The Effect of Albumin/Glutaraldehyde Glue (Bioglue) on Colonic Anastomosis Under Intestinal Obstruction: An Experimental Study in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Study Groups
- Control1 (10 animals): the sacrifice took place on the 4th postoperative day.
- Control2 (10 animals): the sacrifice took place on the 8th postoperative day.
- Ileus1 (10 animals): the sacrifice took place on the 4th postoperative day.
- Ileus2 (10 animals): the sacrifice took place on the 8th postoperative day.
- Bioglue1 (10 animals): the sacrifice took place on the 4th postoperative day.
- Bioglue2 (10 animals): the sacrifice took place on the 8th postoperative day.
- Ileus + Bioglue1 (10 animals): the sacrifice took place on the 4th postoperative day.
- Ileus + Bioglue2 (10 animals): the sacrifice took place on the 8th postoperative day.
2.3. Anesthesia and Operative Technique
2.4. Body Weight Measurement
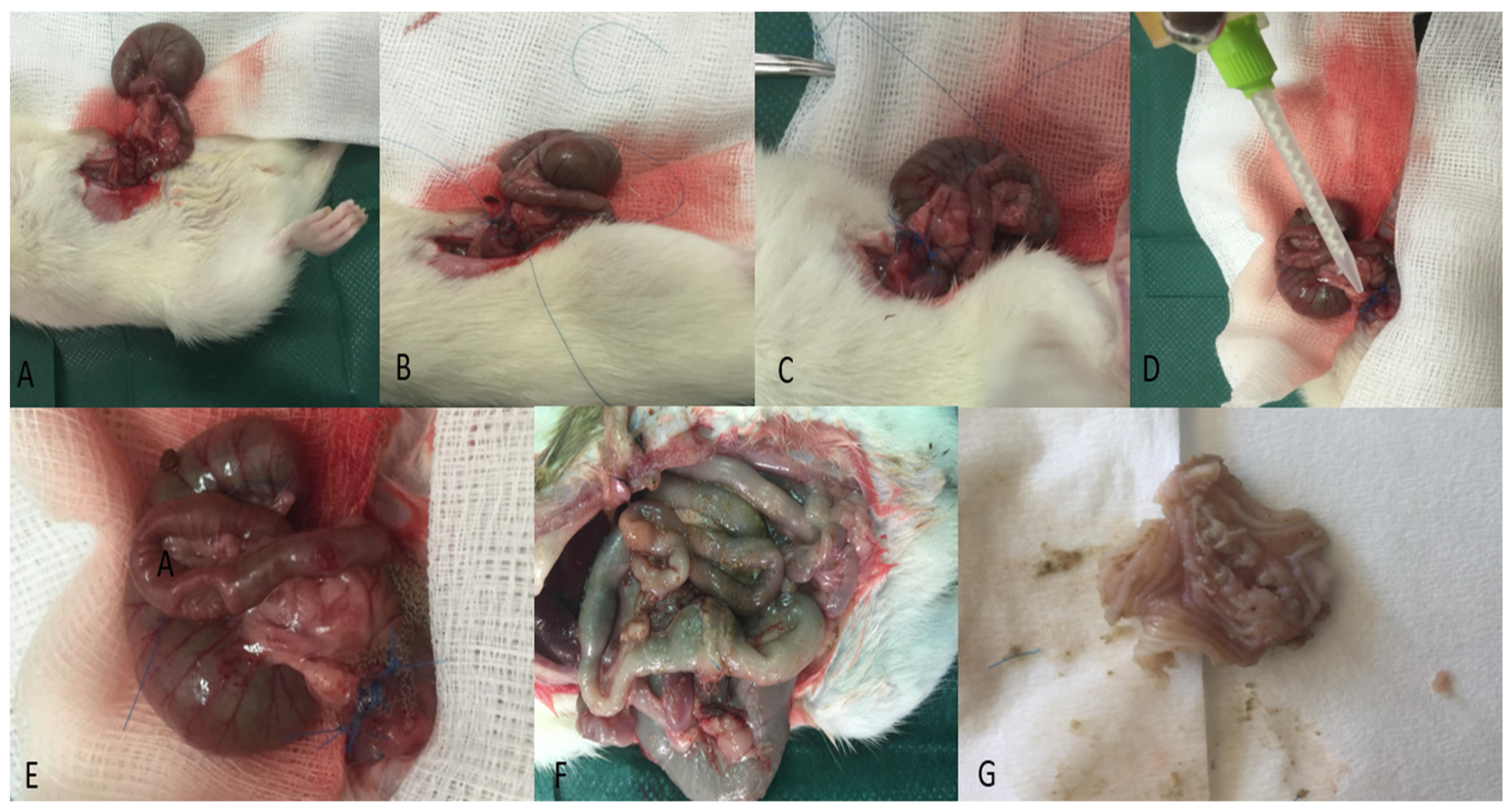
2.5. Macroscopic Examination
2.6. Bursting Pressure Measurement
2.7. Histopathological Assessment
2.8. Hydroxyproline Measurement
2.9. Type I Collagenase Measurement
2.10. Statistical Analysis
3. Results
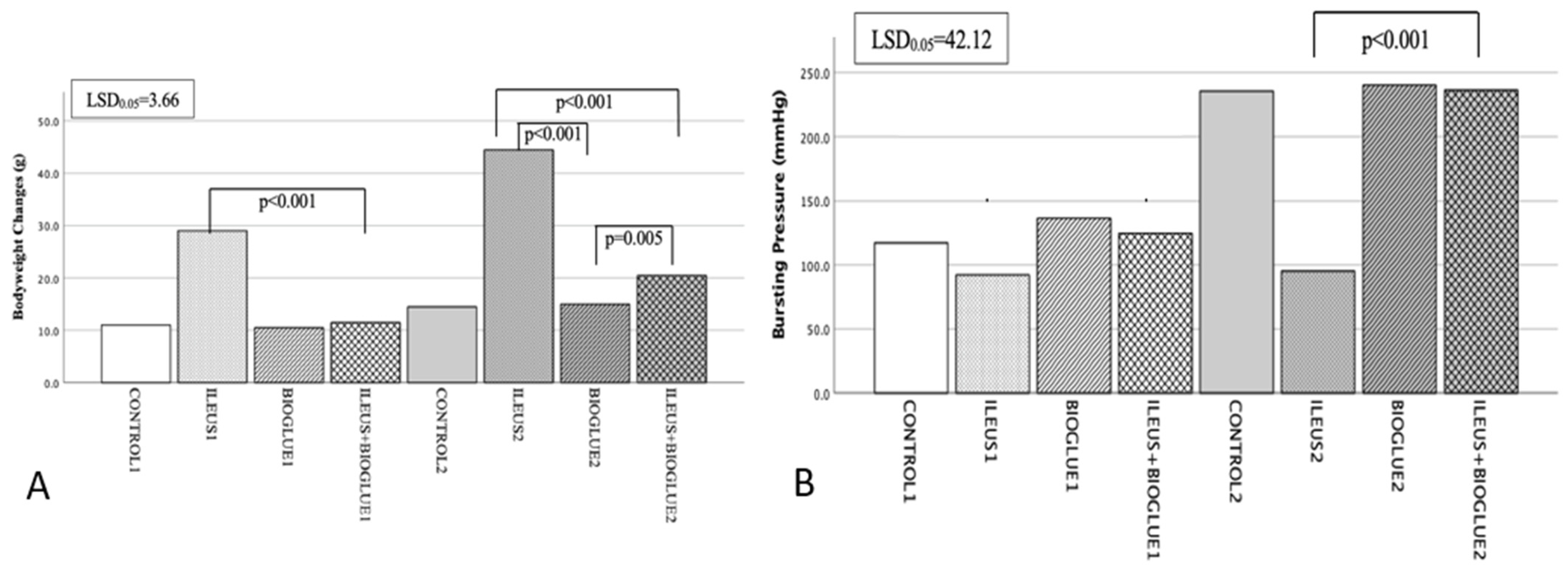
3.1. Body Weight
3.2. Bursting Pressure
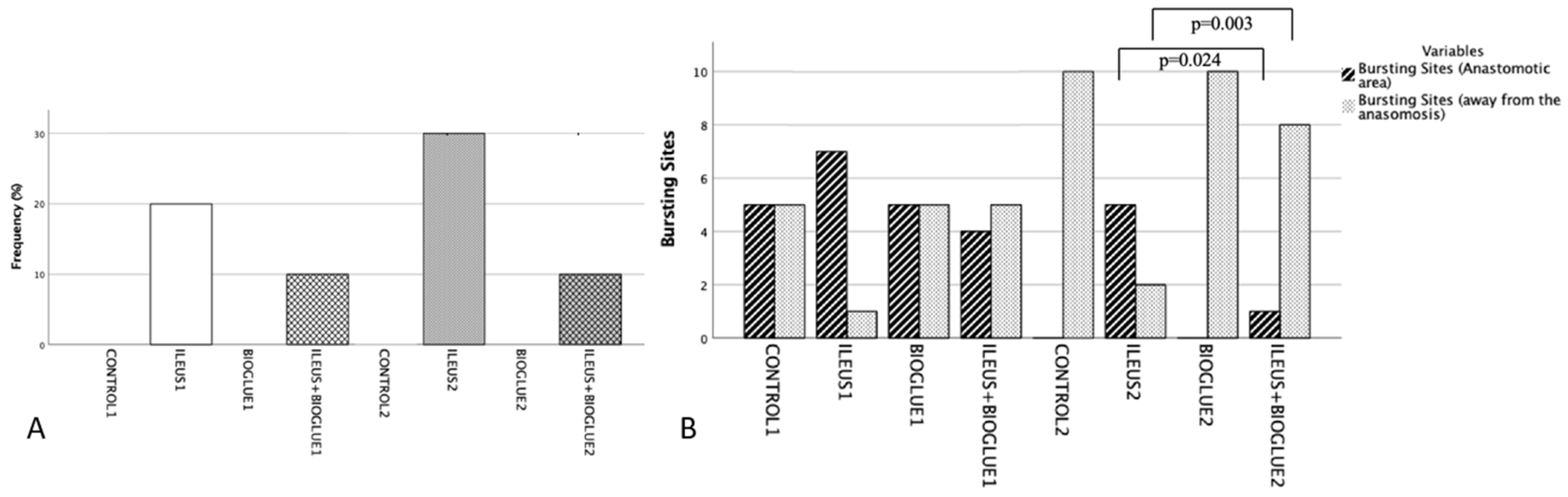
3.3. Anastomotic Dehiscence
3.4. Rupture Site
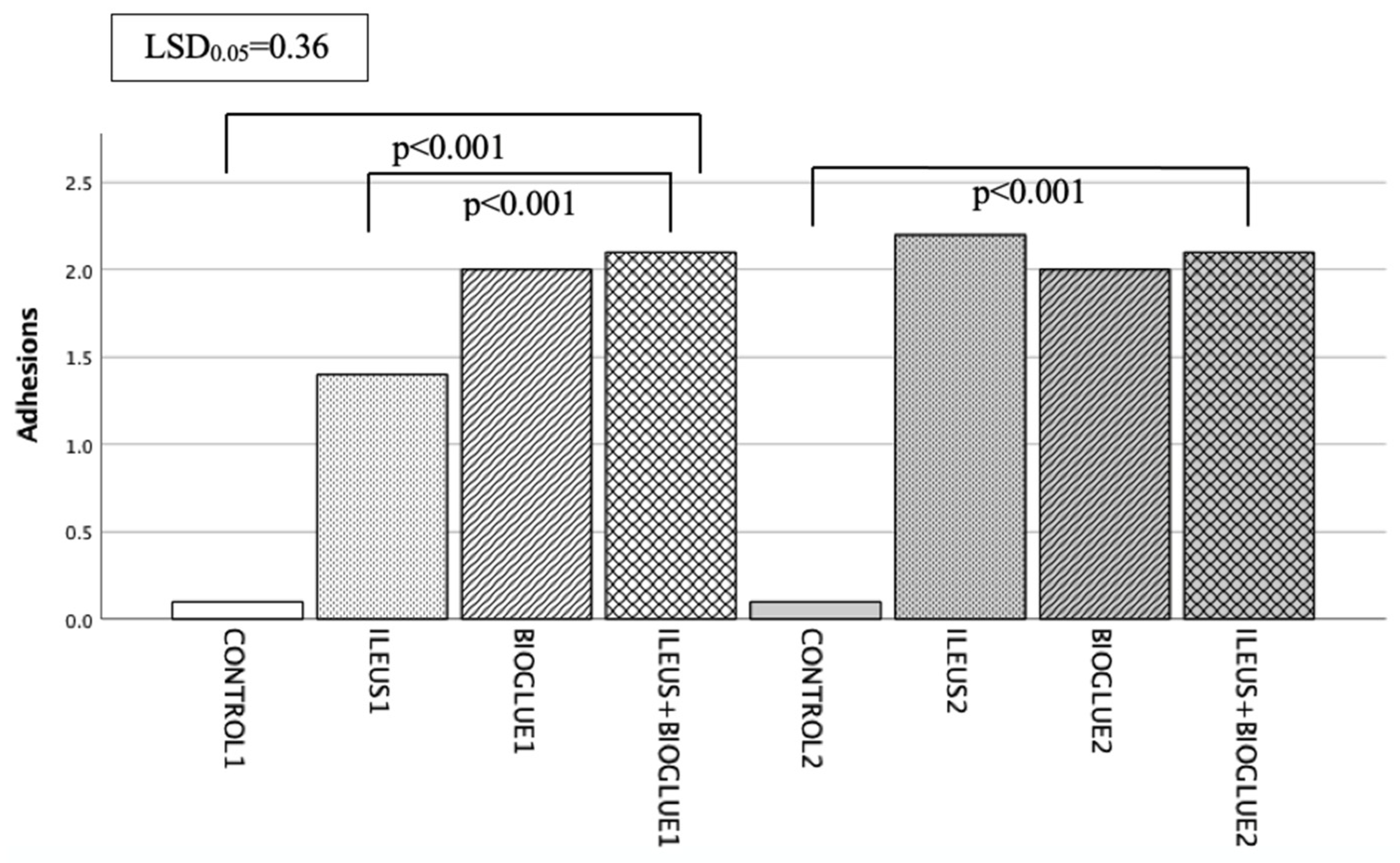
3.5. Adhesion Formation
3.6. Histological Assessment
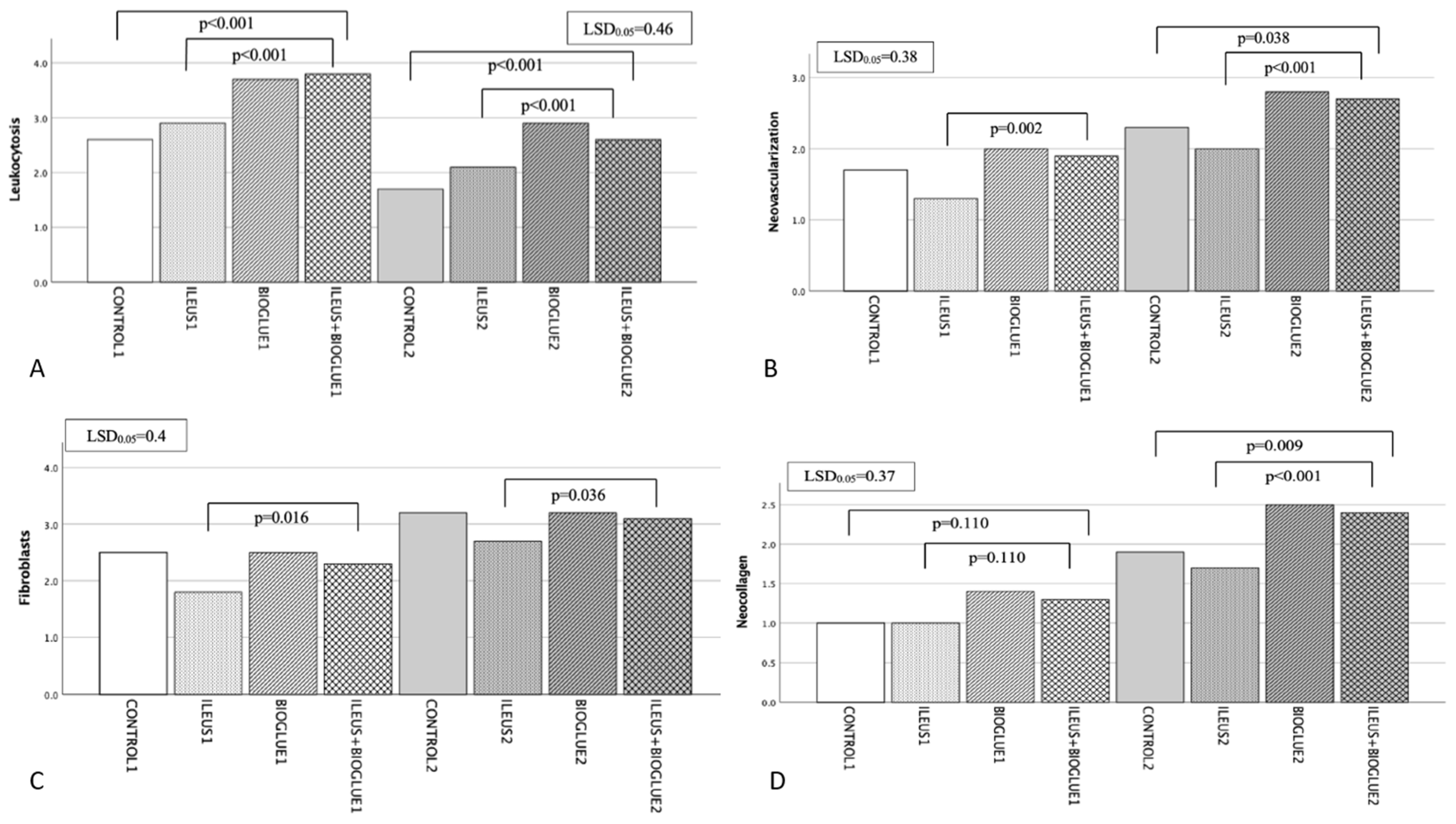
3.6.1. Leukocytosis
3.6.2. Neovascularization
3.6.3. Fibroblast Activity
3.6.4. Neocollagen Production
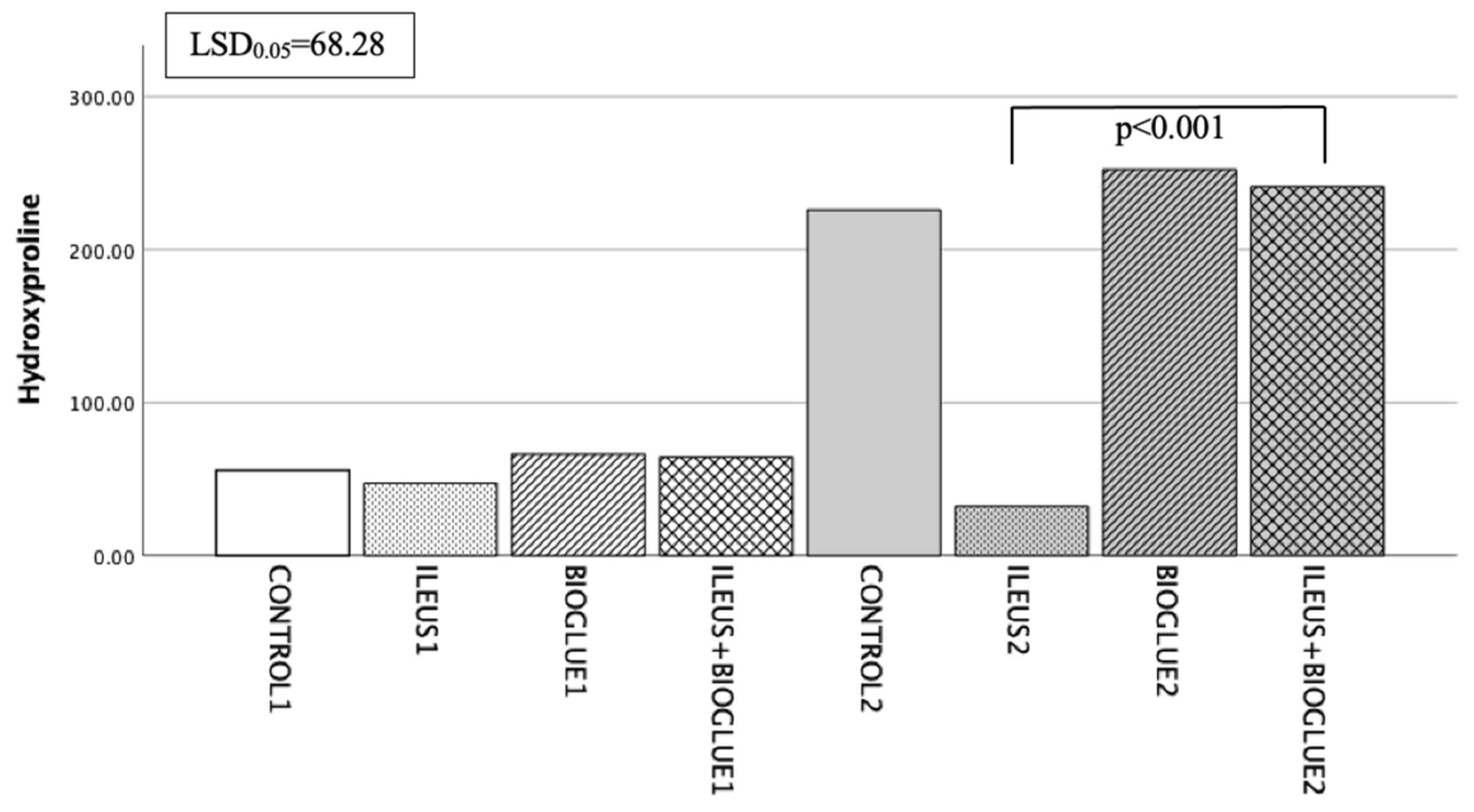
3.6.5. Hydroxyproline Levels
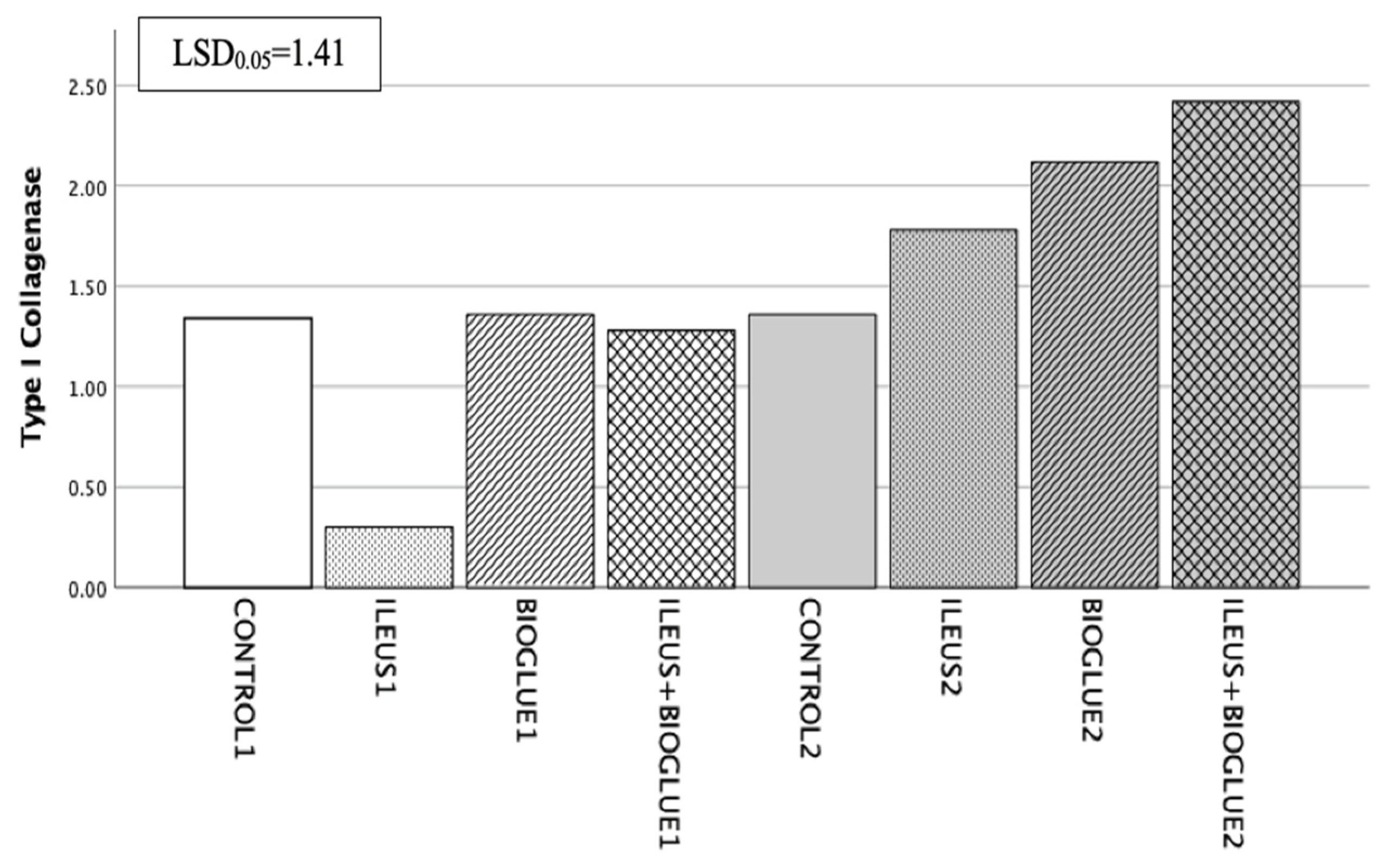
3.6.6. Type I Collagenase
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, I.Y.; Kim, B.R.; Kim, Y.W. The Impact of Anastomotic Leakage on Oncologic Outcomes and the Receipt and Timing of Adjuvant Chemotherapy after Colorectal Cancer Surgery. Int. J. Surg. 2015, 22, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Gallo, G.; Pata, F.; Vennix, S.; Laurberg, S.; Morton, D.; Rubbini, M.; Vaizey, C.; Magill, L.; Perry, R.; Sheward, N.; et al. Predictors for Anastomotic Leak, Postoperative Complications, and Mortality after Right Colectomy for Cancer: Results from an International Snapshot Audit. Dis. Colon Rectum 2020, 63, 606–618. [Google Scholar] [CrossRef]
- Zarnescu, E.C.; Zarnescu, N.O.; Costea, R. Updates of Risk Factors for Anastomotic Leakage after Colorectal Surgery. Diagnostics 2021, 11, 2382. [Google Scholar] [CrossRef]
- Geropoulos, G.; Psarras, K.; Giannis, D.; Martzivanou, E.C.; Papaioannou, M.; Kakos, C.D.; Pavlidis, E.T.; Symeonidis, N.; Koliakos, G.; Pavlidis, T.E. Platelet Rich Plasma Effectiveness in Bowel Anastomoses: A Systematic Review. World J. Gastrointest. Surg. 2021, 13, 1736–1753. [Google Scholar] [CrossRef]
- Buchs, N.C.; Gervaz, P.; Secic, M.; Bucher, P.; Mugnier-Konrad, B.; Morel, P. Incidence, Consequences, and Risk Factors for Anastomotic Dehiscence after Colorectal Surgery: A Prospective Monocentric Study. Int. J. Color. Dis. 2008, 23, 265–270. [Google Scholar] [CrossRef]
- Rahbari, N.N.; Weitz, J.; Hohenberger, W.; Heald, R.J.; Moran, B.; Ulrich, A.; Holm, T.; Wong, W.D.; Tiret, E.; Moriya, Y.; et al. Definition and Grading of Anastomotic Leakage Following Anterior Resection of the Rectum: A Proposal by the International Study Group of Rectal Cancer. Surgery 2010, 147, 339–351. [Google Scholar] [CrossRef]
- Lim, S.B.; Yu, C.S.; Kim, C.W.; Yoon, Y.S.; Park, I.J.; Kim, J.C. The Types of Anastomotic Leakage That Develop Following Anterior Resection for Rectal Cancer Demonstrate Distinct Characteristics and Oncologic Outcomes. Int. J. Color. Dis. 2015, 30, 1533–1540. [Google Scholar] [CrossRef]
- Kang, J.; Kim, H.; Park, H.J.; Lee, B.; Lee, K.Y. Risk Factors and Economic Burden of Postoperative Anastomotic Leakage Related Events in Patients Who Underwent Surgeries for Colorectal Cancer. PLoS ONE 2022, 17, e0267950. [Google Scholar] [CrossRef]
- McDermott, F.D.; Heeney, A.; Kelly, M.E.; Steele, R.J.; Carlson, G.L.; Winter, D.C. Systematic Review of Preoperative, Intraoperative and Postoperative Risk Factors for Colorectal Anastomotic Leaks. Br. J. Surg. 2015, 102, 462–479. [Google Scholar] [CrossRef]
- Sakr, A.; Emile, S.H.; Abdallah, E.; Thabet, W.; Khafagy, W. Predictive Factors for Small Intestinal and Colonic Anastomotic Leak: A Multivariate Analysis. Indian J. Surg. 2017, 79, 555–562. [Google Scholar] [CrossRef]
- Niu, L.; Wang, J.; Zhang, P.; Zhao, X. Protective Ileostomy Does Not Prevent Anastomotic Leakage after Anterior Resection of Rectal Cancer. J. Int. Med. Res. 2020, 48, 300060520946520. [Google Scholar] [CrossRef]
- Abernethy, E.K.; Aly, E.H. Postoperative Ileus after Minimally Invasive Colorectal Surgery: A Summary of Current Strategies for Prevention and Management. Dig. Surg. 2024, 41, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Lluis, N.; Biondo, S. Prolonged Postoperative Ileus after Colorectal Surgery: Still an Unresolved Problem. Ann. Laparosc. Endosc. Surg. 2018, 3, 15. [Google Scholar] [CrossRef]
- Venara, A.; Hamel, J.-F.; Beyer-Berjot, L.; Vignaud, T.; Slim, K.; Abderrazak, M.; Abolo, H.; Abras, N.; Aissou, M.; Albertini, S.; et al. Link between Postoperative Ileus and Anastomotic Leakage: A Structural Equation Modelling Approach. Surg. Open Dig. Adv. 2021, 2, 100009. [Google Scholar] [CrossRef]
- Pavlidis, T.E. Carcinoma of the Colon Caused Intussusception Presenting with Obstructive Ileus. Int. J. Color. Dis. 2007, 22, 337. [Google Scholar] [CrossRef]
- Peters, E.G.; Dekkers, M.; van Leeuwen-Hilbers, F.W.; Daams, F.; Hulsewé, K.W.E.; de Jonge, W.J.; Buurman, W.A.; Luyer, M.D.P. Relation between Postoperative Ileus and Anastomotic Leakage after Colorectal Resection: A Post Hoc Analysis of a Prospective Randomized Controlled Trial. Color. Dis. 2017, 19, 667–674. [Google Scholar] [CrossRef]
- Vakalopoulos, K.A.; Daams, F.; Wu, Z.; Timmermans, L.; Jeekel, J.J.; Kleinrensink, G.J.; Van Der Ham, A.; Lange, J.F. Tissue Adhesives in Gastrointestinal Anastomosis: A Systematic Review. J. Surg. Res. 2013, 180, 290–300. [Google Scholar] [CrossRef]
- Nordentoft, T. Sealing of Gastrointestinal Anastomoses with Fibrin Glue Coated Collagen Patch. Dan. Med. J. 2015, 62, B5081. [Google Scholar]
- Cira, K.; Stocker, F.; Reischl, S.; Obermeier, A.; Friess, H.; Burgkart, R.; Neumann, P.A. Coating of Intestinal Anastomoses for Prevention of Postoperative Leakage: A Systematic Review and Meta-Analysis. Front. Surg. 2022, 9, 882173. [Google Scholar] [CrossRef]
- Panda, S.; Connolly, M.P.; Ramirez, M.G.; de Heredia, J.B. Costs Analysis of Fibrin Sealant for Prevention of Anastomotic Leakage in Lower Colorectal Surgery. Risk Manag. Healthc. Policy 2020, 13, 5–11. [Google Scholar] [CrossRef]
- Stanojković, Z.; Stanojević, G.; Stojanović, M.; Milić, D.; Zivić, S. Determination of fibrin glue with antibiotics on collagen production in colon anastomosis. Vojnosanit. Pregl. 2008, 65, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Houston, K.A.; Rotstein, O.D. Fibrin Sealant in High-Risk Colonic Anastomoses. Arch. Surg. 1988, 123, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Voloudakis, N.; Koutelidakis, I.; Christoforidis, E.; Atmatzidis, S.; Kotoreni, G.; Papaziogas, B.; Schizas, D.; Zavos, C.; Papalois, A.; Chatzimavroudis, G. Fibrin Glue and Coats Compromise the Integrity of Colonic Anastomosis: An Experimental Trial on Rats. Ann. Gastroenterol. 2024, 37, 216–224. [Google Scholar] [CrossRef]
- Leggat, P.A.; Smith, D.R.; Kedjarune, U. Surgical Applications of Cyanoacrylate Adhesives: A Review of Toxicity. ANZ J. Surg. 2007, 77, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Passage, J.; Jalali, H.; Tam, R.K.W.; Harrocks, S.; O’Brien, M.F. BioGlue Surgical Adhesive—An Appraisal of Its Indications in Cardiac Surgery. Ann. Thorac. Surg. 2002, 74, 432–437. [Google Scholar] [CrossRef]
- Gundry, S.R.; Black, K.; Izutani, H. Sutureless Coronary Artery Bypass with Biologic Glued Anastomoses: Preliminary in Vivo and in Vitro Results. J. Thorac. Cardiovasc. Surg. 2000, 120, 473–477. [Google Scholar] [CrossRef]
- Bavaria, J.E.; Brinster, D.R.; Gorman, R.C.; Woo, Y.J.; Gleason, T.; Pochettino, A. Advances in the Treatment of Acute Type A Dissection: An Integrated Approach. Ann. Thorac. Surg. 2002, 74, 2–6. [Google Scholar] [CrossRef]
- Hewitt, C.W.; Marra, S.W.; Kann, B.R.; Tran, H.S.; Puc, M.M.; Chrzanowski, F.A.J.; Tran, J.L.; Lenz, S.D.; Cilley, J.H.J.; Simonetti, V.A.; et al. BioGlue Surgical Adhesive for Thoracic Aortic Repair during Coagulopathy: Efficacy and Histopathology. Ann. Thorac. Surg. 2001, 71, 1609–1612. [Google Scholar] [CrossRef]
- Zehr, K.J. Use of Bovine Albumin-Glutaraldehyde Glue in Cardiovascular Surgery. Ann. Thorac. Surg. 2007, 84, 1048–1052. [Google Scholar] [CrossRef]
- Raanani, E.; Latter, D.A.; Errett, L.E.; Bonneau, D.B.; Leclerc, Y.; Salasidis, G.C. Use of “BioGlue” in Aortic Surgical Repair. Ann. Thorac. Surg. 2001, 72, 638–640. [Google Scholar] [CrossRef]
- Rathinam, S.; Naidu, B.V.; Nanjaiah, P.; Loubani, M.; Kalkat, M.S.; Rajesh, P.B. BioGlue and Peri-Strips in Lung Volume Reduction Surgery: Pilot Randomised Controlled Trial. J. Cardiothorac. Surg. 2009, 4, 37. [Google Scholar] [CrossRef]
- Kocherov, S.; Lev, G.; Chertin, B. Use of BioGlue Surgical Adhesive in Hypospadias Repair. Curr. Urol. 2014, 7, 132–135. [Google Scholar] [CrossRef]
- de Lucena, M.T.; Mathias, C.A.; de Pontes Filho, N.T.; Cândido, A.C.L.; Vasconcelos, E. Influência Da Cola Bioglue® Na Deiscência de Anastomose Colônica: Estudo Experimental. Rev. Bras. De Coloproctol. 2007, 27, 158–166. [Google Scholar] [CrossRef]
- Ekici, Y.; Yılmaz Akçay, E.; Moray, G. Effect of the Bioadhesive, BioGlue, on Impaired Colonic Anastomose Healing in Rats. Int. Surg. 2015, 100, 1375–1381. [Google Scholar] [CrossRef]
- Despoudi, K.; Mantzoros, I.; Ioannidis, O.; Loutzidou, L.; Christidis, P.; Chatzakis, C.; Gkasdaris, G.; Raptis, D.; Pramateftakis, M.G.; Angelopoulos, S.; et al. Healing of Colonic Anastomosis in Rats under Obstructive Ileus Conditions. Discoveries 2021, 9, e129. [Google Scholar] [CrossRef]
- Despoudi, K.; Mantzoros, I.; Ioannidis, O.; Cheva, A.; Antoniou, N.; Konstantaras, D.; Symeonidis, S.; Pramateftakis, M.G.; Kotidis, E.; Angelopoulos, S.; et al. Effects of Albumin/Glutaraldehyde Glue on Healing of Colonic Anastomosis in Rats. World J. Gastroenterol. 2017, 23, 5680–5691. [Google Scholar] [CrossRef]
- de la Portilla, F.; Rada, R.; León, E.; Cisneros, N.; Maldonado, V.H.; Espinosa, E. Evaluation of the Use of BioGlue in the Treatment of High Anal Fistulas: Preliminary Results of a Pilot Study. Dis. Colon Rectum 2007, 50, 218–222. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE Guidelines 2.0: Updated Guidelines for Reporting Animal Research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Aguilar-Nascimento, J.E.; Mathie, R.T.; Man, W.K.; Williamson, R.C. Enhanced Intra-Anastomotic Healing by Operative Lavage with Nutrient Solutions in Experimental Left-Sided Colonic Obstruction. Br. J. Surg. 1995, 82, 461–464. [Google Scholar] [CrossRef]
- Galanopoulos, G.; Raptis, D.; Pramateftakis, M.-G.; Mantzoros, I.; Kanellos, I.; Lazarides, C. The Effects of Iloprost on Colonic Anastomotic Healing in Rats under Obstructive Ileus Conditions. J. Surg. Res. 2014, 189, 22–31. [Google Scholar] [CrossRef]
- Malone, P.S. An Experimental Single Layer Colonic Anastomosis. Ir. J. Med. Sci. 1985, 154, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Ceran, C.; Aksoy, R.T.; Gülbahar, Ö.; Öztürk, F. The Effects of Ghrelin on Colonic Anastomosis Healing in Rats. Clinics 2013, 68, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Krasniqi, A.; Gashi-Luci, L.; Krasniqi, S.; Jakupi, M.; Hashani, S.; Limani, D.; Dreshaj, I.A. A Comparison of Three Single Layer Anastomotic Techniques in the Colon of the Rat. Int. J. Surg. 2009, 7, 31–35. [Google Scholar] [CrossRef]
- Kanellos, I.; Mantzoros, I.; Demetriades, H.; Kalfadis, S.; Sakkas, L.; Kelpis, T.; Betsis, D. Sutureless Colonic Anastomosis in the Rat: A Randomized Controlled Study. Tech. Coloproctol. 2002, 6, 143–146. [Google Scholar] [CrossRef]
- van der Ham, A.C.; Kort, W.J.; Weijma, I.M.; van den Ingh, H.F.; Jeekel, H. Effect of Antibiotics in Fibrin Sealant on Healing Colonic Anastomoses in the Rat. Br. J. Surg. 1992, 79, 525–528. [Google Scholar] [CrossRef]
- Sapidis, N.; Tziouvaras, C.; Ioannidis, O.; Kalaitsidou, I.; Botsios, D. The Effect of Glutamine and Synbiotics on the Healing of Colonic Anastomosis. Rev. Esp. Enfermedades Dig. 2014, 106, 255–262. [Google Scholar]
- Ntampakis, G.; Pramateftakis, M.-G.; Ioannidis, O.; Bitsianis, S.; Christidis, P.; Symeonidis, S.; Koliakos, G.; Karakota, M.; Bekiari, C.; Tsakona, A.; et al. The Role of Adipose Tissue Mesenchymal Stem Cells in Colonic Anastomosis Healing in Inflammatory Bowel Disease: Experimental Study in Rats. J. Clin. Med. 2023, 12, 6336. [Google Scholar] [CrossRef]
- Phillips, J.D.; Kim, C.S.; Fonkalsrud, E.W.; Zeng, H.; Dindar, H. Effects of Chronic Corticosteroids and Vitamin A on the Healing of Intestinal Anastomoses. Am. J. Surg. 1992, 163, 71–77. [Google Scholar] [CrossRef]
- Shomaf, M. Histopathology of Human Intestinal Anastomosis. East. Mediterr. Health J. 2003, 9, 413–421. [Google Scholar] [CrossRef]
- Akyuz, C.; Yasar, N.F.; Uzun, O.; Peker, K.D.; Sunamak, O.; Duman, M.; Sehirli, A.O.; Yol, S. Effects of Melatonin on Colonic Anastomosis Healing Following Chemotherapy in Rats. Singap. Med. J. 2018, 59, 545–549. [Google Scholar] [CrossRef]
- Reddy, G.K.; Enwemeka, C.S. A Simplified Method for the Analysis of Hydroxyproline in Biological Tissues. Clin. Biochem. 1996, 29, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Rosendorf, J.; Klicova, M.; Herrmann, I.; Anthis, A.; Cervenkova, L.; Palek, R.; Treska, V.; Liska, V. Intestinal Anastomotic Healing: What Do We Know About Processes Behind Anastomotic Complications. Front. Surg. 2022, 9, 904810. [Google Scholar] [CrossRef] [PubMed]
- Reischl, S.; Wilhelm, D.; Friess, H.; Neumann, P.-A. Innovative Approaches for Induction of Gastrointestinal Anastomotic Healing: An Update on Experimental and Clinical Aspects. Langenbeck’s Arch. Surg. 2021, 406, 971–980. [Google Scholar] [CrossRef]
- Morgan, R.B.; Shogan, B.D. The Science of Anastomotic Healing. Semin. Colon Rectal Surg. 2022, 33, 100879. [Google Scholar] [CrossRef]
- Jongen, A.C.; Bosmans, J.W.; Kartal, S.; Lubbers, T.; Sosef, M.; Slooter, G.D.; Stoot, J.H.; van Schooten, F.-J.; Bouvy, N.D.; Derikx, J.P. Predictive Factors for Anastomotic Leakage After Colorectal Surgery: Study Protocol for a Prospective Observational Study (REVEAL Study). JMIR Res. Protoc. 2016, 5, e90. [Google Scholar] [CrossRef]
- Tsalikidis, C.; Mitsala, A.; Mentonis, V.I.; Romanidis, K.; Pappas-Gogos, G.; Tsaroucha, A.K.; Pitiakoudis, M. Predictive Factors for Anastomotic Leakage Following Colorectal Cancer Surgery: Where Are We and Where Are We Going? Curr. Oncol. 2023, 30, 3111–3137. [Google Scholar] [CrossRef]
- Kevin Emeka, C. Prolonged Post-Operative Paralytic Ileus and Anastomotic Leak: Guilty or Not Guilty. Biomed. J. Sci. Tech. Res. 2022, 46, 37954–37957. [Google Scholar] [CrossRef]
- Ntampakis, G.; Pramateftakis, M.-G.; Anestiadou, E.; Bitsianis, S.; Ioannidis, O.; Bekiari, C.; Koliakos, G.; Karakota, M.; Tsakona, A.; Cheva, A.; et al. Experimental Models of High-Risk Bowel Anastomosis in Rats: A Systematic Review. World J. Exp. Med. 2024, 14, 94135. [Google Scholar] [CrossRef]
- Boeding, J.R.E.; Ramphal, W.; Crolla, R.M.P.H.; Boonman-de Winter, L.J.M.; Gobardhan, P.D.; Schreinemakers, J.M.J. Ileus Caused by Obstructing Colorectal Cancer—Impact on Long-Term Survival. Int. J. Color. Dis. 2018, 33, 1393–1400. [Google Scholar] [CrossRef]
- Gainant, A. Emergency Management of Acute Colonic Cancer Obstruction. J. Visc. Surg. 2012, 149, e3–e10. [Google Scholar] [CrossRef]
- Ioannidis, O.; Ramirez, J.M.; Ubieto, J.M.; Feo, C.V.; Arroyo, A.; Kocián, P.; Sánchez-Guillén, L.; Bellosta, A.P.; Whitley, A.; Enguita, A.B.; et al. The EUPEMEN (EUropean PErioperative MEdical Networking) Protocol for Bowel Obstruction: Recommendations for Perioperative Care. J. Clin. Med. 2023, 12, 4185. [Google Scholar] [CrossRef] [PubMed]
- Lindeström, L.M.; Ekblad, E. Structural and Neuronal Changes in Rat Ileum after Ischemia with Reperfusion. Dig. Dis. Sci. 2004, 49, 1212–1222. [Google Scholar] [CrossRef] [PubMed]
- Pommergaard, H.C.; Achiam, M.P.; Burcharth, J.; Rosenberg, J. Impaired Blood Supply in the Colonic Anastomosis in Mice Compromises Healing. Int. Surg. 2015, 100, 70–76. [Google Scholar] [CrossRef]
- Farias Rolim, M.; Riger, C.J.; Eleutherio, E.C.; da Fonseca Colão, C.; Cotta Pereira, G.; Schanaider, A. Colonic Healing after Portal Ischemia and Reperfusion: An Experimental Study with Oxidative Stress Biomarkers. Redox Rep. 2007, 12, 267–274. [Google Scholar] [CrossRef]
- Renner, P.; Kienle, K.; Dahlke, M.H.; Heiss, P.; Pfister, K.; Stroszczynski, C.; Piso, P.; Schlitt, H.J. Intestinal Ischemia: Current Treatment Concepts. Langenbeck’s Arch. Surg. 2011, 396, 3–11. [Google Scholar] [CrossRef]
- Grootjans, J.; Lenaerts, K.; Derikx, J.P.M.; Matthijsen, R.A.; De Bruïne, A.P.; Van Bijnen, A.A.; Van Dam, R.M.; Dejong, C.H.C.; Buurman, W.A. Human Intestinal Ischemia-Reperfusion-Induced Inflammation Characterized: Experiences from a New Translational Model. Am. J. Pathol. 2010, 176, 2283–2291. [Google Scholar] [CrossRef]
- Sand, E.; Themner-Persson, A.; Ekblad, E. Infiltration of Mast Cells in Rat Colon Is a Consequence of Ischemia/Reperfusion. Dig. Dis. Sci. 2008, 53, 3158–3169. [Google Scholar] [CrossRef]
- Raptis, D.; Pramateftakis, M.-G.; Kanellos, I. Our 20-Year Experience with Experimental Colonic Anastomotic Healing. J. Med. Life 2018, 11, 5–14. [Google Scholar]
- Kanellos, D.; Blouhos, K.; Pramateftakis, M.G.; Kanellos, I.; Demetriades, H.; Sakkas, L.; Betsis, D. Effect of 5-Fluorouracil plus Interferon on the Integrity of Colonic Anastomoses Covering with Fibrin Glue. World J. Surg. 2007, 31, 186–191. [Google Scholar] [CrossRef]
- Rijcken, E.; Sachs, L.; Fuchs, T.; Spiegel, H.U.; Neumann, P.A. Growth Factors and Gastrointestinal Anastomotic Healing. J. Surg. Res. 2014, 187, 202–210. [Google Scholar] [CrossRef]
- Bobkiewicz, A.; Studniarek, A.; Krokowicz, L.; Szmyt, K.; Borejsza-Wysocki, M.; Szmeja, J.; Marciniak, R.; Drews, M.; Banasiewicz, T. Gastrointestinal Tract Anastomoses with the Biofragmentable Anastomosis Ring: Is It Still a Valid Technique for Bowel Anastomosis? Analysis of 203 Cases and Review of the Literature. Int. J. Color. Dis. 2017, 32, 107–111. [Google Scholar] [CrossRef]
- Gocoł, R.; Bis, J.; Hudziak, D.; Morkisz, Ł.; Deja, M.A. Aortic Root Reconstruction with Tachosil Fibrin Sealant Patch in Acute Type a Aortic Dissection. Ann. Thorac. Cardiovasc. Surg. 2021, 27, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Prodromidou, A.; Iavazzo, C.; Fotiou, A.; Psomiadou, V.; Drakou, M.; Vorgias, G.; Kalinoglou, N. The Application of Fibrin Sealant for the Prevention of Lymphocele after Lymphadenectomy in Patients with Gynecological Malignancies: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Gynecol. Oncol. 2019, 153, 201–208. [Google Scholar] [CrossRef]
- Nerstrøm, M.; Krarup, P.-M.; Jorgensen, L.N.; Ågren, M.S. Therapeutic Improvement of Colonic Anastomotic Healing under Complicated Conditions: A Systematic Review. World J. Gastrointest. Surg. 2016, 8, 389. [Google Scholar] [CrossRef]
- Erbil, Y.; Calis, A.; Berber, E.; Mercan, S. The Effect of Intraoperative Colonic Lavage with NG-Nitro-L-Arginine Methyl Ester (L-NAME) on Anastomotic Healing in the Presence of Left-Sided Colonic Obstruction in the Rat. Surg. Today 2000, 30, 421–425. [Google Scholar] [CrossRef]
- Faruquzzaman; Mazumder, S.K. The Healing Role of Erythropoietin in the Obstructive vs. Nonobstructive Left Colonic Anastomosis. Bratisl. Lek. Listy 2009, 110, 530–535. [Google Scholar]
- Gulcelik, M.A.; Dinc, S.; Bir, F.; Elitok, O.; Alagol, H.; Oz, M. Locally Applied Molgramostim Improves Wound Healing at Colonic Anastomoses in Rats after Ligation of the Common Bile Duct. Can. J. Surg. 2005, 48, 213–218. [Google Scholar]
- Bahouth, Z.; Moskovitz, B.; Halachmi, S.; Nativ, O. Bovine Serum Albumin-Glutaraldehyde (BioGlue®) Tissue Adhesive versus Standard Renorrhaphy Following Renal Mass Enucleation: A Retrospective Comparison. Ther. Adv. Urol. 2017, 9, 67–72. [Google Scholar] [CrossRef]
- Abbas, M.A.; Tejirian, T. Bioglue for the Treatment of Anal Fistula Is Associated with Acute Anal Sepsis. Dis. Colon Rectum 2008, 51, 1155. [Google Scholar] [CrossRef]
- Giuratrabocchetta, S.; Rinaldi, M.; Cuccia, F.; Lemma, M.; Piscitelli, D.; Polidoro, P.; Altomare, D.F. Protection of Intestinal Anastomosis with Biological Glues: An Experimental Randomized Controlled Trial. Tech. Coloproctol. 2011, 15, 153–158. [Google Scholar] [CrossRef]
- Saunders, M.M.; Baxter, Z.C.; Abou-Elella, A.; Kunselman, A.R.; Trussell, J.C. BioGlue and Dermabond Save Time, Leak Less, and Are Not Mechanically Inferior to Two-Layer and Modified One-Layer Vasovasostomy. Fertil. Steril. 2009, 91, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.D.; Doty, D.B.; Doty, J.R.; Yuksel, U.; Flinner, R. BioGlue®: A Protective Barrier after Pericardiotomy. J. Card. Surg. 2007, 22, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Mohan, H.; Beddy, D.; Latif, A.; Bangash, T.; Quill, D.; Traynor, O. The “Flying” Bile Duct: Avulsion of the Common Bile Duct in a Plane Crash Survivor. Ir. J. Med. Sci. 2009, 178, 523–525. [Google Scholar] [CrossRef]
- De Somer, F.; Delanghe, J.; Somers, P.; Debrouwere, M.; Van Nooten, G. Mechanical and Chemical Characteristics of an Autologous Glue. J. Biomed. Mater. Res.-Part A 2008, 86, 1106–1112. [Google Scholar] [CrossRef]
- Glickman, M.; Gheissari, A.; Money, S.; Martin, J.; Ballard, J.L. A Polymeric Sealant Inhibits Anastomotic Suture Hole Bleeding More Rapidly than Gelfoam/Thrombin: Results of a Randomized Controlled Trial. Arch. Surg. 2002, 137, 326–332. [Google Scholar] [CrossRef]
- Chen, X.; Qin, R.; Liu, B.; Ma, Y.; Su, Y.; Yang, C.S.; Glickman, J.N.; Odze, R.D.; Shaheen, N.J. Multilayered Epithelium in a Rat Model and Human Barrett’s Esophagus: Similar Expression Patterns of Transcription Factors and Differentiation Markers. BMC Gastroenterol. 2008, 8, 1. [Google Scholar] [CrossRef]
- Dusick, J.R.; Mattozo, C.A.; Esposito, F.; Kelly, D.F. BioGlue® for Prevention of Postoperative Cerebrospinal Fluid Leaks in Transsphenoidal Surgery: A Case Series. Surg. Neurol. 2006, 66, 371–376. [Google Scholar] [CrossRef]
- Seretis, C.; Kaisari, P.; Wanigasooriya, K.; Shariff, U.; Youssef, H. Malnutrition Is Associated with Adverse Postoperative Outcome in Patients Undergoing Elective Colorectal Cancer Resections. J. BUON 2018, 23, 36–41. [Google Scholar]
- Sripathi, S.; Khan, M.I.; Patel, N.; Meda, R.T.; Nuguru, S.P.; Rachakonda, S. Factors Contributing to Anastomotic Leakage Following Colorectal Surgery: Why, When, and Who Leaks? Cureus 2022, 14, e29964. [Google Scholar] [CrossRef]
- Krarup, P.-M.; Eld, M.; Jorgensen, L.N.; Hansen, M.B.; Ågren, M.S. Selective Matrix Metalloproteinase Inhibition Increases Breaking Strength and Reduces Anastomotic Leakage in Experimentally Obstructed Colon. Int. J. Color. Dis. 2017, 32, 1277–1284. [Google Scholar] [CrossRef]
- Raptis, D.; Mantzoros, I.; Pramateftakis, M.G.; Despoudi, K.; Zaraboukas, T.; Koliakos, G.; Kanellos, I.; Lazarides, C. The Effects of Tacrolimus on Colonic Anastomotic Healing in Rats. Int. J. Color. Dis. 2012, 27, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Ghiselli, R.; Lucarini, G.; Ortenzi, M.; Salvolini, E.; Saccomanno, S.; Orlando, F.; Provinciali, M.; Casciani, F.; Guerrieri, M. Anastomotic Healing in a Rat Model of Peritonitis after Non-Steroidal Anti-Inflammatory Drug Administration. Eur. J. Histochem. 2020, 64, 3085. [Google Scholar] [CrossRef] [PubMed]
- van der Vijver, R.J.; van Laarhoven, C.J.H.M.; de Man, B.M.; Lomme, R.M.L.M.; Hendriks, T. The Effect of Fibrin Glue on the Early Healing Phase of Intestinal Anastomoses in the Rat. Int. J. Color. Dis. 2012, 27, 1101–1107. [Google Scholar] [CrossRef]
- Desmoulière, A.; Chaponnier, C.; Gabbiani, G. Tissue Repair, Contraction, and the Myofibroblast. Wound Repair Regen. 2005, 13, 7–12. [Google Scholar] [CrossRef]
- Sousa, A.A.; Petroianu, A.; Trapiello Neto, V.; Rios, V.S.; Barbosa, A.J.A. Effect of Sodium Carboxymethylcellulose and Methylprednisolone on the Healing of Jejunal Anastomoses in Rats. Braz. J. Med. Biol. Res. 2001, 34, 519–523. [Google Scholar] [CrossRef]
- Gerogiannis, I.; Papalois, A.; Psalla, D.; Kambaroudis, A. Beneficiary Effect of Fibrin Glue on Healing of Ileoileal Anastomoses in Rats. In Vivo 2022, 36, 221–226. [Google Scholar] [CrossRef]
- Deiters, U.; Barsig, J.; Tawil, B.; Mühlradt, P.F. The Macrophage-Activating Lipopeptide-2 Accelerates Wound Healing in Diabetic Mice. Exp. Dermatol. 2004, 13, 731–739. [Google Scholar] [CrossRef]
- Vakalopoulos, K.A.; Wu, Z.; Kroese, L.F.; van der Horst, P.H.; Lam, K.H.; Dodou, D.; Jeekel, J.J.; Lange, J.F. Clinical, Mechanical, and Immunohistopathological Effects of Tissue Adhesives on the Colon: An in-Vivo Study. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2017, 105, 846–854. [Google Scholar] [CrossRef]
- Distler, J.H.W.; Hirth, A.; Kurowska-Stolarska, M.; Gay, R.E.; Gay, S.; Distler, O. Angiogenic and Angiostatic Factors in the Molecular Control of Angiogenesis. Q. J. Nucl. Med. 2003, 47, 149–161. [Google Scholar]
- Citgez, B.; Cengiz, A.N.; Akgun, I.; Uludag, M.; Yetkin, G.; Bahat, N.; Ozcan, O.; Polat, N.; Akcakaya, A.; Karatepe, O. Effects of Chitosan on Healing and Strength of Colonic Anastomosis in Rats|Efeitos de Quitosana Na Cicatrização e Resistência de Anastomose Colônica Em Ratos. Acta Cir. Bras. 2012, 27, 707–712. [Google Scholar] [CrossRef]
- Stergios, K.; Frountzas, M.; Pergialiotis, V.; Korou, L.M.; Kontzoglou, K.; Stefanidis, K.; Nikiteas, N.; Perrea, D.N.; Vaos, G. The Effect of TISSEEL® on Colorectal Anastomosis Healing Process in a Diabetic Animal Experimental Model. In Vivo 2020, 34, 659–665. [Google Scholar] [CrossRef]
- Yol, S.; Tekin, A.; Yilmaz, H.; Küçükkartallar, T.; Esen, H.; Çaǧlayan, O.; Tatkan, Y. Effects of Platelet Rich Plasma on Colonic Anastomosis. J. Surg. Res. 2008, 146, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Göksu, M.; Alakuş, H.; Ertan, S.; Akgün, S. Effect of Platelet-Rich Plasma on Colon Anastomosis in Rats in Which Hyperthermic Intra-Peritoneal Chemotherapy Was Performed Using 5-Fluorouracil. ANZ J. Surg. 2020, 90, 2290–2297. [Google Scholar] [CrossRef] [PubMed]
- Chowcat, N.L.; Savage, F.J.; Lewin, M.R.; Boulos, P.B. Direct Measurement of Collagenase in Colonic Anastomosis. Br. J. Surg. 1990, 77, 1284–1287. [Google Scholar] [CrossRef]
- Erasmi, A.W.; Sievers, H.H.; Wolschläger, C. Inflammatory Response after BioGlue Application. Ann. Thorac. Surg. 2002, 73, 1025–1026. [Google Scholar]
- Slieker, J.C.; Vakalopoulos, K.A.; Komen, N.A.; Jeekel, J.; Lange, J.F. Prevention of Leakage by Sealing Colon Anastomosis: Experimental Study in a Mouse Model. J. Surg. Res. 2013, 184, 819–824. [Google Scholar] [CrossRef]
- Nordentoft, T.; Pommergaard, H.-C.; Rosenberg, J.; Achiam, M.P. Fibrin Glue Does Not Improve Healing of Gastrointestinal Anastomoses: A Systematic Review. Eur. Surg. Res. 2015, 54, 1–13. [Google Scholar] [CrossRef]
- Petrie, E.M. Cyanoacrylate Adhesives in Surgical Applications Cyanoacrylate Adhesives in Surgical Applications: A Critical Review. In Progress in Adhesion and Adhesives; Wiley: Hoboken, NJ, USA, 2018. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Despoudi, K.; Mantzoros, I.; Ioannidis, O.; Anestiadou, E.; Symeonidis, S.; Bitsianis, S.; Kotidis, E.; Pramateftakis, M.G.; Bourtzinakou, A.A.; Salta-Poupnara, E.; et al. The Effect of Albumin/Glutaraldehyde Glue (Bioglue) on Colonic Anastomosis Under Intestinal Obstruction: An Experimental Study in Rats. J. Clin. Med. 2025, 14, 2457. https://doi.org/10.3390/jcm14072457
Despoudi K, Mantzoros I, Ioannidis O, Anestiadou E, Symeonidis S, Bitsianis S, Kotidis E, Pramateftakis MG, Bourtzinakou AA, Salta-Poupnara E, et al. The Effect of Albumin/Glutaraldehyde Glue (Bioglue) on Colonic Anastomosis Under Intestinal Obstruction: An Experimental Study in Rats. Journal of Clinical Medicine. 2025; 14(7):2457. https://doi.org/10.3390/jcm14072457
Chicago/Turabian StyleDespoudi, Kalliopi, Ioannis Mantzoros, Orestis Ioannidis, Elissavet Anestiadou, Savvas Symeonidis, Stefanos Bitsianis, Efstathios Kotidis, Manousos George Pramateftakis, Antonia Aikaterini Bourtzinakou, Eleni Salta-Poupnara, and et al. 2025. "The Effect of Albumin/Glutaraldehyde Glue (Bioglue) on Colonic Anastomosis Under Intestinal Obstruction: An Experimental Study in Rats" Journal of Clinical Medicine 14, no. 7: 2457. https://doi.org/10.3390/jcm14072457
APA StyleDespoudi, K., Mantzoros, I., Ioannidis, O., Anestiadou, E., Symeonidis, S., Bitsianis, S., Kotidis, E., Pramateftakis, M. G., Bourtzinakou, A. A., Salta-Poupnara, E., Angelopoulos, K., Driagka, B., Tserkezidis, F., & Angelopoulos, S. (2025). The Effect of Albumin/Glutaraldehyde Glue (Bioglue) on Colonic Anastomosis Under Intestinal Obstruction: An Experimental Study in Rats. Journal of Clinical Medicine, 14(7), 2457. https://doi.org/10.3390/jcm14072457





