Elevated Levels of IL–1Ra, IL–1β, and Oxidative Stress in COVID-19: Implications for Inflammatory Pathogenesis
Abstract
1. Introduction
2. Material and Methods
2.1. Patients
- Those with a confirmed history of SARS-CoV-2 infection (COVID) (n = 33).
- Control group: Subjects who declared that they had not experienced COVID-19 (non-COVID) (n = 24).
2.2. Procedure
2.3. Data Analysis
2.4. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.; Ou, C.; He, J.; Liu, L.; Shan, H.; Lei, C.; Hui, D.S.C.; et al. China Medical Treatment Expert Group for COVID-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [PubMed]
- Available online: https://data.who.int/dashboards/covid19/cases?n=o (accessed on 8 January 2025).
- Bikdeli, B.; Madhavan, M.V.; Jimenez, D.; Chuich, T.; Dreyfus, I.; Driggin, E.; Nigoghossian, C.D.; Ageno, W.; Madjid, M.; Guo, Y.; et al. COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-Up: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 2950–2973. [Google Scholar] [CrossRef]
- Cui, S.; Chen, S.; Li, X.; Liu, S.; Wang, F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J. Thromb. Haemost. 2020, 18, 1421–1424. [Google Scholar] [PubMed]
- Libby, P. The changing landscape of atherosclerosis. Nature 2021, 592, 524–533. [Google Scholar] [CrossRef]
- Lusis, A.J. Atherosclerosis. Nature 2000, 407, 233–241. [Google Scholar]
- Venkata, K.N.; Pothineni, N.V.K.; Subramany, S.; Kuriakose, K.; Shirazi, L.F.; Romeo, F.; Shah, P.K.; Mehta, J.L. Infections, atherosclerosis, and coronary heart disease. Eur. Heart J. 2017, 38, 3195–3201. [Google Scholar]
- Kadosh, B.S.; Garshick, M.S.; Gaztanaga, J.; Moore, K.J.; Newman, J.D.; Pillinger, M.; Ramasamy, R.; Reynolds, H.R.; Shah, B.; Hochman, J.; et al. COVID-19 and the Heart and Vasculature: Novel Approaches to Reduce Virus-Induced Inflammation in Patients with Cardiovascular Disease. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 2045–2053. [Google Scholar]
- Seang-Hwan, J.; Kyung-Tae, L. Atherosclerosis by Virus Infection—A Short Review. Biomedicines 2022, 10, 2634. [Google Scholar] [CrossRef]
- Brzezińska-Błaszczyk, E.; Zawilska, J.B.; Hille, N.; Szparowska, J.; Małecka, A.; Grabia, M.; Chojnacka, Z. Zrozumieć immunopatologię zakażenia wirusem SARS-CoV-2–klucz do skutecznej terapii COVID-19. Farm. Pol. 2021, 77, 155–165. [Google Scholar]
- Chousterman, B.G.; Swirski, F.K.; Weber, G.F. Cytokine storm and sepsis disease pathogenesis. Semin. Immunopathol. 2017, 39, 517–528. [Google Scholar]
- Liu, Q.; Zhou, Y.H.; Yang, Z.Q. The cytokine storm of severe influenza and development of immunomodulatory therapy. Cell. Mol. Immunol. 2016, 13, 3–10. [Google Scholar] [PubMed]
- Channappanavar, R.; Perlman, S. Pathogenic human coronavirus infections: Causes and consequences of cytokine storm and immunopathology. Semin. Immunopathol. 2017, 39, 529–539. [Google Scholar] [PubMed]
- Liu, J.; Li, S.; Liu, J.; Liang, B.; Wang, X.; Wang, H.; Li, W.; Tong, Q.; Yi, J.; Zhao, L.; et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine 2020, 55, 102763. [Google Scholar]
- Wang, Y.; Wang, J.; Zheng, W.; Zhang, J.; Wang, J.; Jin, T.; Tao, P.; Wang, Y.; Liu, C.; Huang, J.; et al. Identification of an IL-1 receptor mutation driving autoinflammation directs IL-1-targeted drug design. Immunity 2023, 56, 1485–1501. [Google Scholar]
- Dinarello, C.A. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol. Rev. 2018, 281, 8–27. [Google Scholar]
- Broderick, L.; Hoffman, H.M. IL-1 and autoinflammatory disease: Biology, pathogenesis and therapeutic targeting. Nat. Rev. Rheumatol. 2022, 18, 448–463. [Google Scholar] [PubMed]
- McCullough, M.J.; Bose, P.G.; Mock, J.R. Regulatory T cells: Supporting lung homeostasis and promoting resolution and repair after lung injury. Int. J. Biochem. Cell Biol. 2024, 170, 106568. [Google Scholar] [CrossRef]
- Shin, D.S.; Ratnapriya, S.; Cashin, C.N.; Kuhn, L.F.; Rahimi, R.A.; Anthony, R.M.; Moon, J.J. Lung injury induces a polarized immune response by self-antigen-specific CD4+ Foxp3+ regulatory T cells. Cell Rep. 2023, 42, 112839. [Google Scholar]
- Griffith, J.W.; Faustino, L.D.; Cottrell, V.I.; Nepal, K.; Hariri, L.P.; Chiu, R.S.; Jones, M.C.; Julé, A.; Gabay, C.; Luster, A.D. Regulatory T cell-derived IL-1Ra suppresses the innate response to respiratory viral infection. Nat. Immunol. 2023, 24, 2091–2107. [Google Scholar]
- Faustino, L.D.; Griffith, J.W.; Rahimi, R.A.; Nepal, K.; Hamilos, D.L.; Cho, J.L.; Medoff, B.D.; Moon, J.J.; Vignali, D.A.A.; Luster, A.D. Interleukin-33 activates regulatory T cells to suppress innate γδ T cell responses in the lung. Nat. Immunol. 2020, 21, 1371–1383. [Google Scholar]
- De Benedetti, F.; Gattorno, M.; Anton, J.; Ben-Chetrit, E.; Frenkel, J.; Hoffman, H.M.; Koné-Paut, I.; Lachmann, H.J.; Ozen, S.; Simon, A.; et al. Canakinumab for the treatment of autoinflammatory recurrent fever syndromes. N. Engl. J. Med. 2018, 378, 1908–1919. [Google Scholar] [CrossRef] [PubMed]
- Garg, M.; de Jesus, A.A.; Chapelle, D.; Dancey, P.; Herzog, R.; Rivas-Chacon, R.; Muskardin, T.L.W.; Reed, A.; Reynolds, J.C.; Goldbach-Mansky, R.; et al. Rilonacept maintains long-term inflammatory remission in patients with deficiency of the IL-1 receptor antagonist. JCI Insight 2017, 2, e94838. [Google Scholar] [CrossRef]
- Klein, A.L.; Imazio, M.; Cremer, P.; Brucato, A.; Abbate, A.; Fang, F.; Insalaco, A.; LeWinter, M.; Lewis, B.S.; Lin, D.; et al. Phase 3 trial of interleukin-1 trap rilonacept in recurrent pericarditis. N. Engl. J. Med. 2021, 384, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Moghaddas, F.; Llamas, R.; De Nardo, D. A novel Pyrin-Associated Autoinflammation with Neutrophilic Dermatosis mutation further defines 14-3-3 binding of pyrin and distinction to Familial Mediterranean fever. Ann. Rheum. Dis. 2017, 76, 2085–2094. [Google Scholar] [CrossRef]
- Liu, X.; Lv, Z.; Wang, Q.; Yu, J.; Wang, J.; Zhou, Y.; Sui, M.; Hao, C.; Xue, D.; Zhang, Y. IL1RA mediated the effects of aspirin on COVID-19 severity: A Mendelian randomization study. J. Infect. 2023, 86, 410–411. [Google Scholar] [CrossRef]
- Kow, C.S.; Hasan, S.S. Use of antiplatelet drugs and the risk of mortality in patients with COVID-19: A meta-analysis. J. Thromb. Thrombolysis 2021, 52, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Dennis, E.A.; Norris, P.C. Eicosanoid storm in infection and inflammation. Nat. Rev. Immunol. 2015, 15, 511–523. [Google Scholar] [CrossRef]
- Palomo, J.; Dietrich, D.; Martin, P.; Palmer, G.; Gabay, C. The interleukin (IL)-1 cytokine family–Balance between agonists and antagonists in inflammatory diseases. Cytokine 2015, 76, 25–37. [Google Scholar] [CrossRef]
- Kalinina, O.; Golovkin, A.; Zaikova, E.; Aquino, A.; Bezrukikh, V.; Melnik, O.; Vasilieva, E.; Karonova, T.; Kudryavtsev, I.; Shlyakhto, E. Cytokine storm signature in patients with moderate and severe COVID-19. Int. J. Mol. Sci. 2022, 23, 8879. [Google Scholar] [CrossRef]
- Jacobs, L.M.C.; Wintjens, M.S.J.N.; Nagy, M.; Willems, L.; ten Cate, H.; Spronk, H.M.H.; van Kuijk, S.M.J.; Ghossein-Doha, C.; Netea, M.G.; Groh, L.A.; et al. Biomarkers of sustained systemic inflammation and microvascular dysfunction associated with post-COVID-19 condition symptoms at 24 months after SARS-CoV-2-infection. Front. Immunol. 2023, 14, 1182182. [Google Scholar] [CrossRef]
- Schultheiß, C.; Willscher, E.; Paschold, L.; Gottschick, C.; Klee, B.; Henkes, S.; Bosurgi, L.; Dutzmann, J.; Sedding, D.; Frese, T.; et al. The IL-1β, IL-6, and TNF cytokine triad is associated with post-acute sequelae of COVID-19. Cell Rep. Med. 2022, 3, 100663. [Google Scholar] [PubMed]
- Delorey, T.M.; Ziegler, C.G.K.; Heimberg, G.; Normand, R.; Yang, Y.; Segerstolpe, Å.; Abbondanza, D.; Fleming, S.J.; Subramanian, A.; Montoro, D.T.; et al. COVID-19 tissue atlases reveal SARS-CoV-2 pathology and cellular targets. Nature 2021, 595, 107–113. [Google Scholar] [CrossRef]
- Liao, M.; Liu, Y.; Yuan, J.; Wen, Y.; Xu, G.; Zhao, J.; Cheng, L.; Li, J.; Wang, X.; Wang, F.; et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat. Med. 2020, 26, 842–844. [Google Scholar] [PubMed]
- Kowis, R.; Badeau, R.; Kuennen, M. Prior sars-cov-2 infection does not increase risk of exertional heat stroke or cause detrimental changes in plasma cytokines. Int. J. Exerc. Sci. Conf. Proc. 2022, 16, 24. [Google Scholar]
- Darenskaya, M.A.; Kolesnikova, L.I.; Kolesnikov, S.I. COVID-19: Oxidative stress and the relevance of antioxidant therapy. Ann. Russ. Acad. Med. Sci. 2020, 75, 318–325. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–777. [Google Scholar] [CrossRef]
- Hosakote, Y.M.; Rayavara, K. Respiratory Syncytial Virus-Induced Oxidative Stress in Lung Pathogenesis. In Oxidative Stress in Lung Diseases; Springer: Singapore, 2020; pp. 297–330. [Google Scholar]
- Komaravelli, N.; Casola, A. Respiratory Viral Infections and Subversion of Cellular Antioxidant Defenses. J. Pharmacogenomics Pharmacoproteomics 2014, 5, 1000141. [Google Scholar]
- Pincemail, J.; Cavalier, E.; Charlier, C.; Cheramy–Bien, J.; Brevers, E.; Courtois, A.; Fadeur, M.; Meziane, S.; Le Goff, C.; Misset, B.; et al. Oxidative Stress Status in COVID-19 Patients Hospitalized in Intensive Care Unit for Severe Pneumonia. A Pilot Study. Antioxidants 2021, 10, 257. [Google Scholar] [CrossRef]
- Singer, P.; Blaser, A.R.; Berger, M.M.; Alhazzani, W.; Calder, P.C.; Casaer, M.P.; Hiesmayr, M.; Mayer, K.; Montejo, J.C.; Pichard, C.; et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin. Nutr. 2019, 38, 48–79. [Google Scholar]
- Carr, A.C.; Rosengrave, P.C.; Bayer, S.; Chambers, S.; Mehrtens, J.; Shaw, G.M. Hypovitaminosis C and vitamin C deficiency in critically ill patients despite recommended enteral and parenteral intakes. Crit. Care 2017, 21, 300. [Google Scholar]
- Erol, A. High-dose Intravenous Vitamin C Treatment for COVID-19. OSF Prepr. 2020, 10, e039519. [Google Scholar]
- Zhang, J.; Rao, X.; Li, Y.; Zhu, Y.; Liu, F.; Guo, G.; Luo, G.; Meng, Z.; De Backer, D.; Xiang, H.; et al. High-dose vitamin C infusion for the treatment of critically ill COVID-19. Res. Sq. 2020, 100, e25876. [Google Scholar]
- Mehri, F.; Rahbar, A.H.; Ghane, E.T.; Souri, B.; Esfahani, M. Changes in oxidative markers in COVID-19 patients. Arch. Med. Res. 2021, 52, 843–849. [Google Scholar] [PubMed]
- Chernyak, B.V.; Popova, E.N.; Prikhodko, A.S.; Grebenchikov, O.A.; Zinovkina, L.A.; Zinovkin, R.A. COVID-19 and Oxidative Stress. Biochemistry 2020, 85, 1543–1553. [Google Scholar] [CrossRef]
- Delgado-Roche, L.; Mesta, F. Oxidative stress as key player in severe acute respiratory syndrome coronavirus (SARS-CoV) infection. Arch. Med. Res. 2020, 51, 384–387. [Google Scholar] [CrossRef]
- Cecchini, R.; Cecchini, A.L. SARS-CoV-2 infection pathogenesis is related to oxidative stress as a response to aggression. Med. Hypotheses 2020, 143, 110102. [Google Scholar]
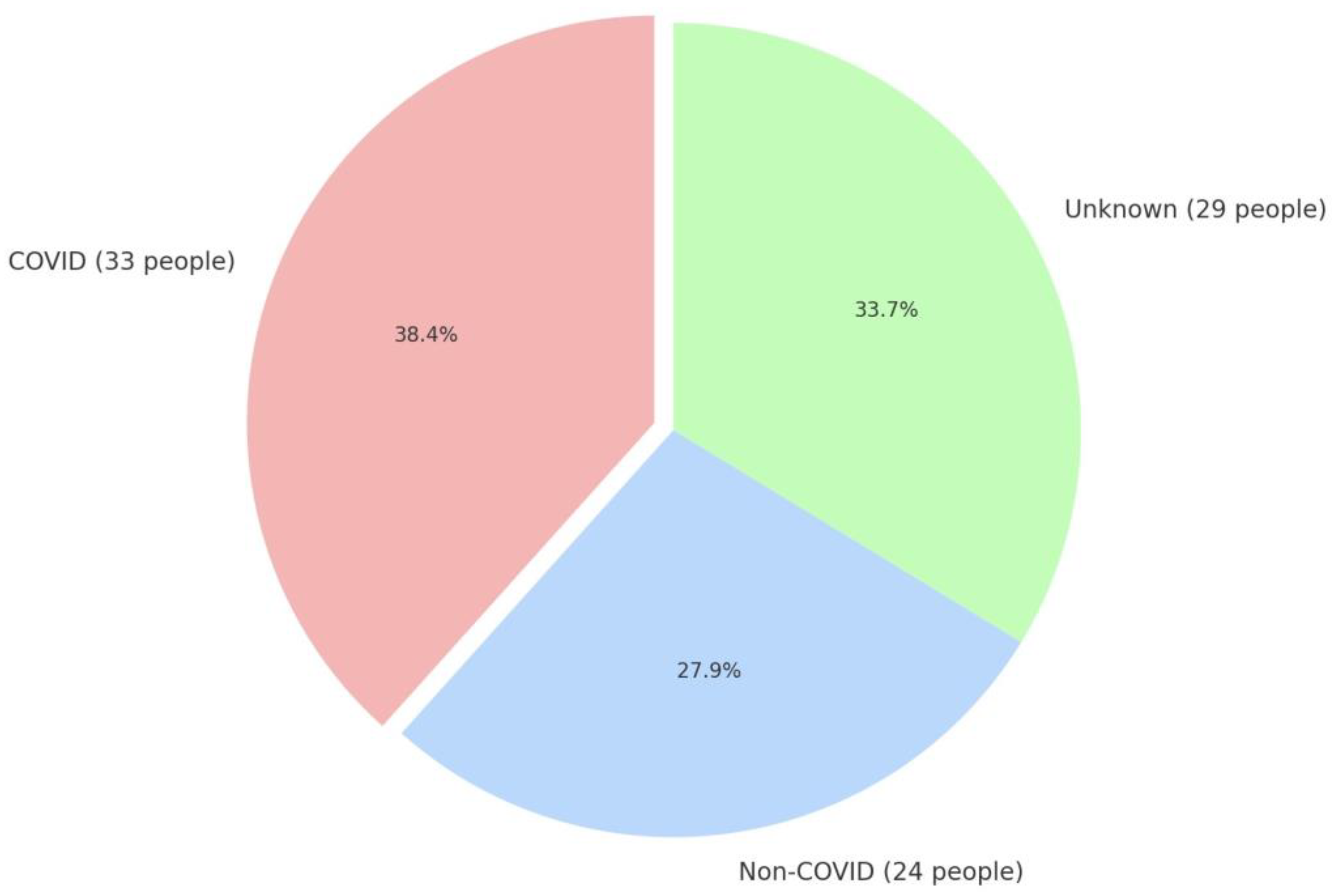


| Parameter | Non-COVID Group | COVID Group | p |
|---|---|---|---|
| ABUNDANCE | 24 | 33 | |
| GENDER | 0.516 * | ||
| F/M (%) | 13/11 (45.8/54.2) | 15/18 (45.5/54.5) | |
| Age [years] | 0.625 | ||
| Medium ± SD | 52.21 ± 9.38 | 50.97 ± 9.83 | |
| Median (min–max) | 48.5 (41.0–75.0) | 49.0 (35.0–86.0) | |
| Height [cm] | 0.184 | ||
| Medium ± SD | 169.46 ± 8.29 | 172.55 ± 9.54 | |
| Median (min–max) | 170.0 (156.0–186.0) | 174.0 (154.0–190.0) | |
| Weight [kg] | 0.547 | ||
| Medium ± SD | 77.17 ± 14.98 | 78.85 ± 15.29 | |
| Median (min–max) | 77.0 (53.0–115.0) | 82.0 (54.0–108.0) | |
| BMI [kg/m2] | 0.917 | ||
| Medium ± SD | 26.85 ± 4.99 | 26.31 ± 3.63 | |
| Median (min–max) | 26.03 (21.19–39.84) | 26.09 (19.94–34.09) | |
| Waist circumference [cm] | 0.943 | ||
| Medium ± SD | 91.45 ± 10.49 | 90.77 ± 10.06 | |
| Median (min–max) | 94.0 (76.0–111.0) | 92.0 (71.0–107.0) | |
| Smoking | 0.208 * | ||
| YES/NO (%) | 19/5 (79.2/20.8) | 30/3 (90.9/9.1) | |
| Alcohol consumption | 0.352 * | ||
| YES/NO (%) | 6/18 (25.0/75.0) | 5/28 (15.1/84.9) | |
| Physical activity | 0.254 * | ||
| Low (%) | 4(16.7) | 8 (24.2) | |
| Moderate (%) | 14 (58.3) | 22 (66.7) | |
| High (%) | 6 (25.0) | 3 (9.1) |
| Parameter | Non–COVID Group | COVID Group | p |
|---|---|---|---|
| AST [UL] | 0.540 | ||
| Medium ± SD | 20.66 ± 6.05 | 22.4 ± 10.21 | |
| Median (min–max) | 19.0 (13.0–35.0) | 21.0 (11.0–68.0) | |
| GGTP [U/L] | 0.924 | ||
| Medium ± SD | 27.5 ± 26.0 | 51.03 ± 148.3 | |
| Median (min–max) | 22.0 (6.0–135.0) | 19.0 (9.0–828.0) | |
| Cholesterol [mg/dL] | 0.355 | ||
| Medium ± SD | 209.22 ± 30.72 | 199.5 ± 33.25 | |
| Median (min–max) | 207.11 (157.6–281.88) | 204.42 (120.0–277.58) | |
| Cholesterol HDL [mg/dL] | 0.389 | ||
| Medium ± SD | 52.11 ± 13.51 | 53.9 ± 12.53 | |
| Median (min–max) | 45.4 (31.9–80.02) | 56.3 (29.13–80.7) | |
| Cholesterol nie–HDL [mg/dL] | 0.360 | ||
| Medium ± SD | 156.16 ± 36.41 | 146.71 ± 35.45 | |
| Median (min–max) | 155.15 (77.58–241.42) | 145.0 (77.74–248.45) | |
| Triglycerides [mg/dL] | 0.008 | ||
| Medium ± SD | 151.64 ± 70.21 | 148.34 ± 237.32 | |
| Median (min–max) | 140.7 (50.43–370.6) | 92.56 (43.25–1366.67) | |
| Creatinine [μmol/L] | 0.048 | ||
| Medium ± SD | 66.54 ± 10.77 | 74.23 ± 14.53 | |
| Median (min–max) | 64.88 (48.32–89.63) | 72.66 (49.21–109.07) | |
| Uric acid [μmol/L] | 0.965 | ||
| Medium ± SD | 298.98 ± 64.64 | 313.05 ± 97.36 | |
| Median (min–max) | 296.35 (161.6–474.6) | 288.9 (189.3–559.6) | |
| Glucose [mol/L] | 0.563 | ||
| Medium ± SD | 5.37 ± 0.64 | 5.52 ± 1.83 | |
| Median (min–max) | 5.38 (4.48–7.0) | 5.22 (4.48–14.92) |
| Parameter | Non-COVID Group | COVID Group | p |
|---|---|---|---|
| IL–1Ra | 0.004 | ||
| Medium ± SD | 173.87 ± 73.55 | 276.31 ± 170.34 | |
| Median (min–max) | 166.09 (73.95–368.64) | 214.72 (88.77–901.91) | |
| IL–1β | <0.001 | ||
| Medium ± SD | 5.68 ± 3.8 | 17.83 ± 15.47 | |
| Median (min–max) | 4.79 (1.42–15.35) | 13.88 (1.51–74.76) |
| Parameter | Non–COVID Group | COVID Group | p |
|---|---|---|---|
| CER mg/dL | 0.179 | ||
| Medium ± SD | 46.49 ± 12.79 | 49.61 ± 14.04 | |
| Median (min–max) | 45.25 (32.27–95.11) | 48.72 (16.18–85.09) | |
| SH µmol/L | 0.812 | ||
| Medium ± SD | 190.9 ± 51.58 | 185.86 ± 58.82 | |
| Median (min–max) | 191.26 (71.09–282.27) | 195.69 (0–281.65) | |
| SH µmol/g proteins | 0.454 | ||
| Medium ± SD | 2.31 ± 0.6 | 2.2 ± 0.65 | |
| Median (min–max) | 2.31 (0.82–3.31) | 2.27 (0–3.14) | |
| TOS µmol/L | 0.153 | ||
| Medium ± SD | 141.81 ± 101.29 | 108.92 ± 92.04 | |
| Median (min–max) | 114.97 (15.3–351.7) | 74.7 (12.36–323.23) | |
| LPH µmol/L | 0.114 | ||
| Medium ± SD | 40.7 ± 27.48 | 30.25 ± 21.07 | |
| Median (min–max) | 34.66 (4.62–123.68) | 23.17 (6.48–94.5) | |
| TAC mmol/L | 0.495 | ||
| Medium ± SD | 1.11 ± 0.15 | 1.12 ± 0.18 | |
| Median (min–max) | 1.11 (0.77–1.57) | 1.14 (0.7–1.48) | |
| SOD NU/mL | 0.675 | ||
| Medium ± SD | 20.47 ± 1.79 | 20.29 ± 2.75 | |
| Median (min–max) | 20.82 (16.25–23.44) | 20.4 (10.02–28.41) | |
| MnSOD NU/mL | 0.749 | ||
| Medium ± SD | 10.07 ± 1.69 | 9.67 ± 3.48 | |
| Median (min–max) | 10.19 (7.34–13.11) | 10.34 (–8.17–12.37) | |
| CuZnSOD NU/mL | 0.749 | ||
| Medium ± SD | 10.39 ± 1.54 | 10.62 ± 2.56 | |
| Median (min–max) | 10.52 (7.83–14.25) | 10.1 (7.07–18.67) | |
| LPS RF | 0.812 | ||
| Medium ± SD | 350.89 ± 124.17 | 365.03 ± 134.06 | |
| Median (min–max) | 349.78 (152.17–655.08) | 345.53 (152.17–719.41) | |
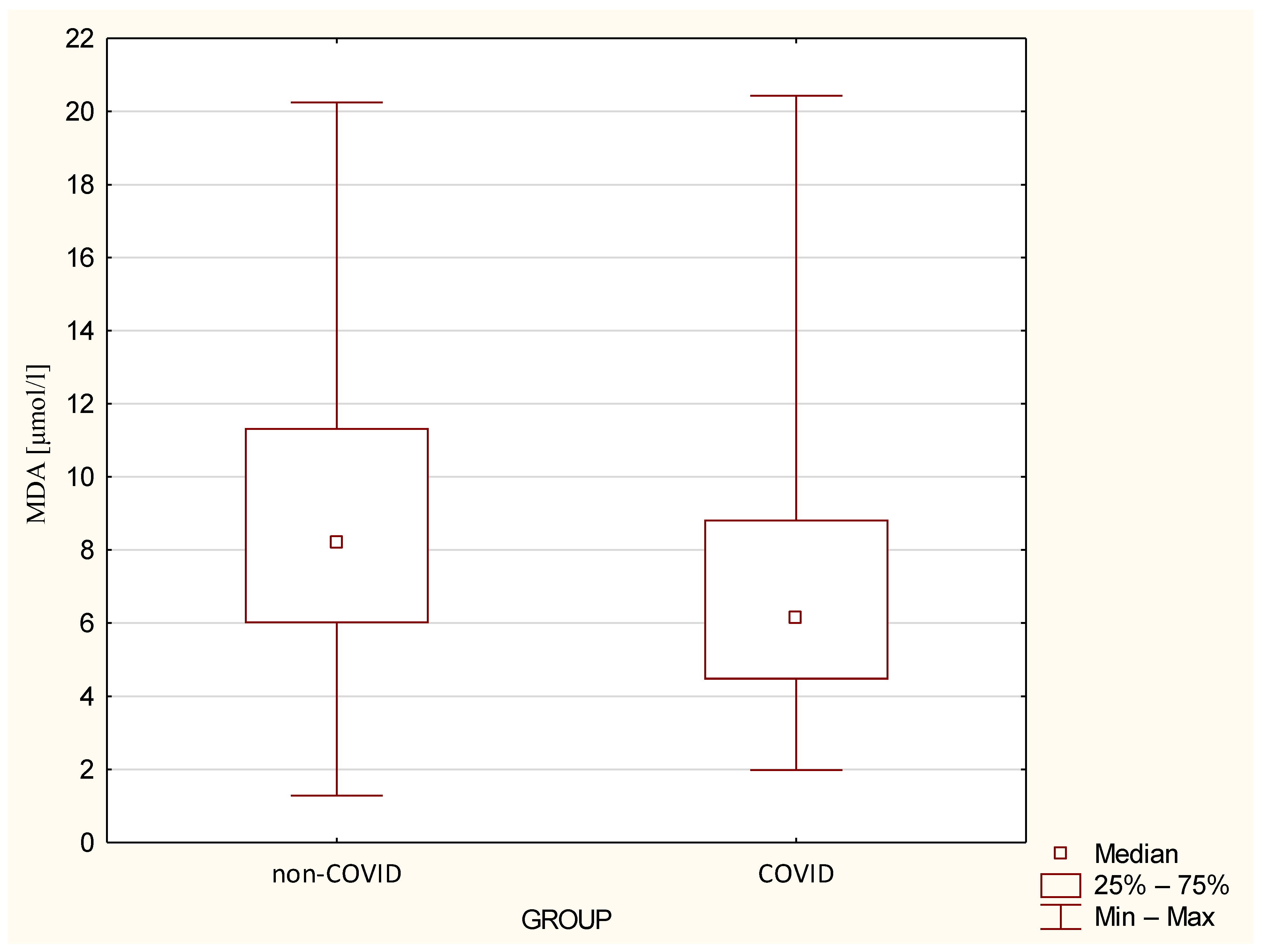
| MDA µmol/L | 0.037 | ||
| Medium ± SD | 9.03 ± 4.69 | 6.77 ± 40 | |
| Median (min–max) | 8.2 (1.28–20.25) | 6.15 (1.98–20.43) | |
| AGE10 [µg/mL] | 0.442 | ||
| Medium ± SD | 782.24 ± 361.43 | 810.89 ± 614.21 | |
| Median (min–max) | 748.05 (226.35–1726.8) | 568.65 (40.65–2311.8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marczewska, A.; Wojciechowska, C.; Marczewski, K.; Gospodarczyk, N.; Dolibog, P.; Czuba, Z.; Wróbel, K.; Zalejska-Fiolka, J. Elevated Levels of IL–1Ra, IL–1β, and Oxidative Stress in COVID-19: Implications for Inflammatory Pathogenesis. J. Clin. Med. 2025, 14, 2489. https://doi.org/10.3390/jcm14072489
Marczewska A, Wojciechowska C, Marczewski K, Gospodarczyk N, Dolibog P, Czuba Z, Wróbel K, Zalejska-Fiolka J. Elevated Levels of IL–1Ra, IL–1β, and Oxidative Stress in COVID-19: Implications for Inflammatory Pathogenesis. Journal of Clinical Medicine. 2025; 14(7):2489. https://doi.org/10.3390/jcm14072489
Chicago/Turabian StyleMarczewska, Alicja, Celina Wojciechowska, Kamil Marczewski, Natalia Gospodarczyk, Paweł Dolibog, Zenon Czuba, Karolina Wróbel, and Jolanta Zalejska-Fiolka. 2025. "Elevated Levels of IL–1Ra, IL–1β, and Oxidative Stress in COVID-19: Implications for Inflammatory Pathogenesis" Journal of Clinical Medicine 14, no. 7: 2489. https://doi.org/10.3390/jcm14072489
APA StyleMarczewska, A., Wojciechowska, C., Marczewski, K., Gospodarczyk, N., Dolibog, P., Czuba, Z., Wróbel, K., & Zalejska-Fiolka, J. (2025). Elevated Levels of IL–1Ra, IL–1β, and Oxidative Stress in COVID-19: Implications for Inflammatory Pathogenesis. Journal of Clinical Medicine, 14(7), 2489. https://doi.org/10.3390/jcm14072489





