Non-Curative Endoscopic Submucosal Dissection: Current Concepts, Pitfalls and Future Perspectives
Abstract
1. Introduction
2. The Development of ESD and Its Establishment as a Mainstay Treatment for Gastrointestinal Early Neoplasia
3. ESD Along the Gastrointestinal Tract
3.1. Esophagus
3.2. Stomach
3.3. Colon and Rectum
4. The Problematic of the Non-Curative ESDs (NC-ESD)
4.1. NC-ESD Definition
- (a)
- For esophageal SCC, curability criteria may be stricter, due to the probable higher risk of LNM for the same staging comparing to other organs. Japanese and European guidelines consider an en bloc and R0 resection of a pT1a SCC without lymphovascular invasion curative, particularly if limited to the epithelium or lamina propria (m1 or m2); no evidence-based recommendation could be made for pT1a with muscularis mucosa invasion (m3), but generally no additional treatment is warranted, particularly in smaller lesions. There is no consensus regarding pT1bSM1 SCC: in well-differentiated lesions smaller than 2 cm without other risk criteria, the rate of LNM may be very small, so endoscopic follow-up (after proper staging) may be sufficient (LRR); nevertheless, Japanese guidelines suggest complementary treatment. Whenever other high-risk criteria are present (lymphovascular invasion, deep submucosal invasion, or positive vertical margin), adjuvant treatment is highly recommended.
- (b)
- Regarding gastric neoplasia, a large study on surgical specimens from Japan found and validated a scoring system that aimed to help decision-making after an NC-ESD [47]. This score, the “eCura system”, included five risk factors for the development of LNM found in the ESD specimens, and weighed them according to the relative risk: three points for lymphatic permeation and one point each for lesion size above 30 mm, positive vertical margins, venous invasion and submucosal invasion equal or above 500 μm. Patients were categorized in three groups: low-risk group (0–1 point, 2.5% risk of LNM), intermediate group (2–4 points, 6.7% risk), and high-risk group (5–7 points, 22.7% risk). Accordingly, lesions removed in an en bloc fashion that follows one of these conditions are considered curative (eCuraA curative resection according to Japanese Guidelines and VLRR or LRR resections in European Guidelines): (1) Predominantly differentiated type, intramucosal (pT1a), non-ulcerated, with free horizontal and vertical margins and without lymphovascular invasion, regardless of the size; (2) Predominantly undifferentiated type, measuring 2 cm or less, intramucosal (pT1a), non-ulcerated, with free horizontal and vertical margins and without lymphovascular invasion; (3) Ulcerated, measuring 3 cm or less, predominantly differentiated type, intramucosal (pT1a), with free horizontal and vertical margins and without lymphovascular invasion. Lesions with superficial submucosal invasion (pT1bSM1), measuring 3 cm or less, predominantly differentiated type, with free horizontal and vertical margins and without lymphovascular invasion would also probably be curative (Japanese eCuraB, European LRR). If these criteria are not fulfilled, those will be non-curative resections and the likelihood of remnant lesion is high (eCuraC). If the only criterion among differentiated lesions that was not considered for being included in eCuraA or eCuraB was positive horizontal margin or piecemeal resection, these are eCuraC-1 lesions, and endoscopic follow-up could be considered due to a low risk of LNM, provided that the submucosal invasive part of the lesion was en bloc resected and with free margins, and the lesion was not ulcerated (European LocRR). All the others are eCuraC-2 lesions (European HRR) and complementary treatment is warranted (Table 2).
- (c)
- Regarding colorectal lesions, ESD resections of benign lesions are curative if removed en bloc and R0 (VLRR); the others (piecemeal-resected or with positive horizontal margin, LocRR) should be managed by endoscopy. T1 (submucosal) carcinomas are considered radically removed if the following conditions are satisfied: free vertical margins, papillary or tubular adenocarcinoma, SM1 invasion, no lymphovascular invasion, and low-grade tumor budding (LRR). Endoscopic follow-up and treatment may be sufficient if removed in piecemeal or with positive horizontal margins (of a benign component—LocRR). Surgery is usually recommended if high-risk criteria are present (HRR), with the possible exception of deep submucosal invasion as the sole criterion, which may carry a low risk of LNM [48,49].
4.2. The Management of NC-ESD
4.2.1. Esophagus
4.2.2. Stomach
4.2.3. Colon and Rectum
5. New Technologies and Future Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EMR | Endoscopic Mucosal Resection |
| ESD | Endoscopic submucosal dissection |
| LNM | Lymph node metastasis |
| LST | Lateral Spreading Tumor |
| NC-ESD | Non-curative ESD |
| POEM | PerOral Endoscopic Myotomy |
| SCC | Squamous cell carcinoma |
References
- Globocan. Global Cancer Observatory. 2020. Available online: http://gco.iarc.fr/ (accessed on 1 December 2024).
- Hirao, M.; Masuda, K.; Asanuma, T.; Naka, H.; Noda, K.; Matsuura, K.; Yamaguchi, O.; Ueda, N. Endoscopic resection of early gastric cancer and other tumors with local injection of hypertonic saline-epinephrine. Gastrointest. Endosc. 1988, 34, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, K.; Yoshida, S. Recent advances in endoscopic mucosal resection for early gastric cancer. Gan Kagaku Ryoho 1998, 25, 476–483. [Google Scholar]
- Gotoda, T.; Kondo, H.; Ono, H.; Saito, Y.; Yamaguchi, H.; Saito, D.; Yokota, T. A new endoscopic mucosal resection procedure using an insulation-tipped electrosurgical knife for rectal flat lesions: Report of two cases. Gastrointest. Endosc. 1999, 50, 560–563. [Google Scholar] [CrossRef]
- Ono, H.; Kondo, H.; Gotoda, T.; Shirao, K.; Yamaguchi, H.; Saito, D.; Hosokawa, K.; Shimoda, T.; Yoshida, S. Endoscopic mucosal resection for treatment of early gastric cancer. Gut 2001, 48, 225–229. [Google Scholar] [CrossRef]
- Oyama, T.; Kikuchi, Y. Aggressive endoscopic mucosal resection in the upper GI tract—Hook knife EMR method. Minim. Invasive Ther. Allied Technol. 2002, 11, 291–295. [Google Scholar] [CrossRef]
- Oyama, T.; Tomori, A.; Hotta, K.; Morita, S.; Kominato, K.; Tanaka, M.; Miyata, Y. Endoscopic submucosal dissection of early esophageal cancer. Clin. Gastroenterol. Hepatol. 2005, 3 (Suppl. S1), S67–S70. [Google Scholar] [CrossRef]
- Yahagi, N.; Fujishiro, M.; Kakushima, N.; Kobayashi, K.; Hashimoto, T.; Oka, M.; Iguchi, M.; Enomoto, S.; Ichinose, M.; Niwa, H.; et al. Endoscopic submucosal dissection for early gastric cancer using the tip of an electrosurgical snare (thin type). Dig. Endosc. 2004, 16, 34–38. [Google Scholar] [CrossRef]
- Yahagi, N.; Fujishiro, M.; Imagawa, A.; Kakushima, N.; Iguchi, M.; Omata, M. Endoscopic submucosal dissection for the reliable en bloc resection of colorectal mucosal tumors. Dig. Endosc. 2004, 16, S89–S92. [Google Scholar] [CrossRef]
- Yahagi, N.; Uraoka, T.; Ida, Y.; Hosoe, N.; Nakamura, R.; Kitagawa, Y.; Ogata, H.; Hibi, T. Endoscopic submucosal dissection using the Flex and the Dual knives. Tech. Gastrointest. Endosc. 2011, 13, 74–78. [Google Scholar] [CrossRef]
- Berr, F.; Ponchon, T.; Neureiter, D.; Kiesslich, T.; Haringsma, J.; Kaehler, G.F.; Schmoll, F.; Messmann, H.; Yahagi, N.; Oyama, T. Experimental endoscopic submucosal dissection training in a porcine model: Learning experience of skilled Western endoscopists. Dig. Endosc. 2011, 23, 281–289. [Google Scholar] [CrossRef]
- Pimentel-Nunes, P.; Dinis-Ribeiro, M.; Soares, J.B.; Marcos-Pinto, R.; Santos, C.; Rolanda, C.; Bastos, R.P.; Areia, M.; Afonso, L.; Bergman, J.; et al. A multicenter validation of anendoscopic classification with narrow band imagingfor gastric precancerous and cancerous lesions. Endoscopy 2012, 44, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, R.; Arima, M.; Iizuka, T.; Oyama, T.; Katada, C.; Kato, M.; Goda, K.; Goto, O.; Tanaka, K.; Yano, T.; et al. Endoscopic submucosal dissection/endoscopic mucosal resection guidelines for esophageal cancer. Dig. Endosc. 2020, 32, 452–493. [Google Scholar] [CrossRef] [PubMed]
- Beaufort, I.N.; Frederiks, C.N.; Overwater, A.; Brosens, L.A.A.; Koch, A.D.; Pouw, R.E.; Bergman, J.; Weusten, B. Endoscopic submucosal dissection for early esophageal squamous cell carcinoma: Long-term results from a Western cohort. Endoscopy 2024, 56, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Probst, A.; Ebigbo, A.; Eser, S.; Fleischmann, C.; Schaller, T.; Markl, B.; Schiele, S.; Geissler, B.; Muller, G.; Messmann, H. Endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma: Long-term follow-up in a Western center. Clin. Endosc. 2023, 56, 55–64. [Google Scholar] [CrossRef]
- Rodriguez de Santiago, E.; van Tilburg, L.; Deprez, P.H.; Pioche, M.; Pouw, R.E.; Bourke, M.J.; Seewald, S.; Weusten, B.; Jacques, J.; Leblanc, S.; et al. Western outcomes of circumferential endoscopic submucosal dissection for early esophageal squamous cell carcinoma. Gastrointest. Endosc. 2024, 99, 511–524.e6. [Google Scholar] [CrossRef]
- Mejia Perez, L.K.; Yang, D.; Draganov, P.V.; Jawaid, S.; Chak, A.; Dumot, J.; Alaber, O.; Vargo, J.J.; Jang, S.; Mehta, N.; et al. Endoscopic submucosal dissection vs. endoscopic mucosal resection for early Barrett’s neoplasia in the West: A retrospective study. Endoscopy 2022, 54, 439–446. [Google Scholar] [CrossRef]
- Terheggen, G.; Horn, E.M.; Vieth, M.; Gabbert, H.; Enderle, M.; Neugebauer, A.; Schumacher, B.; Neuhaus, H. A randomised trial of endoscopic submucosal dissection versus endoscopic mucosal resection for early Barrett’s neoplasia. Gut 2017, 66, 783–793. [Google Scholar] [CrossRef]
- Zullo, A.; Manta, R.; De Francesco, V.; Manfredi, G.; Buscarini, E.; Fiorini, G.; Vaira, D.; Marmo, R. Endoscopic submucosal dissection of gastric neoplastic lesions in Western countries: Systematic review and meta-analysis. Eur. J. Gastroenterol. Hepatol. 2021, 33, e1–e6. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, C. Long-Term Clinical Efficacy and Perioperative Safety of Endoscopic Submucosal Dissection versus Endoscopic Mucosal Resection for Early Gastric Cancer: An Updated Meta-Analysis. Biomed. Res. Int. 2018, 2018, 3152346. [Google Scholar] [CrossRef]
- Tao, M.; Zhou, X.; Hu, M.; Pan, J. Endoscopic submucosal dissection versus endoscopic mucosal resection for patients with early gastric cancer: A meta-analysis. BMJ Open 2019, 9, e025803. [Google Scholar] [CrossRef]
- Ono, H.; Yao, K.; Fujishiro, M.; Oda, I.; Nimura, S.; Yahagi, N.; Iishi, H.; Oka, M.; Ajioka, Y.; Ichinose, M.; et al. Guidelines for endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer. Dig. Endosc. 2016, 28, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Ono, H.; Yao, K.; Fujishiro, M.; Oda, I.; Uedo, N.; Nimura, S.; Yahagi, N.; Iishi, H.; Oka, M.; Ajioka, Y.; et al. Guidelines for endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer (second edition). Dig. Endosc. 2021, 33, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Bestetti, A.M.; de Moura, D.T.H.; Proenca, I.M.; Junior, E.; Ribeiro, I.B.; Sasso, J.; Kum, A.S.T.; Sanchez-Luna, S.A.; Bernardo, W.M.; de Moura, E.G.H. Endoscopic Resection Versus Surgery in the Treatment of Early Gastric Cancer: A Systematic Review and Meta-Analysis. Front. Oncol. 2022, 12, 939244. [Google Scholar] [CrossRef]
- Yang, H.J.; Kim, J.H.; Kim, N.W.; Choi, I.J. Comparison of long-term outcomes of endoscopic submucosal dissection and surgery for undifferentiated-type early gastric cancer meeting the expanded criteria: A systematic review and meta-analysis. Surg. Endosc. 2022, 36, 3686–3697. [Google Scholar] [CrossRef]
- Jiao, J.; Li, H.; Shang, L.; Ren, H.; Ye, C.; Zhang, R.; Xiao, K.; Dong, K.; Liu, J.; Li, L. Impact of preceding noncurative endoscopic submucosal dissection on patients with early gastric cancer who undergo subsequent surgery: A meta-analysis. Expert. Rev. Gastroenterol. Hepatol. 2022, 16, 373–382. [Google Scholar] [CrossRef]
- Libanio, D.; Ortigao, R.; Pimentel-Nunes, P.; Dinis-Ribeiro, M. Improving the Diagnosis and Treatment of Early Gastric Cancer in the West. GE Port. J. Gastroenterol. 2022, 29, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Ngamruengphong, S.; Ferri, L.; Aihara, H.; Draganov, P.V.; Yang, D.J.; Perbtani, Y.B.; Jue, T.L.; Munroe, C.A.; Boparai, E.S.; Mehta, N.A.; et al. Efficacy of Endoscopic Submucosal Dissection for Superficial Gastric Neoplasia in a Large Cohort in North America. Clin. Gastroenterol. Hepatol. 2021, 19, 1611–1619.e1. [Google Scholar] [CrossRef]
- Tate, D.J.; Klein, A.; Sidhu, M.; Desomer, L.; Awadie, H.; Lee, E.Y.T.; Mahajan, H.; McLeod, D.; Bourke, M.J. Endoscopic submucosal dissection for suspected early gastric cancer: Absolute versus expanded criteria in a large Western cohort (with video). Gastrointest. Endosc. 2019, 90, 467–479.e4. [Google Scholar] [CrossRef]
- Libanio, D.; Braga, V.; Ferraz, S.; Castro, R.; Lage, J.; Pita, I.; Ribeiro, C.; De Sousa, J.A.; Dinis-Ribeiro, M.; Pimentel-Nunes, P. Prospective comparative study of endoscopic submucosal dissection and gastrectomy for early neoplastic lesions including patients’ perspectives. Endoscopy 2019, 51, 30–39. [Google Scholar] [CrossRef]
- Moss, A.; Williams, S.J.; Hourigan, L.F.; Brown, G.; Tam, W.; Singh, R.; Zanati, S.; Burgess, N.G.; Sonson, R.; Byth, K.; et al. Long-term adenoma recurrence following wide-field endoscopic mucosal resection (WF-EMR) for advanced colonic mucosal neoplasia is infrequent: Results and risk factors in 1000 cases from the Australian Colonic EMR (ACE) study. Gut 2015, 64, 57–65. [Google Scholar] [CrossRef]
- Yang, D.; Draganov, P.V.; King, W.; Liu, N.; Sarheed, A.; Bhat, A.; Jiang, P.; Ladna, M.; Ruiz, N.C.; Wilson, J.; et al. Margin marking before colorectal endoscopic mucosal resection and its impact on neoplasia recurrence (with video). Gastrointest. Endosc. 2022, 95, 956–965. [Google Scholar] [CrossRef]
- Brooker, J.C.; Saunders, B.P.; Shah, S.G.; Thapar, C.J.; Suzuki, N.; Williams, C.B. Treatment with argon plasma coagulation reduces recurrence after piecemeal resection of large sessile colonic polyps: A randomized trial and recommendations. Gastrointest. Endosc. 2002, 55, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Chandan, S.; Facciorusso, A.; Ramai, D.; Deliwala, S.; Mohan, B.P.; Kassab, L.L.; Draganov, P.V.; Othman, M.O.; Kochhar, G.S. Snare tip soft coagulation (STSC) after endoscopic mucosal resection (EMR) of large (>20 mm) non pedunculated colorectal polyps: A systematic review and meta-analysis. Endosc. Int. Open 2022, 10, E74–E81. [Google Scholar] [CrossRef] [PubMed]
- Yamashina, T.; Uedo, N.; Akasaka, T.; Iwatsubo, T.; Nakatani, Y.; Akamatsu, T.; Kawamura, T.; Takeuchi, Y.; Fujii, S.; Kusaka, T.; et al. Comparison of Underwater vs Conventional Endoscopic Mucosal Resection of Intermediate-Size Colorectal Polyps. Gastroenterology 2019, 157, 451–461.e2. [Google Scholar] [CrossRef]
- Fuccio, L.; Hassan, C.; Ponchon, T.; Mandolesi, D.; Farioli, A.; Cucchetti, A.; Frazzoni, L.; Bhandari, P.; Bellisario, C.; Bazzoli, F.; et al. Clinical outcomes after endoscopic submucosal dissection for colorectal neoplasia: A systematic review and meta-analysis. Gastrointest. Endosc. 2017, 86, 74–86.e17. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Kashida, H.; Saito, Y.; Yahagi, N.; Yamano, H.; Saito, S.; Hisabe, T.; Yao, T.; Watanabe, M.; Yoshida, M.; et al. Japan Gastroenterological Endoscopy Society guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection. Dig. Endosc. 2020, 32, 219–239. [Google Scholar] [CrossRef]
- Schlemper, R.J.; Itabashi, M.; Kato, Y.; Lewin, K.J.; Riddell, R.H.; Shimoda, T.; Sipponen, P.; Stolte, M.; Watanabe, H. Differences in the diagnostic criteria used by Japanese and Western pathologists to diagnose colorectal carcinoma. Cancer 1998, 82, 60–69. [Google Scholar] [CrossRef]
- Pioche, M.; Rivory, J.; Jeremie, J. Colorectal endoscopic submucosal dissection for all LSTs: Histological information loss due to piecemeal EMR is no longer acceptable. Endosc. Int. Open 2019, 7, E1195–E1196. [Google Scholar] [CrossRef]
- Yoshida, N.; Naito, Y.; Murakami, T.; Hirose, R.; Ogiso, K.; Inada, Y.; Rani, R.A.; Kishimoto, M.; Nakanishi, M.; Itoh, Y. Tips for safety in endoscopic submucosal dissection for colorectal tumors. Ann. Transl. Med. 2017, 5, 185. [Google Scholar] [CrossRef]
- Yoshida, N.; Wakabayashi, N.; Kanemasa, K.; Sumida, Y.; Hasegawa, D.; Inoue, K.; Morimoto, Y.; Kashiwa, A.; Konishi, H.; Yagi, N.; et al. Endoscopic submucosal dissection for colorectal tumors: Technical difficulties and rate of perforation. Endoscopy 2009, 41, 758–761. [Google Scholar] [CrossRef]
- Yoshida, N.; Yagi, N.; Inada, Y.; Kugai, M.; Yanagisawa, A.; Naito, Y. Prevention and management of complications of and training for colorectal endoscopic submucosal dissection. Gastroenterol. Res. Pract. 2013, 2013, 287173. [Google Scholar] [CrossRef] [PubMed]
- Tidehag, V.; Tornqvist, B.; Pekkari, K.; Marsk, R. Endoscopic submucosal dissection for removal of large colorectal neoplasias in an outpatient setting: A single-center series of 660 procedures in Sweden. Gastrointest. Endosc. 2022, 96, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Stephane, S.; Timothee, W.; Jeremie, A.; Raphael, O.; Martin, D.; Emmanuelle, P.; Elodie, L.; Quentin, D.; Nikki, C.; Sonia, B.; et al. Endoscopic submucosal dissection or piecemeal endoscopic mucosal resection for large superficial colorectal lesions: A cost effectiveness study. Clin. Res. Hepatol. Gastroenterol. 2022, 46, 101969. [Google Scholar] [CrossRef]
- Hong, K.H.; Shin, S.J.; Kim, J.H. Learning curve for endoscopic submucosal dissection of gastric neoplasms. Eur. J. Gastroenterol. Hepatol. 2014, 26, 949–954. [Google Scholar] [CrossRef]
- Yoshida, M.; Kakushima, N.; Mori, K.; Igarashi, K.; Kawata, N.; Tanaka, M.; Takizawa, K.; Ito, S.; Imai, K.; Hotta, K.; et al. Learning curve and clinical outcome of gastric endoscopic submucosal dissection performed by trainee operators. Surg. Endosc. 2017, 31, 3614–3622. [Google Scholar] [CrossRef]
- Hatta, W.; Gotoda, T.; Oyama, T.; Kawata, N.; Takahashi, A.; Yoshifuku, Y.; Hoteya, S.; Nakagawa, M.; Hirano, M.; Esaki, M.; et al. A Scoring System to Stratify Curability after Endoscopic Submucosal Dissection for Early Gastric Cancer: “eCura system”. Am. J. Gastroenterol. 2017, 112, 874–881. [Google Scholar] [CrossRef] [PubMed]
- Nakadoi, K.; Tanaka, S.; Kanao, H.; Terasaki, M.; Takata, S.; Oka, S.; Yoshida, S.; Arihiro, K.; Chayama, K. Management of T1 colorectal carcinoma with special reference to criteria for curative endoscopic resection. J. Gastroenterol. Hepatol. 2012, 27, 1057–1062. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, S.; Nojima, M.; Nosho, K.; Omori, S.; Kusumi, T.; Okuda, H.; Tsukagoshi, H.; Fujita, M.; Yamamoto, H.; Hosokawa, M. Factors associated with risk for colorectal cancer recurrence after endoscopic resection of T1 tumors. Clin. Gastroenterol. Hepatol. 2014, 12, 292–302.e3. [Google Scholar] [CrossRef]
- Haasnoot, K.; Baldaque-Silva, F.; Koch, A.D.; Ferreira, M.F.; Santos-Antunes, J.; Dias, E.; Omae, M.; van Tilburg, L.; Dang, H.; Lemmers, A.; et al. Low risk of local recurrence after a successful en bloc Endoscopic Submucosal Dissection for non-invasive colorectal lesions with positive horizontal resection margins(R-ESD study). Endoscopy 2023, 55, 245–251. [Google Scholar] [CrossRef]
- Spadaccini, M.; Bourke, M.J.; Maselli, R.; Pioche, M.; Bhandari, P.; Jacques, J.; Haji, A.; Yang, D.; Albeniz, E.; Kaminski, M.F.; et al. Clinical outcome of non-curative endoscopic submucosal dissection for early colorectal cancer. Gut 2022, 71, 1998–2004. [Google Scholar] [CrossRef]
- Morais, R.; Libanio, D.; Ribeiro, M.D.; Ferreira, A.; Barreiro, P.; Bourke, M.J.; Gupta, S.; Amaro, P.; Magalhaes, R.K.; Cecinato, P.; et al. Predicting residual neoplasia after a non-curative gastric ESD: Validation and modification of the eCura system in the Western setting: The W-eCura score. Gut 2023, 73, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Santos-Antunes, J.; Pioche, M.; Ramos-Zabala, F.; Cecinato, P.; Gallego, F.; Barreiro, P.; Mascarenhas, A.; Sferrazza, S.; Berr, F.; Wagner, A.; et al. Risk of Residual Neoplasia after a Local-Risk Resection of Colorectal Lesions by Endoscopic Submucosal Dissection: A Multinational Study. J. Clin. Med. 2023, 12, 5356. [Google Scholar] [CrossRef] [PubMed]
- Santos-Antunes, J.; Pioche, M.; Ramos-Zabala, F.; Cecinato, P.; Rojo, F.J.G.; Barreiro, P.; Felix, C.; Sferrazza, S.; Berr, F.; Wagner, A.; et al. Risk of residual neoplasia after a noncurative colorectal endoscopic submucosal dissection for malignant lesions: A multinational study. Endoscopy 2023, 55, 235–244. [Google Scholar] [CrossRef]
- Morais, R.; Libanio, D.; Santos-Antunes, J.; Group, N.-E.S. eCura and W-eCura: Different scores, different populations, same goal. Gut 2024, 73, e29. [Google Scholar] [CrossRef] [PubMed]
- Santos-Antunes, J.; Berr, F.; Pioche, M.; Ramos-Zabala, F.; Cecinato, P.; Gallego, F.; Barreiro, P.; Felix, C.; Sferrazza, S.; Wagner, A.; et al. Deep submucosal invasion as a risk factor for recurrence after endoscopic submucosal dissection for T1 colorectal cancer. Endoscopy 2023, 55, 881–882. [Google Scholar] [CrossRef]
- Lee, J.W.; Cho, C.J.; Kim, D.H.; Ahn, J.Y.; Lee, J.H.; Choi, K.D.; Song, H.J.; Park, S.R.; Lee, H.J.; Kim, Y.H.; et al. Long-Term Survival and Tumor Recurrence in Patients with Superficial Esophageal Cancer after Complete Non-Curative Endoscopic Resection: A Single-Center Case Series. Clin. Endosc. 2018, 51, 470–477. [Google Scholar] [CrossRef]
- Flor de Lima, M.; Castro, B.; Rodriguez-Carrasco, M.; Libanio, D.; Pimentel-Nunes, P.; Sousa, O.; Dinis-Ribeiro, M. Best additional management after non-curative endoscopic resection of esophageal squamous cell carcinoma: A systematic review and meta-analysis. Scand. J. Gastroenterol. 2022, 57, 525–533. [Google Scholar] [CrossRef]
- Kanie, Y.; Okamura, A.; Asari, T.; Maruyama, S.; Sakamoto, K.; Fujiwara, D.; Kanamori, J.; Imamura, Y.; Ishiyama, A.; Yoshio, T.; et al. Additional Treatment Following Noncurative Endoscopic Resection for Esophageal Squamous Cell Carcinoma: A Comparison of Outcomes between Esophagectomy and Chemoradiotherapy. Ann. Surg. Oncol. 2021, 28, 8428–8435. [Google Scholar] [CrossRef]
- Minashi, K.; Nihei, K.; Mizusawa, J.; Takizawa, K.; Yano, T.; Ezoe, Y.; Tsuchida, T.; Ono, H.; Iizuka, T.; Hanaoka, N.; et al. Efficacy of Endoscopic Resection and Selective Chemoradiotherapy for Stage I Esophageal Squamous Cell Carcinoma. Gastroenterology 2019, 157, 382–390.e3. [Google Scholar] [CrossRef]
- van Tilburg, L.; Verheij, E.P.D.; van de Ven, S.E.M.; van Munster, S.N.; Weusten, B.; Herrero, L.A.; Nagengast, W.B.; Schoon, E.J.; Alkhalaf, A.; Bergman, J.J.G.H.M.; et al. Vertical tumor-positive resection margins and the risk of residual neoplasia after endoscopic resection of Barrett’s neoplasia: A nationwide cohort with pathology reassessment. Endoscopy 2024, 56, 559–568. [Google Scholar] [CrossRef]
- Othman, M.O.; Bahdi, F.; Ahmed, Y.; Gagneja, H.; Andrawes, S.; Groth, S.; Dhingra, S. Short-term clinical outcomes of non-curative endoscopic submucosal dissection for early esophageal adenocarcinoma. Eur. J. Gastroenterol. Hepatol. 2021, 33 (Suppl. S1), e700–e708. [Google Scholar] [CrossRef]
- Tankel, J.; Ijner, T.; Ferri, C.; Trottenberg, T.; Dehghani, M.; Najmeh, S.; Fiset, P.O.; Alsaddah, S.; Cools-Lartigue, J.; Spicer, J.; et al. Esophagectomy versus observation following endoscopic submucosal dissection of pT1b esophageal adenocarcinoma. Surg. Endosc. 2024, 38, 1342–1350. [Google Scholar] [CrossRef] [PubMed]
- Weusten, B.; Bisschops, R.; Dinis-Ribeiro, M.; di Pietro, M.; Pech, O.; Spaander, M.C.W.; Baldaque-Silva, F.; Barret, M.; Coron, E.; Fernandez-Esparrach, G.; et al. Diagnosis and management of Barrett esophagus: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2023, 55, 1124–1146. [Google Scholar] [CrossRef]
- Chan, M.W.; Haidry, R.; Norton, B.; di Pietro, M.; Hadjinicolaou, A.V.; Barret, M.; Mandengue, P.D.; Seewald, S.; Bisschops, R.; Nafteux, P.; et al. Outcomes after radical endoscopic resection of high-risk T1 esophageal adenocarcinoma: An international multicenter retrospective cohort study. Endoscopy 2025, online ahead of print. [Google Scholar] [CrossRef]
- Kim, T.S.; Min, B.H.; Kim, K.M.; Yoo, H.; Kim, K.; Min, Y.W.; Lee, H.; Rhee, P.L.; Kim, J.J.; Lee, J.H. Risk-Scoring System for Prediction of Non-Curative Endoscopic Submucosal Dissection Requiring Additional Gastrectomy in Patients with Early Gastric Cancer. J. Gastric Cancer 2021, 21, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Lee, H. Management Strategy of Non-curative ESD in Gastric Cancer: Curative Criteria, and the Critical Building Block for Determining Beyond It. J. Gastric Cancer 2024, 25, 210–227. [Google Scholar] [CrossRef] [PubMed]
- Niwa, H.; Ozawa, R.; Kurahashi, Y.; Kumamoto, T.; Nakanishi, Y.; Okumura, K.; Matsuda, I.; Ishida, Y.; Hirota, S.; Shinohara, H. The eCura system as a novel indicator for the necessity of salvage surgery after non-curative ESD for gastric cancer: A case-control study. PLoS ONE 2018, 13, e0204039. [Google Scholar] [CrossRef]
- Lee, S.; Song, J.H.; Park, S.H.; Cho, M.; Kim, Y.M.; Kim, H.I.; Hyung, W.J. Determination of Additional Surgery after Non-Curative Endoscopic Submucosal Dissection in Patients with Early Gastric Cancer: A Practically Modified Application of the eCura System. Cancers 2021, 13, 5768. [Google Scholar] [CrossRef]
- Li, S.; Tian, X.; Wei, J.; Shi, Y.; Zhang, H.; Huang, Y. Long-term outcomes of additional surgery versus non-gastrectomy treatment for early gastric cancer after non-curative endoscopic submucosal dissection: A meta-analysis. Chin. Med. J. 2023, 136, 528–535. [Google Scholar] [CrossRef]
- Hatta, W.; Gotoda, T.; Oyama, T.; Kawata, N.; Takahashi, A.; Yoshifuku, Y.; Hoteya, S.; Nakagawa, M.; Hirano, M.; Esaki, M.; et al. Is the eCura system useful for selecting patients who require radical surgery after noncurative endoscopic submucosal dissection for early gastric cancer? A comparative study. Gastric Cancer 2018, 21, 481–489. [Google Scholar] [CrossRef]
- Jeon, M.Y.; Park, J.C.; Hahn, K.Y.; Shin, S.K.; Lee, S.K.; Lee, Y.C. Long-term outcomes after noncurative endoscopic resection of early gastric cancer: The optimal time for additional endoscopic treatment. Gastrointest. Endosc. 2018, 87, 1003–1013.e2. [Google Scholar] [CrossRef]
- Pimingstorfer, P.; Biebl, M.; Gregus, M.; Kurz, F.; Schoefl, R.; Shamiyeh, A.; Spaun, G.O.; Ziachehabi, A.; Fuegger, R. Endoscopic Submucosal Dissection in the Upper Gastrointestinal Tract and the Need for Rescue Surgery—A Multicenter Analysis. J. Clin. Med. 2023, 12, 6940. [Google Scholar] [CrossRef]
- Sun, F.; Huang, Y.; Sun, Y.; Wang, X.; Ai, S.; Guan, W.; Wang, M. Risk factors of additional surgery after non-curative endoscopic submucosal dissection for early gastric cancer. BMC Gastroenterol. 2023, 23, 383. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kim, M.C.; Jeon, S.W.; Lee, K.N.; Park, J.J.; Hong, S.J. Risk Factors and Clinical Outcomes of Non-Curative Resection in Patients with Early Gastric Cancer Treated with Endoscopic Submucosal Dissection: A Retrospective Multicenter Study in Korea. Clin. Endosc. 2020, 53, 196–205. [Google Scholar] [CrossRef]
- Figueiredo, P.C.; Pimentel-Nunes, P.; Libanio, D.; Dinis-Ribeiro, M. A systematic review and meta-analysis on outcomes after Rx or R1 endoscopic resection of superficial gastric cancer. Eur. J. Gastroenterol. Hepatol. 2015, 27, 1249–1258. [Google Scholar] [CrossRef]
- Beaton, C.; Twine, C.P.; Williams, G.L.; Radcliffe, A.G. Systematic review and meta-analysis of histopathological factors influencing the risk of lymph node metastasis in early colorectal cancer. Color. Dis. 2013, 15, 788–797. [Google Scholar] [CrossRef]
- Tateishi, Y.; Nakanishi, Y.; Taniguchi, H.; Shimoda, T.; Umemura, S. Pathological prognostic factors predicting lymph node metastasis in submucosal invasive (T1) colorectal carcinoma. Mod. Pathol. 2010, 23, 1068–1072. [Google Scholar] [CrossRef] [PubMed]
- Sakuragi, M.; Togashi, K.; Konishi, F.; Koinuma, K.; Kawamura, Y.; Okada, M.; Nagai, H. Predictive factors for lymph node metastasis in T1 stage colorectal carcinomas. Dis. Colon. Rectum 2003, 46, 1626–1632. [Google Scholar] [CrossRef]
- Dang, H.; Hardwick, J.C.H.; Boonstra, J.J. Endoscopic intermuscular dissection with intermuscular tunneling for local resection of rectal cancer with deep submucosal invasion. VideoGIE 2022, 7, 273–277. [Google Scholar] [CrossRef]
- Moons, L.M.G.; Bastiaansen, B.A.J.; Richir, M.C.; Hazen, W.L.; Tuynman, J.; Elias, S.G.; Schrauwen, R.W.M.; Vleggaar, F.P.; Dekker, E.; Bos, P.; et al. Endoscopic intermuscular dissection for deep submucosal invasive cancer in the rectum: A new endoscopic approach. Endoscopy 2022, 54, 993–998. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, K.; Inaba, T.; Morimoto, T.; Aya, Y.; Colvin, H.S.; Nagahara, T.; Ishikawa, S.; Wato, M.; Imagawa, A. The Risk of Metastatic Recurrence after Non-Curative Endoscopic Resection with Negative Deep Margins for Early Colorectal Cancer: Two-Center Retrospective Cohort Study. Digestion 2024, 105, 320–330. [Google Scholar] [CrossRef]
- Messmann, H.; Bisschops, R.; Antonelli, G.; Libanio, D.; Sinonquel, P.; Abdelrahim, M.; Ahmad, O.F.; Areia, M.; Bergman, J.; Bhandari, P.; et al. Expected value of artificial intelligence in gastrointestinal endoscopy: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy 2022, 54, 1211–1231. [Google Scholar] [CrossRef] [PubMed]
- An, P.; Yang, D.; Wang, J.; Wu, L.; Zhou, J.; Zeng, Z.; Huang, X.; Xiao, Y.; Hu, S.; Chen, Y.; et al. A deep learning method for delineating early gastric cancer resection margin under chromoendoscopy and white light endoscopy. Gastric Cancer 2020, 23, 884–892. [Google Scholar] [CrossRef]
- Na, J.E.; Lee, Y.C.; Kim, T.J.; Lee, H.; Won, H.H.; Min, Y.W.; Min, B.H.; Lee, J.H.; Rhee, P.L.; Kim, J.J. Utility of a deep learning model and a clinical model for predicting bleeding after endoscopic submucosal dissection in patients with early gastric cancer. World J. Gastroenterol. 2022, 28, 2721–2732. [Google Scholar] [CrossRef]
- Zhao, L.; Han, W.; Niu, P.; Lu, Y.; Zhang, F.; Jiao, F.; Zhou, X.; Wang, W.; Luan, X.; He, M.; et al. Using nomogram, decision tree, and deep learning models to predict lymph node metastasis in patients with early gastric cancer: A multi-cohort study. Am. J. Cancer Res. 2023, 13, 204–215. [Google Scholar]
- Kato, M.; Hayashi, Y.; Uema, R.; Kanesaka, T.; Yamaguchi, S.; Maekawa, A.; Yamada, T.; Yamamoto, M.; Kitamura, S.; Inoue, T.; et al. A machine learning model for predicting the lymph node metastasis of early gastric cancer not meeting the endoscopic curability criteria. Gastric Cancer 2024, 27, 1069–1077. [Google Scholar] [CrossRef]
- Chen, T.-H.; Kuo, C.-F.; Lee, C.; Yeh, T.-S.; Lan, J.; Huang, S.-C. Artificial Intelligence Model for a Distinction between Early-Stage Gastric Cancer Invasive Depth T1a and T1b. J. Cancer 2024, 15, 3085–3094. [Google Scholar] [CrossRef]
- Wu, R.; Qin, K.; Fang, Y.; Xu, Y.; Zhang, H.; Li, W.; Luo, X.; Han, Z.; Liu, S.; Li, Q. Application of the convolution neural network in determining the depth of invasion of gastrointestinal cancer: A systematic review and meta-analysis. J. Gastrointest. Surg. 2024, 28, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Fujinami, H.; Kuraishi, S.; Teramoto, A.; Shimada, S.; Takahashi, S.; Ando, T.; Yasuda, I. Development of a novel endoscopic hemostasis-assisted navigation AI system in the standardization of post-ESD coagulation. Endosc. Int. Open 2024, 12, E520–E525. [Google Scholar] [CrossRef] [PubMed]
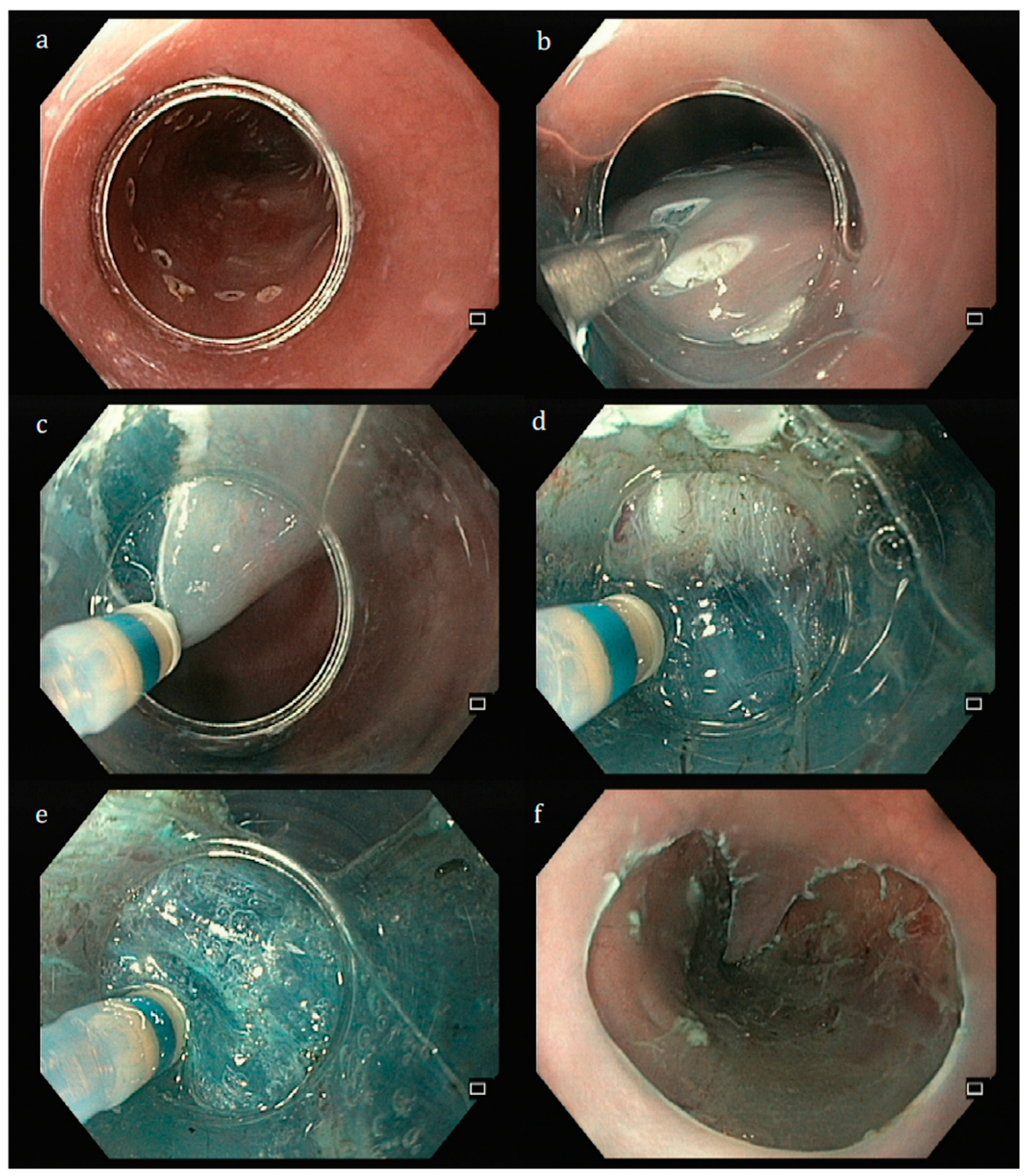

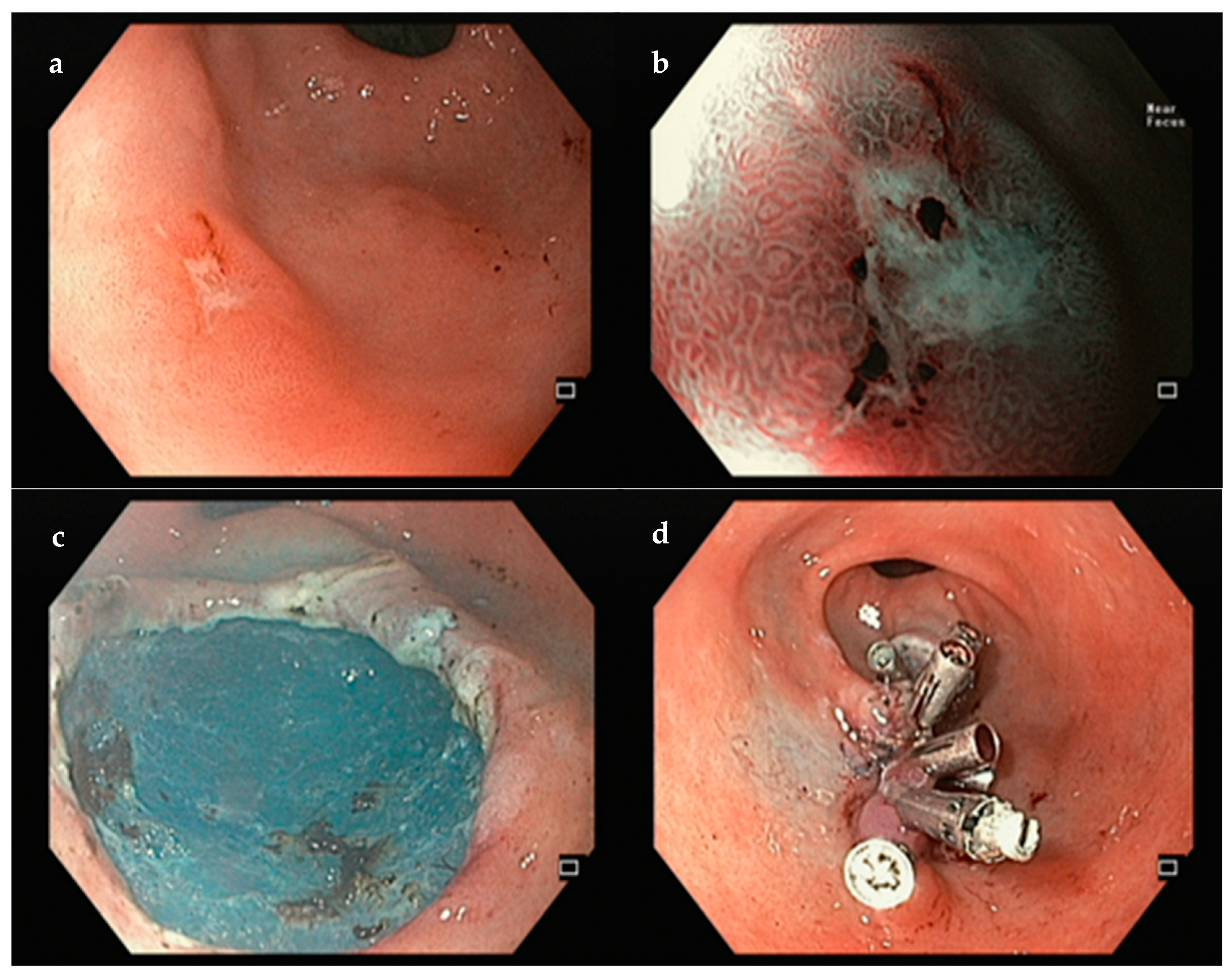
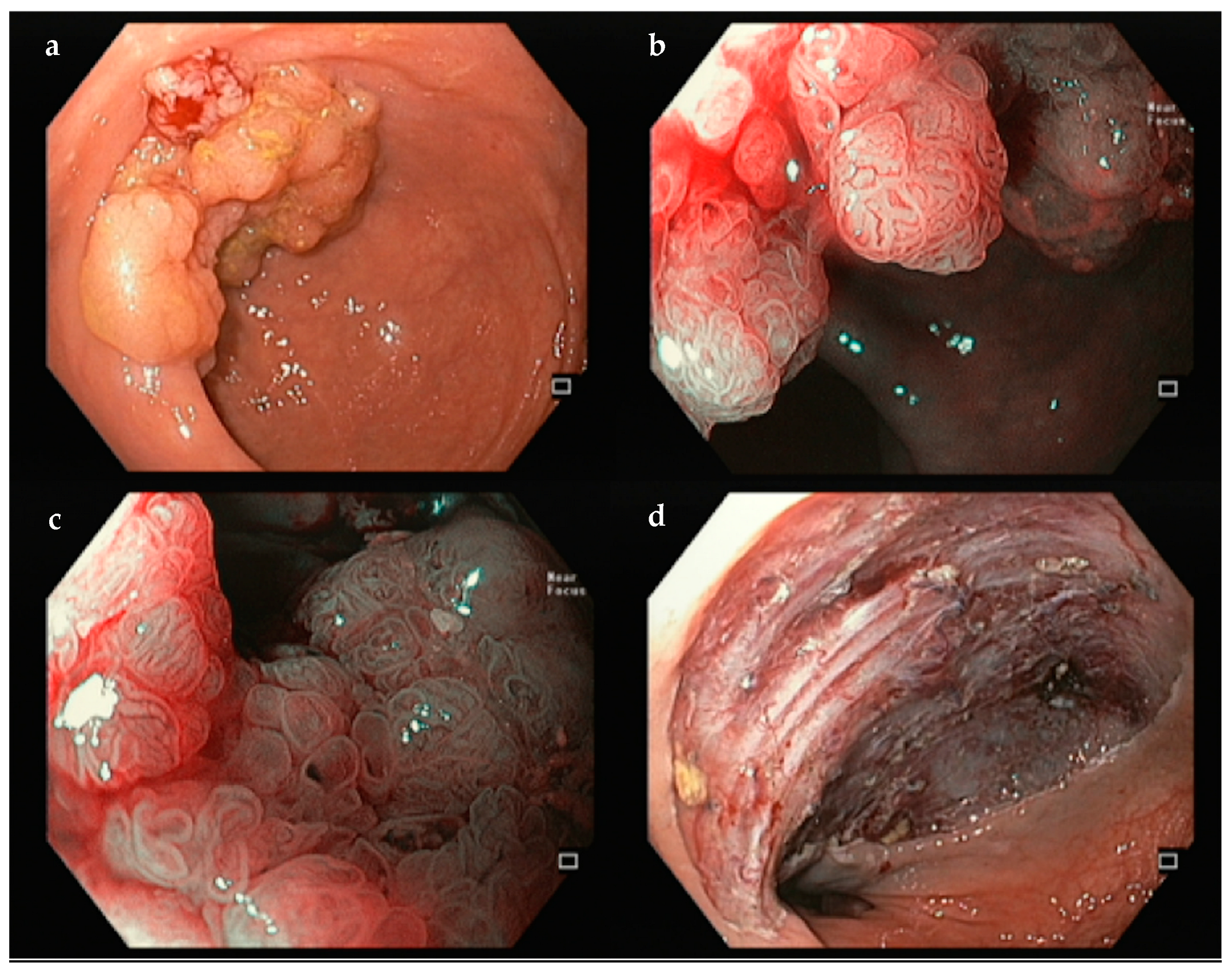
| Resection | ESGE Classification | Features | Interpretation |
|---|---|---|---|
| Curative | VLRR | Lesions en bloc removed, with free horizontal margins, mucosal (VLRR) or submucosal (LRR), without high-risk features. | Very low risk of LNM |
| LRR | Low risk of LNM | ||
| Non-curative | LocRR | Piecemeal-resected benign lesions, positive horizontal margins of benign component, without high-risk features. | High risk of local recurrence |
| HRR | Presence of high-risk features: Lymphovascular invasion, poor differentiation, deep submucosal invasion, positive vertical margins | High risk of LNM |
| Resection | ESGE Classification | e-Cura | Features |
|---|---|---|---|
| Curative | VLRR/LRR | A |
|
| LRR | B | Lesions with superficial submucosal invasion (pT1bSM1), measuring 3 cm or less, predominantly differentiated type, with free horizontal and vertical margins and without lymphovascular invasion | |
| Non-curative | LocRR | C-1 | If the only criteria among differentiated lesions that was not respected for being included in eCuraA or eCuraB were positive horizontal margin or piecemeal resection, provided that the submucosal invasive part of the lesion was en bloc resected and with free margins, and the lesion was not ulcerated |
| HRR | C-2 | Lesions not fulfilling other groups’ criteria |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos-Antunes, J. Non-Curative Endoscopic Submucosal Dissection: Current Concepts, Pitfalls and Future Perspectives. J. Clin. Med. 2025, 14, 2488. https://doi.org/10.3390/jcm14072488
Santos-Antunes J. Non-Curative Endoscopic Submucosal Dissection: Current Concepts, Pitfalls and Future Perspectives. Journal of Clinical Medicine. 2025; 14(7):2488. https://doi.org/10.3390/jcm14072488
Chicago/Turabian StyleSantos-Antunes, João. 2025. "Non-Curative Endoscopic Submucosal Dissection: Current Concepts, Pitfalls and Future Perspectives" Journal of Clinical Medicine 14, no. 7: 2488. https://doi.org/10.3390/jcm14072488
APA StyleSantos-Antunes, J. (2025). Non-Curative Endoscopic Submucosal Dissection: Current Concepts, Pitfalls and Future Perspectives. Journal of Clinical Medicine, 14(7), 2488. https://doi.org/10.3390/jcm14072488





