Hepatitis B-Induced Hepatocellular Carcinoma: Understanding Viral Carcinogenesis and Disease Management
Abstract
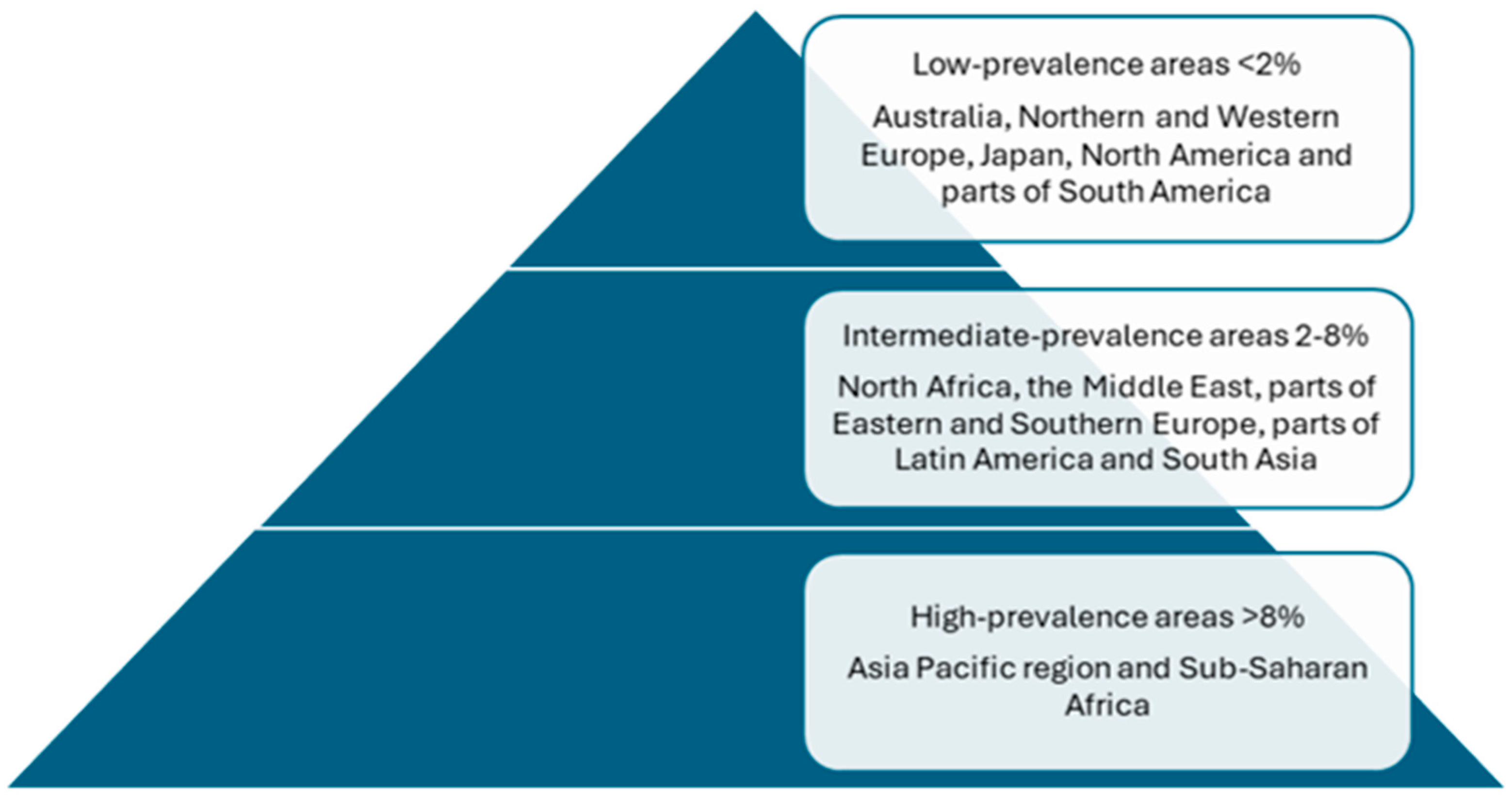
1. Introduction/Epidemiology
2. Incidence and Prevalence
3. Screening Population
4. Hepatitis B Vaccination and HCC
5. HBV Genotypes
6. Mechanism of HBV-Induced HCC
7. Role of Hepatitis Delta Virus Coinfection
8. Does HBV Treatment Reduce the Risk of HCC?
9. Treatment of HCC in HBV Patients and a Spotlight on Prophylaxis
10. Prognosis of HCC in Hepatitis B
11. Conclusions and Remarks
Funding
Data Availability Statement
Conflicts of Interest
References
- Katamba, C.; Onaluwa Philippe, O. Epidemiology of Hepatitis B Virus. In Hepatitis B; Rodrigo, L., Ed.; IntechOpen: Rijeka, Croatia, 2021. [Google Scholar]
- Alberts, C.J.; Clifford, G.M.; Georges, D.; Negro, F.; Lesi, O.A.; Hutin, Y.J.; de Martel, C. Worldwide prevalence of hepatitis B virus and hepatitis C virus among patients with cirrhosis at country, region, and global levels: A systematic review. Lancet Gastroenterol. Hepatol. 2022, 7, 724–735. [Google Scholar] [CrossRef] [PubMed]
- Larke, R.P.; Froese, G.J.; Devine, R.D.; Petruk, M.W. Extension of the epidemiology of hepatitis B in circumpolar regions through a comprehensive serologic study in the Northwest Territories of Canada. J. Med. Virol. 1987, 22, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Center for Disease Control and Prevention. Hepatitis B Surveillance. 2021. Available online: https://www.cdc.gov/hepatitis/statistics/2021surveillance/hepatitis-b.htm (accessed on 1 February 2025).
- World Health Organization. Hepatitis B. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b (accessed on 1 February 2025).
- Government of Canada. Primary Care Management of Hepatitis B—Quick Reference (HBV-QR). Available online: https://www.canada.ca/en/public-health/services/reports-publications/primary-care-management-hepatitis-b-quick-reference.html (accessed on 1 February 2025).
- Government of Canada. Hepatitis B Vaccines: Canadian Immunization Guide. Available online: https://www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide-part-4-active-vaccines/page-7-hepatitis-b-vaccine.html (accessed on 1 February 2025).
- Lok, A.S.; McMahon, B.J. Chronic hepatitis B: Update 2009. Hepatology 2009, 50, 661–662. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.H.; Chen, C.J.; Lai, M.S.; Hsu, H.M.; Wu, T.C.; Kong, M.S.; Liang, D.C.; Shau, W.Y.; Chen, D.S. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. Taiwan Childhood Hepatoma Study Group. N. Engl. J. Med. 1997, 336, 1855–1859. [Google Scholar] [CrossRef]
- Chiang, C.J.; Jhuang, J.R.; Yang, Y.W.; Zhuang, B.Z.; You, S.L.; Lee, W.C.; Chen, C.J. Association of Nationwide Hepatitis B Vaccination and Antiviral Therapy Programs With End-Stage Liver Disease Burden in Taiwan. JAMA Netw. Open 2022, 5, e2222367. [Google Scholar] [CrossRef]
- Wang, X.Y.; Huang, J.M.; Lu, X.M.; Harrison, T.J.; Liu, H.B.; Jia, H.H.; Fang, Z.L. Changing risk factors for hepatocellular carcinoma in hyperendemic regions in the era of universal hepatitis B vaccination. Cancer Epidemiol. 2020, 67, 101775. [Google Scholar] [CrossRef]
- Qu, C.; Chen, T.; Fan, C.; Zhan, Q.; Wang, Y.; Lu, J.; Lu, L.L.; Ni, Z.; Huang, F.; Yao, H.; et al. Efficacy of neonatal HBV vaccination on liver cancer and other liver diseases over 30-year follow-up of the Qidong hepatitis B intervention study: A cluster randomized controlled trial. PLoS Med. 2014, 11, e1001774. [Google Scholar] [CrossRef]
- McMahon, B.J.; Bulkow, L.R.; Singleton, R.J.; Williams, J.; Snowball, M.; Homan, C.; Parkinson, A.J. Elimination of hepatocellular carcinoma and acute hepatitis B in children 25 years after a hepatitis B newborn and catch-up immunization program. Hepatology 2011, 54, 801–807. [Google Scholar] [CrossRef]
- Lin, C.L.; Kao, J.H. Hepatitis B virus genotypes and variants. Cold Spring Harb. Perspect. Med. 2015, 5, a021436. [Google Scholar] [CrossRef]
- Kao, J.H.; Chen, P.J.; Lai, M.Y.; Chen, D.S. Hepatitis B genotypes correlate with clinical outcomes in patients with chronic hepatitis B. Gastroenterology 2000, 118, 554–559. [Google Scholar] [CrossRef]
- Kao, J.H.; Chen, P.J.; Lai, M.Y.; Chen, D.S. Basal core promoter mutations of hepatitis B virus increase the risk of hepatocellular carcinoma in hepatitis B carriers. Gastroenterology 2003, 124, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.L.; Hui, A.Y.; Wong, M.L.; Tse, A.M.; Hung, L.C.; Wong, V.W.; Sung, J.J. Genotype C hepatitis B virus infection is associated with an increased risk of hepatocellular carcinoma. Gut 2004, 53, 1494–1498. [Google Scholar] [CrossRef] [PubMed]
- Yuen, M.F.; Tanaka, Y.; Mizokami, M.; Yuen, J.C.; Wong, D.K.; Yuan, H.J.; Sum, S.M.; Chan, A.O.; Wong, B.C.; Lai, C.L. Role of hepatitis B virus genotypes Ba and C, core promoter and precore mutations on hepatocellular carcinoma: A case control study. Carcinogenesis 2004, 25, 1593–1598. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Eilard, A.; Ringlander, J.; Andersson, M.E.; Nilsson, S.; Norkrans, G.; Lindh, M. Long-Term Outcome of Chronic Hepatitis B-Histological Score and Viral Genotype Are Important Predictors of Hepatocellular Carcinoma. J. Viral Hepat. 2025, 32, e70008. [Google Scholar] [CrossRef]
- Choi, Y.M.; Jang, J.; Kim, D.H.; Kim, Z.; Kim, E.; Choe, W.H.; Kim, B.J. PreS1 deletions in genotype C HBV leads to severe hepatic inflammation and hepatocarcinogenesis via the IRE1-JNK axis. JHEP Rep. Innov. Hepatol. 2025, 7, 101274. [Google Scholar] [CrossRef]
- Zoulim, F.; Chen, P.-J.; Dandri, M.; Kennedy, P.T.; Seeger, C. Hepatitis B virus DNA integration: Implications for diagnostics, therapy, and outcome. J. Hepatol. 2024, 81, 1087–1099. [Google Scholar] [CrossRef]
- Khan, M.N.; Mao, B.; Hu, J.; Shi, M.; Wang, S.; Rehman, A.U.; Li, X. Tumor-associated macrophages and CD8+ T cells: Dual players in the pathogenesis of HBV-related HCC. Front. Immunol. 2024, 15, 1472430. [Google Scholar] [CrossRef]
- Jiang, Y.; Han, Q.; Zhao, H.; Zhang, J. The Mechanisms of HBV-Induced Hepatocellular Carcinoma. J. Hepatocell. Carcinoma 2021, 8, 435–450. [Google Scholar] [CrossRef]
- Sivasudhan, E.; Blake, N.; Lu, Z.; Meng, J.; Rong, R. Hepatitis B Viral Protein HBx and the Molecular Mechanisms Modulating the Hallmarks of Hepatocellular Carcinoma: A Comprehensive Review. Cells 2022, 11, 741. [Google Scholar] [CrossRef]
- Wei, F.; Zheng, Q.; Li, M.; Wu, M. The association between hepatitis B mutants and hepatocellular carcinoma: A meta-analysis. Medicine 2017, 96, e6835. [Google Scholar] [CrossRef]
- Pollicino, T.; Cacciola, I.; Saffioti, F.; Raimondo, G. Hepatitis B virus PreS/S gene variants: Pathobiology and clinical implications. J. Hepatol. 2014, 61, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Xie, D.; Zhang, J.; Jin, W.; Li, Y.; Yao, J.; Pan, Z.; Xie, D. ROS/NF-κB Signaling Pathway-Mediated Transcriptional Activation of TRIM37 Promotes HBV-Associated Hepatic Fibrosis. Mol. Ther. Nucleic Acids 2020, 22, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Witt-Kehati, D.; Fridkin, A.; Alaluf, M.B.; Zemel, R.; Shlomai, A. Inhibition of pMAPK14 Overcomes Resistance to Sorafenib in Hepatoma Cells with Hepatitis B Virus. Transl. Oncol. 2018, 11, 511–517. [Google Scholar] [CrossRef]
- Flavahan, W.A.; Gaskell, E.; Bernstein, B.E. Epigenetic plasticity and the hallmarks of cancer. Science 2017, 357, eaal2380. [Google Scholar] [CrossRef]
- Dandri, M. Epigenetic modulation in chronic hepatitis B virus infection. Semin. Immunopathol. 2020, 42, 173–185. [Google Scholar] [CrossRef]
- Zhang, C.; Huang, C.; Sui, X.; Zhong, X.; Yang, W.; Hu, X.; Li, Y. Association between gene methylation and HBV infection in hepatocellular carcinoma: A meta-analysis. J. Cancer 2019, 10, 6457–6465. [Google Scholar] [CrossRef]
- Song, M.A.; Kwee, S.A.; Tiirikainen, M.; Hernandez, B.Y.; Okimoto, G.; Tsai, N.C.; Wong, L.L.; Yu, H. Comparison of genome-scale DNA methylation profiles in hepatocellular carcinoma by viral status. Epigenetics 2016, 11, 464–474. [Google Scholar] [CrossRef]
- Hsieh, A.; Kim, H.S.; Lim, S.O.; Yu, D.Y.; Jung, G. Hepatitis B viral X protein interacts with tumor suppressor adenomatous polyposis coli to activate Wnt/β-catenin signaling. Cancer Lett. 2011, 300, 162–172. [Google Scholar] [CrossRef]
- Chung, T.-W.; Lee, Y.-C.; Ko, J.-H.; Kim, C.-H. Hepatitis B Virus X Protein Modulates the Expression of PTEN by Inhibiting the Function of p53, a Transcriptional Activator in Liver Cells1. Cancer Res. 2003, 63, 3453–3458. [Google Scholar]
- Ha, H.L.; Yu, D.Y. HBx-induced reactive oxygen species activates hepatocellular carcinogenesis via dysregulation of PTEN/Akt pathway. World J. Gastroenterol. 2010, 16, 4932–4937. [Google Scholar] [CrossRef]
- Kim, G.W.; Imam, H.; Khan, M.; Mir, S.A.; Kim, S.J.; Yoon, S.K.; Hur, W.; Siddiqui, A. HBV-Induced Increased N6 Methyladenosine Modification of PTEN RNA Affects Innate Immunity and Contributes to HCC. Hepatology 2021, 73, 533–547. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Cui, T.; Wei, F.; Zhou, Z.; Sun, Y.; Gao, C.; Xu, X.; Zhang, H. Wnt/β-Catenin signaling pathway in hepatocellular carcinoma: Pathogenic role and therapeutic target. Front. Oncol. 2024, 14, 1367364. [Google Scholar] [CrossRef] [PubMed]
- Alfaiate, D.; Clément, S.; Gomes, D.; Goossens, N.; Negro, F. Chronic hepatitis D and hepatocellular carcinoma: A systematic review and meta-analysis of observational studies. J. Hepatol. 2020, 73, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Romeo, R.; Del Ninno, E.; Rumi, M.; Russo, A.; Sangiovanni, A.; de Franchis, R.; Ronchi, G.; Colombo, M. A 28-Year Study of the Course of Hepatitis Δ Infection: A Risk Factor for Cirrhosis and Hepatocellular Carcinoma. Gastroenterology 2009, 136, 1629–1638. [Google Scholar] [CrossRef]
- Guilhot, S.; Huang, S.N.; Xia, Y.P.; La Monica, N.; Lai, M.M.; Chisari, F.V. Expression of the hepatitis delta virus large and small antigens in transgenic mice. J. Virol. 1994, 68, 1052–1058. [Google Scholar] [CrossRef]
- Maestro, S.; Gómez-Echarte, N.; Camps, G.; Usai, C.; Suárez, L.; Vales, Á.; Olagüe, C.; Aldabe, R.; González-Aseguinolaza, G. AAV-HDV: An Attractive Platform for the In Vivo Study of HDV Biology and the Mechanism of Disease Pathogenesis. Viruses 2021, 13, 788. [Google Scholar] [CrossRef]
- Usai, C.; Maestro, S.; Camps, G.; Olague, C.; Suárez-Amaran, L.; Vales, A.; Aragon, T.; Hommel, M.; Aldabe, R.; Gonzalez-Aseguinolaza, G. TNF-alpha inhibition ameliorates HDV-induced liver damage in a mouse model of acute severe infection. JHEP Rep. Innov. Hepatol. 2020, 2, 100098. [Google Scholar] [CrossRef]
- Park, C.Y.; Oh, S.H.; Kang, S.M.; Lim, Y.S.; Hwang, S.B. Hepatitis delta virus large antigen sensitizes to TNF-alpha-induced NF-kappaB signaling. Mol. Cells 2009, 28, 49–55. [Google Scholar] [CrossRef]
- Lombardo, D.; Franzè, M.S.; Caminiti, G.; Pollicino, T. Hepatitis Delta Virus and Hepatocellular Carcinoma. Pathogens 2024, 13, 362. [Google Scholar] [CrossRef]
- Yang, Y.M.; Seki, E. TNFα in liver fibrosis. Curr. Pathobiol. Rep. 2015, 3, 253–261. [Google Scholar] [CrossRef]
- Majumdar, A.; Curley, S.A.; Wu, X.; Brown, P.; Hwang, J.P.; Shetty, K.; Yao, Z.X.; He, A.R.; Li, S.; Katz, L.; et al. Hepatic stem cells and transforming growth factor β in hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Hosaka, T.; Suzuki, F.; Kobayashi, M.; Seko, Y.; Kawamura, Y.; Sezaki, H.; Akuta, N.; Suzuki, Y.; Saitoh, S.; Arase, Y.; et al. Long-term entecavir treatment reduces hepatocellular carcinoma incidence in patients with hepatitis B virus infection. Hepatology 2013, 58, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.-M.; Yip, T.C.-F.; Wong, G.L.-H.; Kim, W.R.; Yee, L.J.; Brooks-Rooney, C.; Curteis, T.; Cant, H.; Chen, C.-H.; Chen, C.-Y.; et al. Hepatocellular carcinoma risk in patients with chronic hepatitis B receiving tenofovir- vs. entecavir-based regimens: Individual patient data meta-analysis. J. Hepatol. 2023, 78, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Zoutendijk, R.; Reijnders, J.G.; Zoulim, F.; Brown, A.; Mutimer, D.J.; Deterding, K.; Hofmann, W.P.; Petersen, J.; Fasano, M.; Buti, M.; et al. Virological response to entecavir is associated with a better clinical outcome in chronic hepatitis B patients with cirrhosis. Gut 2013, 62, 760–765. [Google Scholar] [CrossRef]
- Singal, A.G.; Llovet, J.M.; Yarchoan, M.; Mehta, N.; Heimbach, J.K.; Dawson, L.A.; Jou, J.H.; Kulik, L.M.; Agopian, V.G.; Marrero, J.A.; et al. AASLD Practice Guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatology 2023, 78, 1922–1965. [Google Scholar] [CrossRef]
- Lamarque, C.; Segaux, L.; Bachellier, P.; Buchard, B.; Chermak, F.; Conti, F.; Decaens, T.; Dharancy, S.; Di Martino, V.; Dumortier, J.; et al. Evaluation of a delayed liver transplantation strategy for patients with HCC receiving bridging therapy: The DELTA-HCC study. J. Hepatol. 2024, 81, 278–288. [Google Scholar] [CrossRef]
- Pazgan-Simon, M.; Simon, K.A.; Jarowicz, E.; Rotter, K.; Szymanek-Pasternak, A.; Zuwała-Jagiełło, J. Hepatitis B virus treatment in hepatocellular carcinoma patients prolongs survival and reduces the risk of cancer recurrence. Clin. Exp. Hepatol. 2018, 4, 210–216. [Google Scholar] [CrossRef]
- Zhang, S.-S.; Liu, J.-X.; Zhu, J.; Xiao, M.-B.; Lu, C.-H.; Ni, R.-Z.; Qu, L.-S. Effects of TACE and preventive antiviral therapy on HBV reactivation and subsequent hepatitis in hepatocellular carcinoma: A meta-analysis. Jpn. J. Clin. Oncol. 2019, 49, 646–655. [Google Scholar] [CrossRef]
- Wang, X.; Yang, X.; Chen, F.; Wu, S.; Song, Z.; Fei, J. Hepatitis B Virus Reactivation Potential Risk Factors in Hepatocellular Carcinoma via Transcatheter Arterial Chemoembolization: A Retrospective Research. Can. J. Gastroenterol. Hepatol. 2021, 2021, 8864655. [Google Scholar] [CrossRef]
- Wu, S.; Yang, L.; Bi, X.; Lin, Y.; Deng, W.; Jiang, T.; Li, M.; Xie, Y. Attach importance to antiviral therapy in patients with hepatocellular carcinoma caused by hepatitis virus. Gastroenterol. Endosc. 2023, 1, 5–10. [Google Scholar] [CrossRef]
- Xu, L.; Gao, H.; Huang, J.; Wang, H.; Zhou, Z.; Zhang, Y.; Li, S.; Chen, M. Antiviral therapy in the improvement of survival of patients with hepatitis B virus-related hepatocellular carcinoma treated with sorafenib. J. Gastroenterol. Hepatol. 2015, 30, 1032–1039. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Yang, Y.; Wen, F.; Wheeler, J.; Fu, P.; Li, Q. Cost-effectiveness analysis of antiviral therapy in patients with advanced hepatitis B virus-related hepatocellular carcinoma treated with sorafenib. J. Gastroenterol. Hepatol. 2016, 31, 1978–1985. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.-C.; Chao, Y.; Chen, M.-H.; Lan, K.-H.; Lee, I.-C.; Hou, M.-C.; Huang, Y.-H. Risk of HBV reactivation in patients with immune checkpoint inhibitor-treated unresectable hepatocellular carcinoma. J. Immunother. Cancer 2020, 8, e001072. [Google Scholar] [CrossRef]
- Yuan, P.; Chen, P.; Qian, Y. Evaluation of Antiviral Therapy Performed after Curative Therapy in Patients with HBV-Related Hepatocellular Carcinoma: An Updated Meta-Analysis. Can. J. Gastroenterol. Hepatol. 2016, 2016, 5234969. [Google Scholar] [CrossRef][Green Version]
- Zhang, H.; Zhou, Y.; Yuan, G.; Zhou, G.; Yang, D.; Zhou, Y. Antiviral therapy improves the survival rate and decreases recurrences and fatalities in liver cancer patients following curative resection: A meta-analysis. Mol. Clin. Oncol. 2015, 3, 1239–1247. [Google Scholar] [CrossRef]
- Yin, J.; Li, N.; Han, Y.; Xue, J.; Deng, Y.; Shi, J.; Guo, W.; Zhang, H.; Wang, H.; Cheng, S.; et al. Effect of antiviral treatment with nucleotide/nucleoside analogs on postoperative prognosis of hepatitis B virus-related hepatocellular carcinoma: A two-stage longitudinal clinical study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2013, 31, 3647–3655. [Google Scholar] [CrossRef]
| Mechanism | Description |
|---|---|
| HBV Gene Integration and Genomic Instability [21] | HBV integrates into the host genome, causing mutations and instability, potentially leading to HCC. |
| Activation of Cancer-Promoting Pathways [37] | The activation of signaling pathways like Wnt/beta-catenin and PI3K/Akt drive cell proliferation and tumor survival. |
| Epigenetics [30] | Epigenetic modifications, including methylation and histone changes, contribute to liver cancer progression. |
| Exosomes and Autophagy [23] | Exosomal signaling and autophagy contribute to immune suppression and metabolic changes, promoting HCC. |
| Immune Suppression [22] | HBV-induced immune suppression helps tumor cells evade immune responses, promoting cancer progression. This is mainly through CD8+ T cells and tumor-associated macrophages. |
| ORF X [24] | This ORF encodes the HBx protein, a key factor in oncogenesis and liver cancer development. |
| Oxidative Damage and Tumor Survival [23] | HBV proteins (HBx, HBs, and HBc) cause oxidative stress, promoting liver fibrosis and cancer progression. |
| Pro-Oncogenic MAPK14 Activation [23,28] | MAPK14 activation contributes to HBV replication and the survival of tumor cells in liver cancer. |
| Surgical Treatment |
|---|
| Liver transplantation |
| Local ablative therapy Thermal ablation therapies Radiation segmentectomy External beam radiation therapy |
| Transarterial therapy Transarterial chemoembolization Transarterial radioembolization |
| Systemic therapy Antiangiogenic targeted therapies Immunotherapy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farbod, Y.; Kankouni, H.; Moini, M.; Fung, S. Hepatitis B-Induced Hepatocellular Carcinoma: Understanding Viral Carcinogenesis and Disease Management. J. Clin. Med. 2025, 14, 2505. https://doi.org/10.3390/jcm14072505
Farbod Y, Kankouni H, Moini M, Fung S. Hepatitis B-Induced Hepatocellular Carcinoma: Understanding Viral Carcinogenesis and Disease Management. Journal of Clinical Medicine. 2025; 14(7):2505. https://doi.org/10.3390/jcm14072505
Chicago/Turabian StyleFarbod, Yasamin, Husain Kankouni, Maryam Moini, and Scott Fung. 2025. "Hepatitis B-Induced Hepatocellular Carcinoma: Understanding Viral Carcinogenesis and Disease Management" Journal of Clinical Medicine 14, no. 7: 2505. https://doi.org/10.3390/jcm14072505
APA StyleFarbod, Y., Kankouni, H., Moini, M., & Fung, S. (2025). Hepatitis B-Induced Hepatocellular Carcinoma: Understanding Viral Carcinogenesis and Disease Management. Journal of Clinical Medicine, 14(7), 2505. https://doi.org/10.3390/jcm14072505





