Depression in Diarrhea-Predominant IBS Patients: Exploring the Link Between Gut Barrier Dysfunction and Erythrocyte Polyunsaturated Fatty Acid Levels
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Recruitment
2.2. IBS Severity Scoring System (IBS-SSS)
2.3. Psychological Questionnaire
2.4. Intestinal Barrier Function and Integrity Biomarkers
2.5. Biomarkers of Intestinal Dysbiosis, Bacterial Translocation, and the Indices of Inflammation
2.6. PUFAs Analysis
2.7. Statistical Analysis
3. Results
3.1. Study Groups’ Description and the Symptom Profile
3.2. Biomarkers of Intestinal Barrier Function and Integrity
3.3. Biomarkers of Intestinal Dysbiosis, Bacterial Translocation, and the Indices of Inflammation
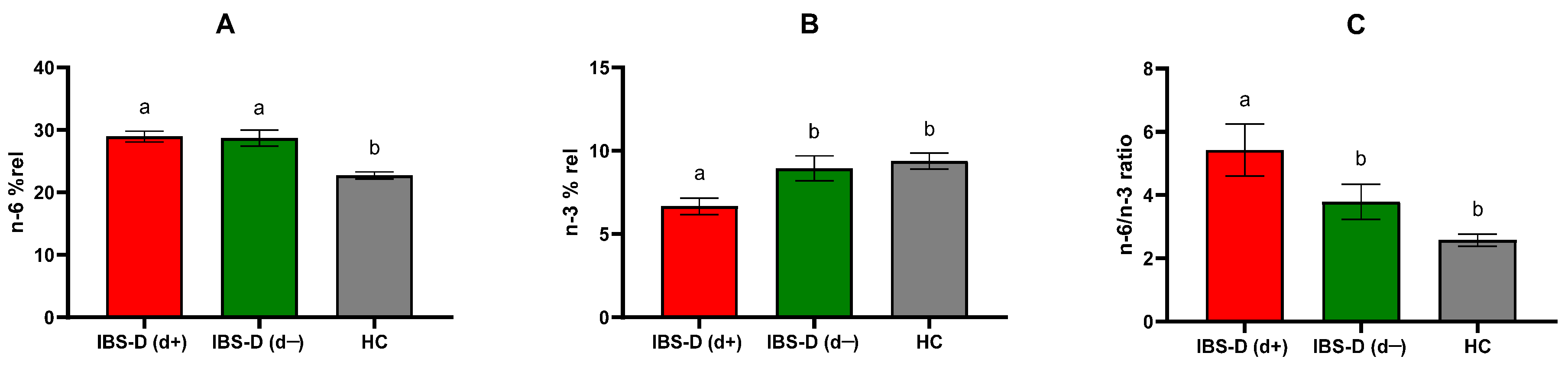
3.4. PUFAs Profile
3.5. Correlations and Regression Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IBS | Irritable bowel syndrome |
| IBS-D | Diarrhea-predominant irritable bowel syndrome |
| PUFAs | Polyunsaturated fatty acids |
| s-IP | Small intestinal permeability |
| GI | Gastrointestinal |
| QoL | Quality of life |
| LPS | Lipopolysaccharide |
| TJs | Tight junctions |
| FAs | Fatty acids |
| n-3 PUFAs | Omega-3 polyunsaturated fatty acids |
| n-6 PUFAs | Omega-6 polyunsaturated fatty acids |
| IBS-C | Constipation-predominant irritable bowel syndrome |
| IBS-M | Mixed-type irritable bowel syndrome |
| IBS-U | Unclassified irritable bowel syndrome |
| Lac | Lactulose |
| Man | Mannitol |
| Suc | Sucrose |
| IBS-SSS | IBS Severity Scoring System |
| GSI | Global Severity Index |
| IBS-QoL | IBS Quality of Life Questionnaire |
| SF-36 | Short Form Health Survey (36 items) |
| EMAs | Anti-endomysial antibodies |
| tTG | Tissue transglutaminase |
| EDTA | Ethylenediaminetetraacetic acid |
| FAMEs | Fatty acid methyl esters |
| I-FABP | Intestinal fatty acid-binding protein |
| IL-6 | Interleukin-6 |
| IL-8 | Interleukin-8 |
| IL-10 | Interleukin-10 |
| TNF-α | Tumor necrosis factor-alpha |
| HCs | Healthy controls |
| ELISA | Enzyme-linked immunosorbent assay |
| HbA1c | Glycated hemoglobin |
| SCL-90-R | Symptom Checklist-90-Revised |
| CFUs | Colony-forming units |
| FAMEs Mix | Fatty acid methyl esters mixture |
| IBD | Inflammatory bowel disease |
| DHA | Docosahexaenoic acid |
| ARA | Arachidonic acid |
| UCR-IBS | Ulcerative colitis in remission exhibiting IBS-like symptomatology |
References
- Black, C.J.; Ford, A.C. Global burden of irritable bowel syndrome: Trends, predictions and risk factors. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.Y.; Wang, F.Y.; Lv, M.; Ma, X.X.; Tang, X.D.; Lv, L. Irritable bowel syndrome: Epidemiology, overlap disorders, pathophysiology and treatment. World J. Gastroenterol. 2023, 29, 4120–4135. [Google Scholar] [CrossRef]
- Saha, L. Irritable bowel syndrome: Pathogenesis, diagnosis, treatment, and evidence-based medicine. World J. Gastroenterol. 2014, 20, 6759–6773. [Google Scholar] [CrossRef] [PubMed]
- Colomier, E.; Algera, J.; Melchior, C. Pharmacological Therapies and Their Clinical Targets in Irritable Bowel Syndrome with Diarrhea. Front. Pharmacol. 2021, 11, 629026. [Google Scholar] [CrossRef]
- Aziz, M.N.M.; Kumar, J.; Muhammad, N.K.N.; Raja, A.R.A.; Mokhtar, N.M. Irritable Bowel Syndrome, Depression, and Neurodegeneration: A Bidirectional Communication from Gut to Brain. Nutrients 2021, 13, 3061. [Google Scholar] [CrossRef] [PubMed]
- Hausteiner-Wiehle, C.; Henningsen, P. Irritable bowel syndrome: Relations with functional, mental, and somatoform disorders. World J. Gastroenterol. 2014, 20, 6024–6030. [Google Scholar]
- Zhang, Q.E.; Wang, F.; Qin, G.; Zheng, W.; Ng, C.H.; Ungvari, G.S.; Yuan, Z.; Mei, S.; Wang, G.; Xiang, Y.T. Depressive symptoms in patients with irritable bowel syndrome: A meta analysis of comparative studies. Int. J. Biol. Sci. 2018, 14, 1504–1512. [Google Scholar] [CrossRef]
- Jia, L.; Lili, S.; Dan, H.; Wenjuan, F.; Xiaoqing, L.; Liming, Z.; Jing, W.; Xiucai, F. Depression and Structural Factors Are Associated with Symptoms in Patients of Irritable Bowel Syndrome with Diarrhea. J. Neurogastroenterol. Motil. 2020, 26, 505–513. [Google Scholar] [CrossRef]
- Bhatt, S.; Kanoujia, J.; Mohana Lakshmi, S.; Patil, C.R.; Gupta, G.; Chellappan, D.K.; Dua, K. Role of Brain-Gut-Microbiota Axis in Depression: Emerging Therapeutic Avenues. CNS Neurol. Disord. Drug Targets 2023, 22, 276–288. [Google Scholar] [CrossRef]
- Clarke, T.B.; Davis, K.M.; Lysenko, E.S.; Zhou, A.Y.; Yu, Y.; Weiser, J.N. Recognition of Peptidoglycan from the Microbiota by Nod1 Enhances Systemic Innate Immunity. Nat. Med. 2010, 16, 228–231. [Google Scholar] [CrossRef]
- Sonali, S.; Ray, B.; Ahmed Tousif, H.; Rathipriya, A.G.; Sunanda, T.; Mahalakshmi, A.M.; Rungratanawanich, W.; Essa, M.M.; Qoronfleh, M.W.; Chidambaram, S.B.; et al. Mechanistic Insights into the Link between Gut Dysbiosis and Major Depression: An Extensive Review. Cells 2022, 11, 1362. [Google Scholar] [CrossRef]
- Aleman, R.S.; Moncada, M.; Aryana, K.J. Leaky Gut and the Ingredients That Help Treat It: A Review. Molecules 2023, 28, 619. [Google Scholar] [CrossRef]
- Hanning, N.; Edwinson, A.L.; Ceuleers, H.; Peters, S.A.; De Man, J.G.; Hassett, L.C.; De Winter, B.Y.; Grover, M. Intestinal barrier dysfunction in irritable bowel syndrome: A systematic review. Therap Adv. Gastroenterol. 2021, 14, 756284821993586. [Google Scholar] [CrossRef]
- Du, Y.; He, C.; An, Y.; Huang, Y.; Zhang, H.; Fu, W.; Wang, M.; Shan, Z.; Xie, J.; Yang, Y.; et al. The Role of Short Chain Fatty Acids in Inflammation and Body Health. Int. J. Mol. Sci. 2024, 25, 7379. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wang, L.; Guo, J.; Zhao, T.; Tang, H.; Dong, F.; Wang, C.; Chen, J.; Tang, M. Erythrocyte Membrane Fatty Acid Composition as a Potential Biomarker for Depression. Int. J. Neuropsychopharmacol. 2023, 26, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Malau, I.A.; Chang, J.P.; Lin, Y.W.; Chang, C.C.; Chiu, W.C.; Su, K.P. Omega-3 Fatty Acids and Neuroinflammation in Depression: Targeting Damage-Associated Molecular Patterns and Neural Biomarkers. Cells 2024, 13, 1791. [Google Scholar] [CrossRef]
- Wang, M.; Yan, X.; Li, Y.; Li, Q.; Xu, Y.; Huang, J.; Gan, J.; Yang, W. Association between plasma polyunsaturated fatty acids and depressive among US adults. Front. Nutr. 2024, 11, 1342304. [Google Scholar]
- Amin, A.A.; Menon, R.A.; Reid, K.J.; Harris, W.S.; Spertus, J.A. Acute coronary syndrome patients with depression have low blood cell membrane omega-3 fatty acid levels. Psychosom. Med. 2008, 70, 856–862. [Google Scholar] [CrossRef]
- Léniz, A.; Fernández-Quintela, A.; Arranz, S.; Portune, K.; Tueros, I.; Arana, E.; Castaño, L.; Velasco, O.; Portillo, M.P. Altered Red Blood Cell Fatty Acid and Serum Adipokine Profiles in Subjects with Obesity. Biomedicines 2023, 11, 3320. [Google Scholar] [CrossRef]
- Norouziasl, R.; Zeraattalab-Motlagh, S.; Jayedi, A.; Shab-Bidar, S. Efficacy and safety of n-3 fatty acids supplementation on depression: A systematic review and dose-response meta-analysis of randomised controlled trials. Br. J. Nutr. 2024, 131, 658–671. [Google Scholar] [CrossRef]
- Grosso, G.; Galvano, F.; Marventano, S.; Malaguarnera, M.; Bucolo, C.; Drago, F.; Caraci, F. Omega-3 fatty acids and depression: Scientific evidence and biological mechanisms. Oxid. Med. Cell Longev. 2014, 2014, 313570. [Google Scholar] [CrossRef] [PubMed]
- Sublette, M.E.; Ellis, S.P.; Geant, A.L.; Mann, J.J. Meta-analysis of the effects of eicosapentaenoic acid (EPA) in clinical trials in depression. J. Clin. Psychiatry 2011, 72, 1577–1584. [Google Scholar] [CrossRef]
- Kilkens, T.O.; Honig, A.; Maes, M.; Lousberg, R.; Brummer, R.J. Fatty acid profile and affective dysregulation in irritable bowel syndrome. Lipids 2004, 39, 425–431. [Google Scholar] [CrossRef]
- Linsalata, M.; Ignazzi, A.; D’Attoma, B.; Riezzo, G.; Mallardi, D.; Orlando, A.; Prospero, L.; Notarnicola, M.; De Nunzio, V.; Pinto, G.; et al. Relationship between Markers of Gut Barrier Function and Erythrocyte Membrane PUFAs in Diarrhea-Predominant IBS Patients Undergoing a Low-FODMAP Diet. Nutrients 2024, 16, 2706. [Google Scholar] [CrossRef] [PubMed]
- Linsalata, M.; Riezzo, G.; Clemente, C.; D’Attoma, B.; Russo, F. Noninvasive Biomarkers of Gut Barrier Function in Patients Suffering from Diarrhea Predominant-IBS: An Update. Dis. Markers 2020, 2020, 2886268. [Google Scholar] [CrossRef] [PubMed]
- Veres-Székely, A.; Szász, C.; Pap, D.; Szebeni, B.; Bokrossy, P.; Vannay, Á. Zonulin as a Potential Therapeutic Target in Microbiota-Gut-Brain Axis Disorders: Encouraging Results and Emerging Questions. Int. J. Mol. Sci. 2023, 24, 7548. [Google Scholar] [CrossRef]
- Huang, X.; Zhou, Y.; Sun, Y.; Wang, Q. Intestinal fatty acid binding protein: A rising therapeutic target in lipid metabolism. Prog. Lipid Res. 2022, 87, 101178. [Google Scholar] [CrossRef] [PubMed]
- Schmulson, M.J.; Drossman, D.A.J. What Is New in Rome IV. Neurogastroenterol. Motil. 2017, 23, 151–163. [Google Scholar] [CrossRef]
- Francis, C.Y.; Morris, J.; Whorwell, P.J. The irritable bowel severity scoring system: A simple method of monitoring irritable bowel syndrome and its progress. Aliment. Pharmacol. Ther. 1997, 11, 395–402. [Google Scholar] [CrossRef]
- Derogatis, L.R.; Lipman, R.S.; Covi, L. SCL-90: An outpatient psychiatric rating scale—Preliminary report. Psychopharmacol. Bull. 1973, 9, 13–28. [Google Scholar] [PubMed]
- Hildenbrand, A.K.; Nicholos, E.G.; Aggarwall, R.; Brody-Bizar, E.; Daly, B.P. Symptom Checklist-90-revised (SCL-90-R). In The Encyclopedia of Clinical Psychology, 1st ed.; Cautin, R.L., Lilienfeld, S.O., Eds.; John Wiley & Sons: Chichester, UK, 2015; pp. 1–5. [Google Scholar]
- Andrae, D.A.; Patrick, D.L.; Drossman, D.A.; Covington, P.S. Evaluation of the Irritable Bowel Syndrome Quality of Life (IBS-QOL) questionnaire in diarrheal-predominant irritable bowel syndrome patients. Health Qual. Life Outcomes 2013, 11, 208. [Google Scholar] [CrossRef] [PubMed]
- Barile, J.P.; Horner-Johnson, W.; Krahn, G.; Zack, M.; Miranda, D.; DeMichele, K.; Ford, D.; Thompson, W.W. Measurement characteristics for two health-related quality of life measures in older adults: The SF-36 and the CDC Healthy Days items. Disabil. Health J. 2016, 9, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Seethaler, B.; Basrai, M.; Neyrinck, A.M.; Nazare, J.A.; Walter, J.; Delzenne, N.M.; Bischoff, S.C. Biomarkers for assessment of intestinal permeability in clinical practice. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 321, G11–G17. [Google Scholar] [CrossRef]
- Linsalata, M.; D’Attoma, B.; Orlando, A.; Guerra, V.; Russo, F. Comparison of an enzymatic assay with liquid chromatography pulsed amperometric detection for the determination of lactulose and mannitol in urine of healthy subjects and patients with active celiac disease. Clin. Chem. Lab. Med. 2014, 52, 61–64. [Google Scholar] [CrossRef]
- Hilsden, R.; Meddings, J.; Sutherland, L. Intestinal permeability changes in response to acetylsalicylic acid in relatives of patients with Crohn’s disease. Gastroenterology 1996, 110, 1395–1403. [Google Scholar] [CrossRef]
- Simeoni, M.; Citraro, M.L.; Cerantonio, A.; Deodato, F.; Provenzano, M.; Cianfrone, P.; Capria, M.; Corrado, S.; Libri, E.; Comi, A.; et al. An open-label, randomized, placebo-controlled study on the effectiveness of a novel probiotics administration protocol (ProbiotiCKD) in patients with mild renal insufficiency (stage 3a of CKD). Eur. J. Nutr. 2019, 58, 2145–2156. [Google Scholar] [CrossRef]
- Breil, C.; Vian, M.A.; Zemb, T.; Kunz, W.; Chemat, F. “Bligh and Dyer” and folch methods for solid–liquid–liquid extraction of lipids from microorganisms. Comprehension of solvatation mechanisms and towards substitution with alternative solvents. Int. J. Mol. Sci. 2017, 18, 708. [Google Scholar] [CrossRef]
- Tutino, V.; Gigante, I.; Scavo, M.P.; Refolo, M.; De Nunzio, V.; Milella, R.A.; Caruso, M.G.; Notarnicola, M. Stearoyl-CoADesaturase-1 enzyme inhibition by grape skin extracts affects membrane fluidity in human colon cancer cell lines. Nutrients 2020, 12, 693. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Wang, X.; Huang, S.; Wang, H.; Shen, G. Low-level inflammation, immunity, and brain-gut axis in IBS: Unraveling the complex relationships. Gut Microbes 2023, 15, 263209. [Google Scholar] [CrossRef]
- Fond, G.; Loundou, A.; Hamdani, N.; Boukouaci, W.; Dargel, A.; Oliveira, J.; Roger, M.; Tamouza, R.; Leboyer, M.; Boyer, L. Anxiety and depression comorbidities in irritable bowel syndrome (IBS): A systematic review and meta-analysis. Eur. Arch. Psychiatry Clin. Neurosci. 2014, 264, 651–660. [Google Scholar] [CrossRef]
- Chen, Y.; Lian, B.; Li, P.; Yao, S.; Hou, Z. Studies on irritable bowel syndrome associated with anxiety or depression in the last 20 years: A bibliometric analysis. Front. Public. Health 2022, 10, 947097. [Google Scholar] [CrossRef]
- Pereyra, F.; Schlottmann, F.; Casas, M.A.; Steinberg, L.; Pereyra, L. Exploring the gut-brain axis in a large cohort of patients with irritable bowel syndrome: Is there a link between depression and intestinal and extra-intestinal symptoms? Gastroenterol. Hepatol. 2025, 3, 502370. [Google Scholar] [CrossRef]
- Simon, G.E.; Moise, N.; Mohr, D.C. Management of Depression in Adults: A Review. JAMA 2024, 332, 141–152. [Google Scholar] [CrossRef]
- Stevens, B.R.; Goel, R.; Seungbum, K.; Richards, E.M.; Holbert, R.C.; Pepine, C.J.; Raizada, M.K. Increased human intestinal barrier permeability plasma biomarkers zonulin and FABP2 correlated with plasma LPS and altered gut microbiome in anxiety or depression. Gut 2018, 67, 1555–1557. [Google Scholar] [CrossRef]
- Naufel, M.F.; Truzzi, G.M.; Ferreira, C.M.; Coelho, F.M.S. The brain-gut-microbiota axis in the treatment of neurologic and psychiatric disorders. Arq. Neuropsiquiatr. 2023, 81, 670–684. [Google Scholar] [CrossRef] [PubMed]
- Linsalata, M.; Riezzo, G.; Orlando, A.; D’Attoma, B.; Prospero, L.; Ignazzi, A.; Losurdo, G.; Di Leo, A.; Giannelli, G.; Russo, F. The Role of Intestinal Barrier Function in Overweight Patients with IBS with Diarrhea Undergoing a Long-Term Low Fermentable Oligo-, Di-, and Monosaccharide and Polyol Diet. Nutrients 2023, 15, 4683. [Google Scholar] [CrossRef]
- Ait Abdellah, S.; Gal, C.; Laterza, L.; Velenza, V.; Settanni, C.R.; Napoli, M.; Schiavoni, E.; Mora, V.; Petito, V.; Gasbarrini, A. Effect of a Multistrain Probiotic on Leaky Gut in Patients with Diarrhea-Predominant Irritable Bowel Syndrome: A Pilot Study. Dig. Dis. 2023, 41, 489–499. [Google Scholar] [CrossRef]
- Calarge, C.A.; Devaraj, S.; Shulman, R.J. Gut permeability and depressive symptom severity in unmedicated adolescents. J. Affect. Disord. 2019, 246, 586–594. [Google Scholar] [CrossRef]
- Alonso, C.; Guilarte, M.; Vicario, M.; Ramos, L.; Ramadan, Z.; Antolín, M.; Martínez, C.; Rezzi, S.; Saperas, E.; Kochhar, S.; et al. Maladaptive intestinal epithelial responses to life stress may predispose healthy women to gut mucosal inflammation. Gastroenterology 2008, 135, 163–172.e1. [Google Scholar] [CrossRef]
- Doney, E.; Cadoret, A.; Dion-Albert, L.; Lebel, M.; Menard, C. Inflammation-driven brain and gut barrier dysfunction in stress and mood disorders. Eur. J. Neurosci. 2022, 55, 2851–2894. [Google Scholar] [CrossRef]
- Sanders, A.E.; Weatherspoon, E.D.; Ehrmann, B.M.; Soma, P.S.; Shaikh, S.R.; Preisser, J.S.; Ohrbach, R.; Fillingim, R.B.; Slade, G.D. Ratio of Omega-6/Omega-3 Polyunsaturated Fatty Acids Associated With Somatic and Depressive Symptoms in People With Painful Temporomandibular Disorder and Irritable Bowel Syndrome. J. Pain. 2022, 23, 1737–1748. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Jiang, T.; Shan, X.; Zhang, M.; Li, Y.; Qi, X.; Bian, Y.; Zhao, L. Pro-inflammatory cytokines in stress-induced depression: Novel insights into mechanisms and promising therapeutic strategies. Prog. Neuropsychopharmacol. Biol. Psychiatry 2024, 131, 110931. [Google Scholar] [CrossRef]
- Liao, Y.; Xie, B.; Zhang, H.; He, Q.; Guo, L.; Subramanieapillai, M.; Fan, B.; Lu, C.; McIntyre, R.S. Efficacy of omega-3 PUFAs in depression: A meta-analysis. Transl. Psychiatry 2019, 9, 190. [Google Scholar] [CrossRef] [PubMed]
- Durkin, L.A.; Childs, C.E.; Calder, P.C. Omega-3 Polyunsaturated Fatty Acids and the Intestinal Epithelium-A Review. Foods 2021, 10, 199. [Google Scholar] [CrossRef]
- Seethaler, B.; Lehnert, K.; Yahiaoui-Doktor, M.; Basrai, M.; Vetter, W.; Kiechle, M.; Bischoff, S.C. Omega-3 polyu nsaturated fatty acids improve intestinal barrier integrity albeit to a lesser degree than short-chain fatty acids: An exploratory analysis of the randomized controlled LIBRE trial. Eur. J. Nutr. 2023, 62, 2779–2791. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.G.; Bryce, R.P.; Horrobin, D.F.; Mansel, R.E. Regulation of tight junction permeability and occludin expression by polyunsaturated fatty acids. Biochem. Biophys. Res. Commun. 1998, 17, 244, 414–420. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, Q.; Zhang, M.; Wang, C.; Zhu, Z.; Li, N.; Li, J. Effect of n-3 polyunsaturated fatty acids on membrane microdomain localization of tight junction proteins in experimental colitis. FEBS J. 2008, 275, 411–420. [Google Scholar] [CrossRef]
- Seethaler, B.; Basrai, M.; Neyrinck, A.M.; Vetter, W.; Delzenne, N.M.; Kiechle, M.; Bischoff, S.C. Effect of the Mediterranean diet on the faecal long-chain fatty acid composition and intestinal barrier integrity: An exploratory analysis of the randomised controlled LIBRE trial. Br. J. Nutr. 2024, 132, 1–9. [Google Scholar] [CrossRef]
- Chen, G.; Wu, X.; Zhu, H.; Li, K.; Zhang, J.; Sun, S.; Wang, H.; Wang, M.; Shao, B.; Li, H.; et al. Multisample lipidomic profiles of irritable bowel syndrome and irritable bowel syndrome-like symptoms in patients with inflammatory bowel disease: New insight into the recognition of the same symptoms in different diseases. J. Gastroenterol. 2024, 59, 1000–1010. [Google Scholar] [CrossRef]
- Medina-Rodríguez, E.M.; Martínez-Raga, J.; Sanz, Y. Intestinal Barrier, Immunity and Microbiome: Partners in the Depression Crime. Pharmacol. Rev. 2024, 76, 956–969. [Google Scholar] [CrossRef]
- Wysoczański, T.; Sokoła-Wysoczańska, E.; Pękala, J.; Lochyński, S.; Czyż, K.; Bodkowski, R.; Herbinger, G.; Patkowska-Sokoła, B.; Librowski, T. Omega-3 Fatty Acids and their Role in Central Nervous System—A Review. Curr. Med. Chem. 2016, 23, 816–831. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.; Reggi, A.; Parini, A.; Borghi, C. Application of polyunsaturated fatty acids in internal medicine: Beyond the established cardiovascular effects. Arch. Med. Sci. 2012, 8, 784–793. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
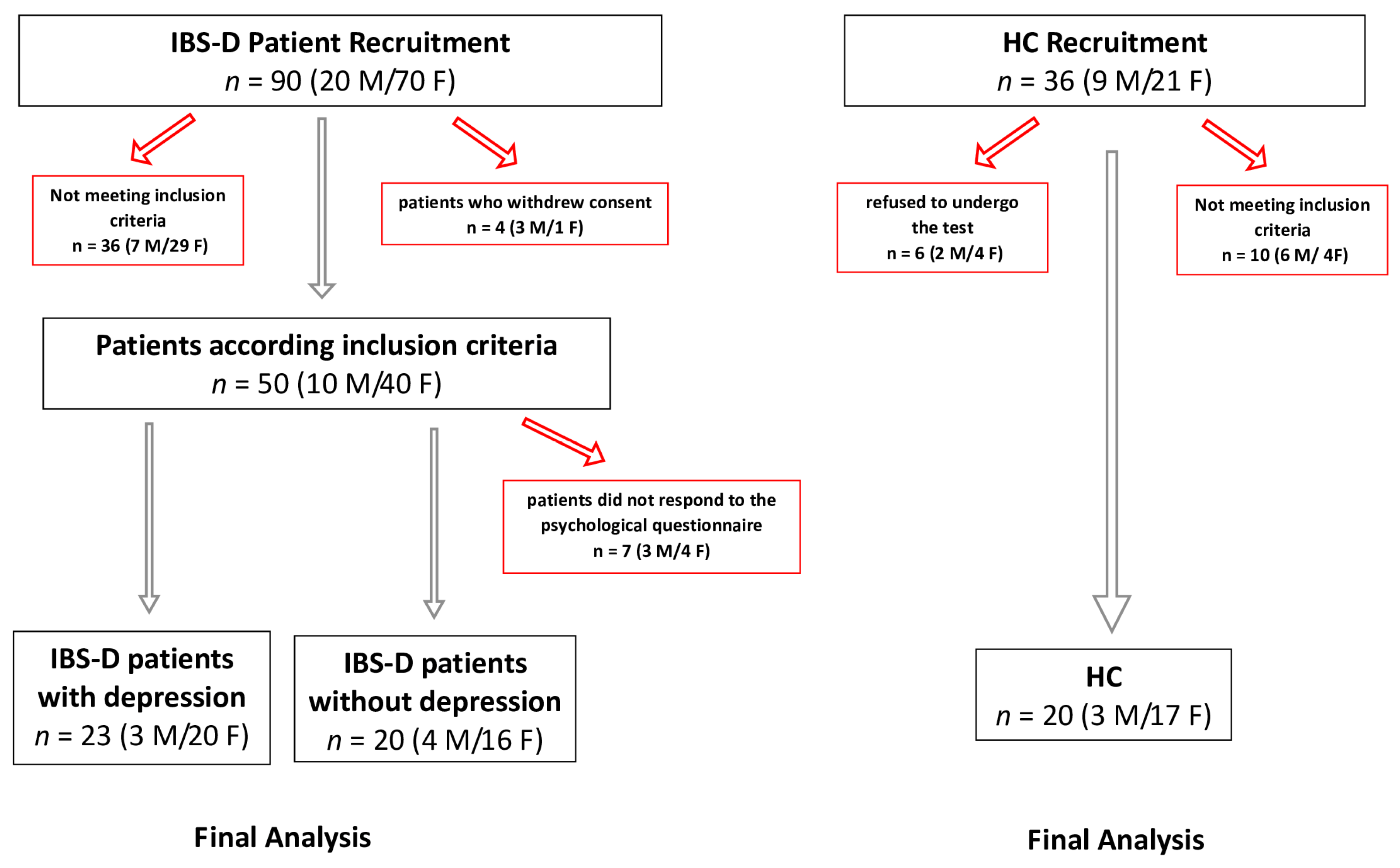


| IBS-D(d+) n = 23 | IBS-D (d−) n = 20 | HCs n = 20 | p-Value | |
|---|---|---|---|---|
| Demographics | ||||
| Sex(F/M) | 20/3 | 16/4 | 17/3 | ns |
| Age (years) | 46.17 ± 1.98 | 42.7 ± 2.31 | 40.2 ± 1.84 | ns |
| BMI (Kg/m2) | 25.38 ± 0.98 | 25.19 ± 1.01 | 24.63 ± 0.97 | ns |
| IBS-SSS | ||||
| Total score | 277.0 ± 16.86 a | 262.1 ± 19.79 a | 47.2 ± 11.41 b | p < 0.0001 |
| Abdominal pain intensity | 48.0 ± 4.84 a | 45.8 ± 5.47 a | 3.0 ± 1.75 b | p < 0.0001 |
| Abdominal pain frequency (days) | 50.0 ± 6.16 a | 43.0 ± 6.61 a | 4.0 ± 2.66 b | p < 0.0001 |
| Abdominal bloating severity | 54.3 ± 3.89 a | 57.7 ± 6.16 a | 3.0 ± 2.10 b | p < 0.0001 |
| Dissatisfaction with Bowel habits | 64.2 ± 5.33 a | 64.2 ± 5.27 a | 28.0 ± 5.74 b | p < 0.0001 |
| Symptom impact on daily life | 60.5 ± 4.35 a | 51.5 ± 5.64 a | 9.25 ± 2.72 b | p < 0.0001 |
| SF-36 | ||||
| Physical health | 45.0 ± 1.58 a | 47.4 ±1.69 a | 58.8 ± 1.08 b | p < 0.0001 |
| Mental health | 26.2 ± 2.93 a | 40.5 ± 2.97 b | 43.3± 3.11 b | p = 0.0003 |
| IBS-QoL | ||||
| Total score | 39.5 ± 3.44 a | 21.18 ± 2.69 b | 3.6 ± 1.23 c | p < 0.0001 |
| SCL-90-R | ||||
| GSI (T scores) | 80.6 ± 2.95 a | 51.7 ±1.98 b | 48.7 ± 2.68 b | p < 0.0001 |
| Somatization (T scores) | 74.5 ± 2.96 a | 60.4 ± 3.43 b | 48.7 ± 2.15 b | p < 0.0001 |
| Anxiety (T score) | 75.3 ± 3.63 a | 49.7 ± 2.23 b | 46.6 ± 2.35 b | p < 0.0001 |
| IBS-D (d+) (n = 23) | IBS-D (d−) (n = 20) | HCs (n = 20) | p-Value | |
|---|---|---|---|---|
| IL-6 (ng/mL) | 7.26 ± 1.55 a | 4.65 ± 0.19 b | 4.05 ± 0.19 c | <0.0001 |
| IL-8 (ng/mL) | 4.33 ± 0.18 a | 5.00 ± 0.73 a | 4.77 ± 0.07 a | n.s. |
| IL-10 (ng/mL) | 2.82 ± 0.05 a | 2.92 ± 0.06 a | 3.46 ± 0.12 b | <0.0001 |
| TNF-α (ng/mL) | 3.73 ± 0.14 a | 3.20 ± 0.11 b | 3.30 ± 0.29 b | p = 0.0007 |
| Parameters | (β) | Std. Error (β) | p | 95% CI |
|---|---|---|---|---|
| %Lac | 17.525 | 6.434 | 0.010 | −0.402–1.800 |
| n-3 PUFAs | −1.585 | 0.767 | 0.046 | −3.088–0.081 |
| n-6 PUFAs | 0.699 | 0.562 | 0.221 | −0.402–1.800 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Linsalata, M.; Prospero, L.; Ignazzi, A.; Riezzo, G.; D’Attoma, B.; Mallardi, D.; Goscilo, F.; Notarnicola, M.; De Nunzio, V.; Pinto, G.; et al. Depression in Diarrhea-Predominant IBS Patients: Exploring the Link Between Gut Barrier Dysfunction and Erythrocyte Polyunsaturated Fatty Acid Levels. J. Clin. Med. 2025, 14, 2483. https://doi.org/10.3390/jcm14072483
Linsalata M, Prospero L, Ignazzi A, Riezzo G, D’Attoma B, Mallardi D, Goscilo F, Notarnicola M, De Nunzio V, Pinto G, et al. Depression in Diarrhea-Predominant IBS Patients: Exploring the Link Between Gut Barrier Dysfunction and Erythrocyte Polyunsaturated Fatty Acid Levels. Journal of Clinical Medicine. 2025; 14(7):2483. https://doi.org/10.3390/jcm14072483
Chicago/Turabian StyleLinsalata, Michele, Laura Prospero, Antonia Ignazzi, Giuseppe Riezzo, Benedetta D’Attoma, Domenica Mallardi, Francesco Goscilo, Maria Notarnicola, Valentina De Nunzio, Giuliano Pinto, and et al. 2025. "Depression in Diarrhea-Predominant IBS Patients: Exploring the Link Between Gut Barrier Dysfunction and Erythrocyte Polyunsaturated Fatty Acid Levels" Journal of Clinical Medicine 14, no. 7: 2483. https://doi.org/10.3390/jcm14072483
APA StyleLinsalata, M., Prospero, L., Ignazzi, A., Riezzo, G., D’Attoma, B., Mallardi, D., Goscilo, F., Notarnicola, M., De Nunzio, V., Pinto, G., & Russo, F. (2025). Depression in Diarrhea-Predominant IBS Patients: Exploring the Link Between Gut Barrier Dysfunction and Erythrocyte Polyunsaturated Fatty Acid Levels. Journal of Clinical Medicine, 14(7), 2483. https://doi.org/10.3390/jcm14072483








