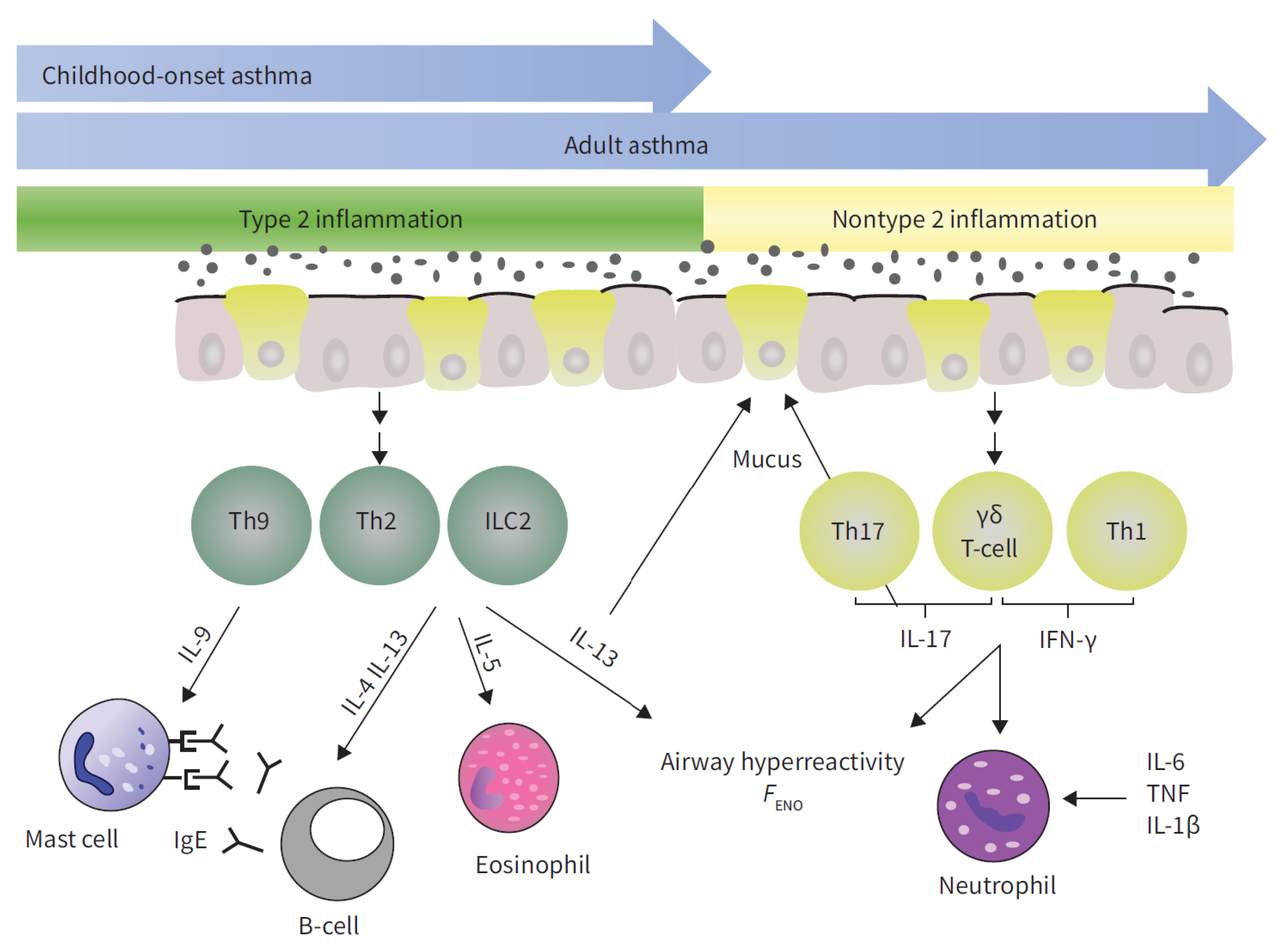
1. Introduction
2. Materials and Methods
2.1. Phase 1: Epidemiological Overview
2.2. Phase 2: Global Literature Review
2.3. Phase 3: Local Insight
- Anatomical Factors;
- Hormonal Factors;
- Environmental Factors;
- Lifestyle Factors.
3. Revisiting Gender Disparities in Asthma
3.1. Biological Influences: Anatomical Factors
3.2. Biological Influences: Hormonal Factors
3.3. Environmental Factors
3.4. Lifestyle Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Institute for Health Metrics and Evaluation. University of Washington. Seattle, WA, USA. Global Burden of Disease Study. 2021. Available online: https://vizhub.healthdata.org/gbd-results (accessed on 26 March 2025).
- World Health Organization. Global Health Estimates 2021: Deaths by Cause, Age, Sex, by Country and by Region, 2000–2021. Available online: https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-leading-causes-of-death (accessed on 22 October 2024).
- World Health Organization. The Global Health Observatory. Available online: https://www.who.int/data/gho (accessed on 2 August 2024).
- World Health Organization. Gender and Health. Available online: https://www.who.int/health-topics/gender#tab=tab_1 (accessed on 2 August 2024).
- Mead, J. Dysanapsis in normal lungs assessed by the relationship between maximal flow, static recoil, and vital capacity. Am. Rev. Respir. Dis. 1980, 121, 339–342. [Google Scholar] [PubMed]
- Nadif, R.; Siroux, V.; Boudier, A.; Le Moual, N.; Just, J.; Gormand, F.; Pison, C.; Matran, R.; Pin, I. Blood granulocyte patterns as predictors of asthma phenotypes in adults from the EGEA study. Eur. Respir. J. 2016, 48, 1040–1051. [Google Scholar] [PubMed]
- Chowdhury, N.U.; Guntur, V.P.; Newcomb, D.C.; Wechsler, M.E. Sex and gender in asthma. Eur. Respir. Rev. 2021, 30, 210067. [Google Scholar] [PubMed]
- Becklake, M.R.; Kauffmann, F. Gender differences in airway behaviour over the human life span. Thorax 1999, 54, 1119–1138. [Google Scholar] [CrossRef]
- Burrows, B.; Barbee, R.A.; Cline, M.G.; Knudson, R.J.; Lebowitz, M.D. Characteristics of asthma among elderly adults in a sample of the general population. Chest 1991, 100, 935–942. [Google Scholar]
- Platts-Mills, T.A.; Carter, M.C. Asthma and indoor exposure to allergens. N. Engl. J. Med. 1997, 336, 1382–1384. [Google Scholar]
- Von Mutius, E.; Martinez, F.D.; Fritzsch, C.; Nicolai, T.; Reitmeir, P.; Thiemann, H.H. Skin test reactivity and number of siblings. BMJ 1994, 308, 692–695. [Google Scholar] [CrossRef]
- Miles, E.A.; Warner, J.A.; Jones, A.C.; Colweel, B.M.; Bryant, T.N.; Warner, J.O. Peripheral blood mononuclear cell proliferative responses in the first year of life in babies born to allergic parents. Clin. Exp. Allergy 1996, 26, 780–788. [Google Scholar]
- Thurlbeck, W.M. Postnatal human lung growth. Thorax 1982, 37, 564–571. [Google Scholar] [CrossRef]
- Hoffstein, V. Relationship between Lung Volume, Maximal Expiratory Flow, Forced Expiratory Volume in One Second, and Tracheal Area in Normal Men and Women. Am. Rev. Respir. Dis. 1986, 134, 956–961. [Google Scholar] [CrossRef]
- LoMauro, A.; Aliverti, A. Sex Differences in Respiratory Function. Breathe 2018, 14, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Green, M.; Mead, J.; Turner, J.M. Variability of maximum expiratory flow-volume curves. J. Appl. Physiol. 1974, 37, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.R.; Castile, R.G.; Fredberg, J.J.; Wohl, M.; Mead, J. Airway size is related to sex but not lung size in normal adults. J. Appl. Physiol. 1987, 63, 2042–2047. [Google Scholar] [CrossRef] [PubMed]
- Sheel, A.W.; Guenette, J.A.; Yuan, R.; Holy, L.; Mayo, J.R.; McWilliams, A.M.; Lam, S.; Coxson, H.O. Evidence for dysanapsis using computed tomographic imaging of the airways in older ex-smokers. J. Appl. Physiol. 2009, 107, 1622–1628. [Google Scholar]
- Colebatch, H.; Greaves, I.; Ng, C. Exponential analysis of elastic recoil and aging in healthy males and females. J. Appl. Physiol. 1979, 47, 683–691. [Google Scholar] [CrossRef]
- Dominelli, P.B.; Molgat-Seon, Y.; Bingham, D.; Swartz, P.M.; Road, J.D.; Foster, G.E.; Sheel, A.W. Dysanapsis and the resistive work of breathing during exercise in healthy men and women. J. Appl. Physiol. 2015, 119, 1105–1113. [Google Scholar] [CrossRef]
- Cerveri, I.; Rossi, A. Manuale di Fisiopatologia Respiratoria; Pacini Editore: Pisa, Italy, 2015; pp. 82–88. [Google Scholar]
- Stanojevic, S.; Kaminsky, D.A.; Miller, M.R.; Thompson, B.; Aliverti, A.; Barjaktarevic, I.; Cooper, B.G.; Culver, B.; Derom, E.; Hall, G.L.; et al. ERS/ATS Technical Standard on Interpretive Strategies for Routine Lung Function Tests. Eur. Respir. J. 2022, 60, 2101499. [Google Scholar] [CrossRef]
- Dos Santos Andreata, L.; Soares, M.R.; Pereira, C.A. Reduced FEV1 /FVC and FEV1 in the Normal Range as a Physiological Variant. Respir. Care 2019, 64, 570–575. [Google Scholar] [CrossRef]
- Krowka, M.J.; Enright, P.L.; Rodarte, J.R.; Hyatt, R.E. Effect of Effort on Measurement of Forced Expiratory Volume in One Second. Am. Rev. Respir. Dis. 1987, 136. [Google Scholar] [CrossRef]
- Carrel, L.; Willard, H.F. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature 2005, 434, 400–404. [Google Scholar]
- Tukiainen, T.; Villani, A.C.; Yen, A.; Rivas, M.A.; Marshall, J.L.; Satija, R.; Aguirre, M.; Gauthier, L.; Fleharty, M.; Kirby, A.; et al. Landscape of X chromosome inactivation across human tissues. Nature 2017, 550, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Laffont, S.; Guéry, J.C. Deconstructing the Sex Bias in Allergy and Autoimmunity: From Sex Hormones and Beyond. Adv. Immunol. 2019, 142, 35–64. [Google Scholar] [CrossRef] [PubMed]
- Kadel, S.; Kovats, S. Sex hormones regulate innate immune cells and promote sex differences in respiratory virus infection. Front. Immunol. 2018, 9, 1653. [Google Scholar] [CrossRef] [PubMed]
- Kuruvilla, M.E.; Lee, F.E.H.; Lee, G.B. Understanding asthma phenotypes, endotypes, and mechanisms of disease. Clin. Rev. Allergy Immunol. 2019, 56, 219–233. [Google Scholar] [CrossRef]
- Keselman, A.; Heller, N. Estrogen signaling modulates allergic inflammation and contributes to sex differences in asthma. Front. Immunol. 2015, 6, 568. [Google Scholar] [CrossRef]
- Fuseini, H.; Newcomb, D.C. Mechanisms Driving Gender Differences in Asthma. Curr. Allergy Asthma Rep. 2017, 17, 19. [Google Scholar] [CrossRef]
- Melgert, B.N.; Oriss, T.B.; Qi, Z.; Dixon-McCarthy, B.; Geerlings, M.; Hylkema, M.N.; Ray, A. Macrophages: Regulators of sex differences in asthma? Am. J. Respir. Cell Mol. Biol. 2010, 42, 595–603. [Google Scholar] [CrossRef]
- Ackerman, L.S. Sex hormones and the genesis of autoimmunity. Arch. Dermatol. 2006, 142, 371–376. [Google Scholar] [CrossRef]
- Miyasaka, T.; Dobashi-Okuyama, K.; Kawakami, K.; Masuda-Suzuki, C.; Takayanagi, M.; Ohno, I. Sex Plays a Multifaceted Role in Asthma Pathogenesis. Biomolecules 2022, 12, 650. [Google Scholar] [CrossRef]
- Global Strategy for Asthma Management and Prevention; Technical Report, Global Initiative for Asthma; WHO: Geneva, Switzerland, 2024.
- Hanley, S. Asthma variation with menstruation. Br. J. Dis. Chest 1981, 75, 306–308. [Google Scholar] [CrossRef]
- Sánchez-Ramos, J.L.; Pereira-Vega, A.R.; Alvarado-Gómez, F.; Maldonado-Pérez, J.A.; Svanes, C.; Gómez-Real, F. Risk factors for premenstrual asthma: A systematic review and meta-analysis. Expert Rev. Respir. Med. 2017, 11, 57–72. [Google Scholar] [CrossRef] [PubMed]
- McCleary, N.; Nwaru, B.I.; Nurmatov, U.B.; Critchley, H.; Sheikh, A. Endogenous and exogenous sex steroid hormones in asthma and allergy in females: A systematic review and meta-analysis. J. Allergy Clin. Immunol. 2018, 141, 1510–1513. [Google Scholar] [PubMed]
- Nwaru, B.I.; Sheikh, A. Hormonal contraceptives and asthma in women of reproductive age: Analysis of data from serial national Scottish Health Surveys. J. R. Soc. Med. 2015, 108, 358–371. [Google Scholar] [PubMed]
- Salam, M.T.; Wenten, M.; Gilliland, F.D. Endogenous and exogenous sex steroid hormones and asthma and wheeze in young women. J. Allergy Clin. Immunol. 2006, 117, 1001–1007. [Google Scholar]
- Nwaru, B.I.; Pillinger, R.; Tibble, H.; Shah, S.A.; Ryan, D.; Critchley, H.; Price, D.; Hawrylowicz, C.M.; Simpson, C.R.; Soyiri, I.N.; et al. Hormonal contraceptives and onset of asthma in reproductive-age women: Population-based cohort study. J. Allergy Clin. Immunol. 2020, 146, 438–446. [Google Scholar]
- Nwaru, B.I.; Tibble, H.; Shah, S.A.; Pillinger, R.; McLean, S.; Ryan, D.P.; Critchley, H.; Price, D.B.; Hawrylowicz, C.M.; Simpson, C.R.; et al. Hormonal contraception and the risk of severe asthma exacerbation: 17-year population-based cohort study. Thorax 2021, 76, 109–115. [Google Scholar]
- Troisi, R.J.; Willett, W.C.; Weiss, S.T.; Trichopoulos, D.; Rosner, B.; Speizer, F.E. A prospective study of diet and adult-onset asthma. Am. J. Respir. Crit. Care Med. 1995, 151, 1401–1408. [Google Scholar]
- Zein, J.G.; Dweik, R.A.; Comhair, S.A.; Bleecker, E.R.; Moore, W.C.; Peters, S.P.; Busse, W.W.; Jarjour, N.N.; Calhoun, W.J.; Castro, M.; et al. Asthma is more severe in older adults. PLoS ONE 2015, 10, e0133490. [Google Scholar]
- Scioscia, G.; Carpagnano, G.E.; Lacedonia, D.; Soccio, P.; Quarato, C.M.I.; Trabace, L.; Fuso, P.; Foschino Barbaro, M.P. The role of airways 17beta-estradiol as a biomarker of severity in postmenopausal asthma: A pilot study. J. Clin. Med. 2020, 9, 2037. [Google Scholar]
- Wenzel, S.E.; Robinson, C.B.; Leonard, J.M.; Panettieri, R.A., Jr. Nebulized dehydroepiandrosterone-3-sulfate improves asthma control in the moderate-to-severe asthma results of a 6-week, randomized, double-blind, placebo-controlled study. Allergy Asthma Proc. 2010, 31, 461–471. [Google Scholar]
- Marozkina, N.; Zein, J.; DeBoer, M.D.; Logan, L.; Veri, L.; Ross, K.; Gaston, B. Dehydroepiandrosterone supplementation may benefit women with asthma who have low androgen levels: A pilot study. Pulm. Ther. 2019, 5, 213–220. [Google Scholar] [PubMed]
- Schatz, M.; Harden, K.; Forsythe, A.; Chilingar, L.; Hoffman, C.; Sperling, W.; Zeiger, R.S. The Course of Asthma during Pregnancy, Post Partum, and with Successive Pregnancies: A Prospective Analysis. J. Allergy Clin. Immunol. 1988, 81, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Yung, J.A.; Fuseini, H.; Newcomb, D.C. Hormones, sex, and asthma. Ann. Allergy Asthma Immunol. 2018, 120, 488–494. [Google Scholar]
- Juniper, E.F.; Daniel, E.E.; Roberts, R.S.; Kline, P.A.; Hargreave, F.E.; Newhouse, M.T. Improvement in Airway Responsiveness and Asthma Severity during Pregnancy: A Prospective Study. Am. Rev. Respir. Dis. 1989, 140, 924–931. [Google Scholar] [CrossRef]
- Belanger, K.; Hellenbrand, M.E.; Holford, T.R.; Bracken, M. Effect of Pregnancy on Maternal Asthma Symptoms and Medication Use. Obstet. Gynecol. 2010, 115, 559–567. [Google Scholar] [CrossRef]
- White, G.E.; Seaman, C.; Filios, M.S.; Mazurek, J.M.; Flattery, J.; Harrison, R.J.; Reilly, M.J.; Rosenman, K.D.; Lumia, M.E.; Stephens, A.C.; et al. Gender differences in work-related asthma: Surveillance data from California, Massachusetts, Michigan, and New Jersey, 1993–2008. J. Asthma 2014, 51, 691–702. [Google Scholar] [PubMed]
- Boulet, L.P.; Lavoie, K.L.; Raherison-Semjen, C.; Kaplan, A.; Singh, D.; Jenkins, C.R. Addressing Sex and Gender to Improve Asthma Management. Prim. Care Respir. Med. 2022, 32, 56. [Google Scholar] [CrossRef]
- Colombo, D.; Zagni, E.; Ferri, F.; Canonica, G.W. Gender differences in asthma perception and its impact on quality of life: A post hoc analysis of the PROXIMA (Patient Reported Outcomes and Xolair® In the Management of Asthma) study. Allergy Asthma Clin. Immunol. 2019, 15, 65. [Google Scholar]
- Borges, R.C.; Alith, M.B.; Nascimento, O.A.; Jardim, J.R. Gender differences in the perception of asthma respiratory symptoms in five Latin American countries. J. Asthma 2022, 59, 1030–1040. [Google Scholar]
- Peters, U.; Dixon, A.E.; Forno, E. Obesity and asthma. J. Allergy Clin. Immunol. 2018, 141, 1169–1179. [Google Scholar] [CrossRef]
- VanKim, N.A.; Corliss, H.L.; Jun, H.J.; Calzo, J.P.; AlAwadhi, M.; Austin, S.B. Gender expression and sexual orientation differences in diet quality and eating habits from adolescence to young adulthood. J. Acad. Nutr. Diet. 2019, 119, 2028–2040. [Google Scholar] [CrossRef] [PubMed]
- Nyenhuis, S.M.; Dixon, A.E.; Ma, J. Impact of lifestyle interventions targeting healthy diet, physical activity, and weight loss on asthma in adults: What is the evidence? J. Allergy Clin. Immunol. Pract. 2018, 6, 751–763. [Google Scholar] [CrossRef] [PubMed]
- Guilleminault, L.; Williams, E.J.; Scott, H.A.; Berthon, B.S.; Jensen, M.; Wood, L.G. Diet and asthma: Is it time to adapt our message? Nutrients 2017, 9, 1227. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, C.S. Sex-dependent differences in voluntary physical activity. J. Neurosci. Res. 2017, 95, 279–290. [Google Scholar] [CrossRef]
- Ritchie, H. Who smokes more, men or women? Our World Data 2019. [Google Scholar]
- Langhammer, A.; Johnsen, R.; Holmen, J.; Gulsvik, A.; Bjermer, L. Cigarette smoking gives more respiratory symptoms among women than among men The Nord-Trøndelag Health Study (HUNT). J. Epidemiol. Community Health 2000, 54, 917–922. [Google Scholar] [CrossRef]
- Park, S.J.; Yi, B.; Lee, H.S.; Oh, W.Y.; Na, H.K.; Lee, M.; Yang, M. To quit or not: Vulnerability of women to smoking tobacco. J. Environ. Sci. Health Part C 2016, 34, 33–56. [Google Scholar] [CrossRef]
- Zein, J.G.; Denson, J.L.; Wechsler, M.E. Asthma over the adult life course: Gender and hormonal influences. Clin. Chest Med. 2019, 40, 149–161. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marinelli, A.; Dragonieri, S.; Portacci, A.; Quaranta, V.N.; Carpagnano, G.E. Reconsidering Gender in Asthma: Is It All About Sex? A Perspective Review. J. Clin. Med. 2025, 14, 2506. https://doi.org/10.3390/jcm14072506
Marinelli A, Dragonieri S, Portacci A, Quaranta VN, Carpagnano GE. Reconsidering Gender in Asthma: Is It All About Sex? A Perspective Review. Journal of Clinical Medicine. 2025; 14(7):2506. https://doi.org/10.3390/jcm14072506
Chicago/Turabian StyleMarinelli, Alessio, Silvano Dragonieri, Andrea Portacci, Vitaliano Nicola Quaranta, and Giovanna Elisiana Carpagnano. 2025. "Reconsidering Gender in Asthma: Is It All About Sex? A Perspective Review" Journal of Clinical Medicine 14, no. 7: 2506. https://doi.org/10.3390/jcm14072506
APA StyleMarinelli, A., Dragonieri, S., Portacci, A., Quaranta, V. N., & Carpagnano, G. E. (2025). Reconsidering Gender in Asthma: Is It All About Sex? A Perspective Review. Journal of Clinical Medicine, 14(7), 2506. https://doi.org/10.3390/jcm14072506







