The Association Between Periodontal Disease and Acute Coronary Syndrome—A Clinical Analysis
Abstract
1. Introduction
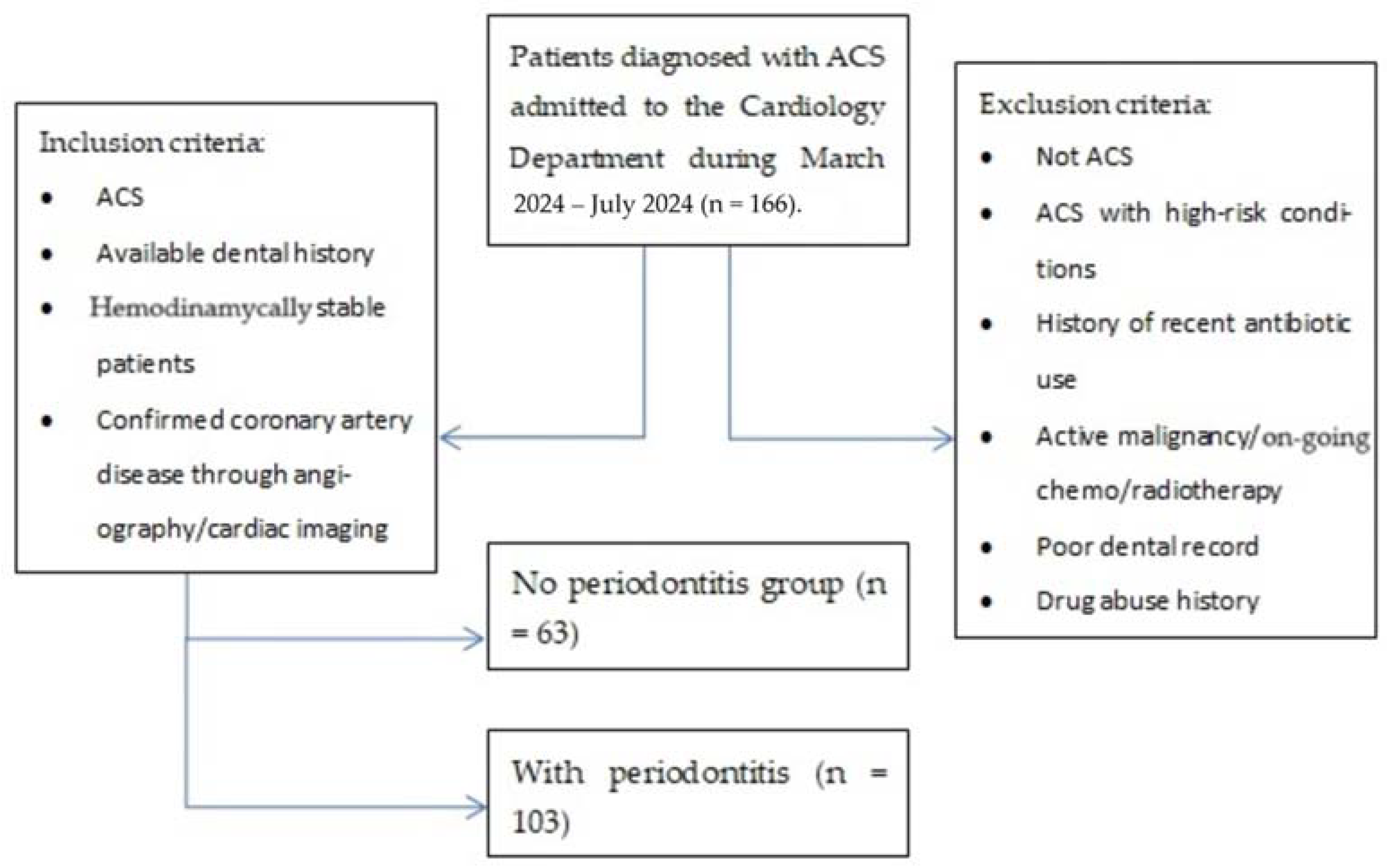
2. Materials and Methods
- An acute coronary syndrome (ACS) diagnosis (unstable angina, non-ST-elevation myocardial infarction—N-STEMI, ST-elevation myocardial infarction—STEMI) based on the clinical presentation (chest pain over 30 min, dyspnea, diaphoresis, etc.), and paraclinical characteristics (ischemic electrocardiographic findings, with or without elevated cardiac enzyme levels);
- The patients’ electronic charts revealed prior presentations to our hospital’s dentistry department as well as the oral and maxillofacial surgery department. All patients included in the study presented a history of early permanent teeth loss, partial edentulism, gingival bleeding, and dental imaging, with complete periodontal charts;
- Patients were hemodynamically stable patients in order to undergo the necessary assessment and data collection without immediate life-threatening complications;
- Patients who underwent coronary angiography or other cardiac imaging to confirm coronary artery disease.
- Patients admitted with suspicions of ACS, which was later denied;
- Patients admitted with ACS with other high-risk conditions (pulmonary embolism, septic shock, cardiac tamponade, etc.) that prevented undergoing coronary intervention during the duration of the study;
- History of recent antibiotic use (last 3 months) given that systemic antibiotics in the past three months could influence periodontal status;
- Active malignancies or patients undergoing chemo/radiotherapy as these conditions affect both CV as well as periodontal health;
- Poor dental records;
- History of drug abuse, particularly cocaine or amphetamines, contributing to ACS through non-atherosclerotic mechanisms.
- Group NP, the group with no periodontitis, consisted of 63 patients (26 men—41.3% and 37 women—58.7%), diagnosed with acute coronary syndrome based on clinical presentations, electrocardiographic findings, and elevated cardiac enzyme levels. Individuals in this group presented no dental history of periodontitis.
- Group WP (with periodontitis) comprised 103 patients (60 men—61.2% and 40 women—38.8%) diagnosed with ACS based on clinical presentations, EKG findings, and cardiac enzyme levels and presenting with a dental history of periodontitis with or without prior treatment.
- The presence of periodontal disease was established using visual inspection, periodontal probing, and bone level evaluations on radiography (in the patients’ medical records).
- Signs of periodontal disease included the following: active bleeding of the gingiva in the presence of absence of mild tissue manipulation, gingival retraction, halitosis, radiographic bone loss according to patients’ history, teeth loss, pain, and dental mobility.
3. Results
Data Analysis
4. Discussion
- Our study establishes an association between periodontal disease and acute coronary syndrome but does not prove causality. Longitudinal or interventional studies are required to determine whether periodontal treatment can directly reduce cardiovascular risk.
- The study population may not be fully representative of the general population, as it includes only patients who underwent coronary evaluation. This could overestimate the association between periodontitis and cardiovascular disease.
- The severity of periodontitis was not uniformly assessed using a single standardized diagnostic method across all participants. Variability in the classification of periodontal disease could affect the reliability of the findings.
- While we accounted for traditional cardiovascular risk factors (such as smoking and diabetes), other unmeasured variables, including genetic predisposition, dietary habits, and socioeconomic status, may influence both periodontal and cardiovascular health.
- Some risk factors, such as smoking habits and adherence to treatment, were based on self-reported data, which may be subject to recall bias or underreporting.
- Differences in study populations, sample sizes, and methodologies across various referenced studies may contribute to inconsistencies in the reported strength of the association between periodontitis and cardiovascular disease.
- The study does not include long-term follow-up to assess how periodontal disease progression influences cardiovascular outcomes over time.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sanz, M.; del Castillo, A.M.; Jepsen, S.; Gonzalez-Juanatey, J.R.; D’Aiuto, F.; Bouchard, P.; Chapple, I.; Dietrich, T.; Gotsman, I.; Graziani, F.; et al. Periodontitis and cardiovascular diseases: Consensus report. J. Clin. Periodontol. 2020, 47, 268–288. [Google Scholar] [CrossRef] [PubMed]
- Liccardo, D.; Cannavo, A.; Spagnuolo, G.; Ferrara, N.; Cittadini, A.; Rengo, C.; Rengo, G. Periodontal Disease: A Risk Factor for Diabetes and Cardiovascular Disease. Int. J. Mol. Sci. 2019, 20, 1414. [Google Scholar] [CrossRef]
- Kilgare, C. Periodontitis an Independent Risk Factor for CHD-coro-nary heart disease. Family Practice News, 1 August 2000. Available online: https://www.thefreelibrary.com/Periodontitis+an+Independent+Risk+Factor+for+CHD.-a065108414 (accessed on 31 March 2025).
- Beck, J.D.; Offenbacher, S. Systemic Effects of Periodontitis: Epidemiology of Periodontal Disease and Cardiovascular Disease. J. Periodontol. 2005, 76, 2089–2100. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, F.; Piepoli, M.; Hoes, A.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.; Cooney, M.; Corra, U.; Cosyns, B.; et al. 2016 European Guidelines on Cardiovascular Disease Prevention in Clinical Practice. Eur. Heart J. 2016, 37, 2315–2381. [Google Scholar] [CrossRef]
- Li, X.; Kolltveit, K.M.; Tronstad, L.; Olsen, I. Systemic Diseases Caused by Oral Infection. Clin. Microbiol. Rev. 2000, 13, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, T.; Jimenez, M.; Krall Kaye, E.A.; Vokonas, P.S.; Garcia, R.I. Age-dependent associations between chronic periodontitis/edentulism and risk of coronary heart disease. Circulation 2008, 117, 1668–1674. [Google Scholar]
- Winning, L.; Patterson, C.C.; Linden, K.; Evans, A.; Yarnel, J.; McKeown, P.P.; Kee, F.; Linden, G.J. Periodontitis and risk of prevalent and incident coronary heart disease events. J. Clin. Periodontol. 2020, 47, 1446–1456. [Google Scholar] [CrossRef] [PubMed]
- Heaton, B.; Applebaum, K.M.; Rothman, K.J.; Brooks, D.R.; Heeren, T.; Dietrich, T.; Garcia, R.I. The influence of prevalent cohort bias in the association between periodontal disease progression and incident coronary heart disease. Ann. Epidemiol. 2014, 24, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Khalid, T.; Awan, K.H. Chronic periodontitis and smoking. Prevalence and dose-response relationship. Saudi Med. J. 2016, 37, 889–894. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Northridge, M.E.; Kumar, A.; Kaur, R. Disparities in Access to Oral Health Care. Annu. Rev. Public Health 2020, 41, 513–535. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, H.F.; Lee, H.E.; Chen, I.T.; Huang, Y.T.; Ho, P.S.; Karim, S.A. Rural-urban disparities in the incidence and treatment intensity of periodontal disease among patients with diabetes. Front. Public Health 2023, 11, 1241150. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Theriault, H.; Bridge, G. Oral health equity for rural communities: Where are we now and where can we go from here? Br. Dent. J. 2023, 235, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Holde, G.E.; Oscarson, N.; Trovik, T.A.; Tillberg, A.; Jönsson, B. Periodontitis Prevalence and Severity in Adults: A Cross-Sectional Study in Norwegian Circumpolar Communities. J. Periodontol. 2017, 88, 1012–1022. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Curtis, J.; Hicklin, D.; Nichols, C.; Glover, S.; Merchant, A.T.; Hardin, J.W.; Logue, M.; Meyer, J.; Mason, E.; et al. Periodontal Disease Treatment After Stroke or Transient Ischemic Attack: The PREMIERS Study, a Randomized Clinical Trial. Stroke 2023, 54, 2214–2222. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wożakowska-Kapłon, B.; Włosowicz, M.; Gorczyca-Michta, I.; Górska, R. Oral health status and the occurrence and clinical course of myocardial infarction in hospital phase: A case-control study. Cardiol. J. 2013, 20, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Kodovazenitis, G.; Pitsavos, C.; Papadimitriou, L.; Vrotsos, I.A.; Stefanadis, C.; Madianos, P.N. Association between periodontitis and acute myocardial infarction: A case-control study of a nondiabetic population. J. Periodontal Res. 2014, 49, 246–252. [Google Scholar] [CrossRef]
- Noguchi, S.; Toyokawa, S.; Miyoshi, Y.; Suyama, Y.; Inoue, K.; Kobayashi, Y. Five-year follow-up study of the association between periodontal disease and myocardial infarction among Japanese male workers: MY Health Up Study. J. Public Health 2015, 37, 605–611. [Google Scholar] [CrossRef]
- Rydén, L.; Buhlin, K.; Ekstrand, E.; de Faire, U.; Gustafsson, A.; Holmer, J.; Kjellström, B.; Lindahl, B.; Norhammar, A.; Nygren, Å.; et al. Periodontitis Increases the Risk of a First Myocardial Infarction: A Report From the PAROKRANK Study. Circulation 2016, 133, 576–578. [Google Scholar] [CrossRef]
- Mattila, K.J.; Nieminen, M.S.; Valtonen, V.V.; Rasi, V.P.; Kesäniemi, Y.A.; Syrjälä, S.L.; Jungell, P.S.; Isoluoma, M.; Hietaniemi, K.; Jokinen, M.J. Association between dental health and acute myocardial infarction. BMJ 1989, 298, 779–781. [Google Scholar] [CrossRef]
- Willershausen, B.; Kasaj, A.; Willershausen, I.; Zahorka, D.; Briseño, B.; Blettner, M.; Genth-Zotz, S.; Münzel, T. Association between chronic dental infection and acute myocardial infarction. J. Endod. 2009, 35, 626–630. [Google Scholar] [CrossRef]
- Jansson, L.; Lavstedt, S.; Frithiof, L.; Theobald, H. Relationship between oral health and mortality in cardiovascular diseases. J. Clin. Periodontol. 2001, 28, 762–768. [Google Scholar] [PubMed]
- Voinescu, I.; Petre, A.; Burlibasa, M.; Oancea, L. Evidence of Connections Between Periodontitis and Ischemic Cardiac Disease—An Updated Systematic Review. Maedica 2019, 14, 384–390. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Spahr, A.; Klein, E.; Khuseyinova, N.; Boeckh, C.; Muche, R.; Kunze, M.; Rothenbacher, D.; Pezeshki, G.; Hoffmeister, A.; Koenig, W. Periodontal Infections and Coronary Heart Disease: Role of Periodontal Bacteria and Importance of Total Pathogen Burden in the Coronary Event and Periodontal Disease (CORODONT) Study. Arch. Intern. Med. 2006, 166, 554–559. [Google Scholar] [CrossRef]
- Hujoel, P.P.; Drangsholt, M.; Spiekerman, C.; Derouen, T.A. Examining the link between coronary heart disease and the elimination of chronic dental infections. J. Am. Dent. Assoc. 2001, 132, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Howell, T.H.; Ridker, P.M.; Ajani, U.A.; Hennekens, C.H.; Christen, W.G. Periodontal disease and risk of subsequent cardiovascular disease in U.S. male physicians. J. Am. Coll. Cardiol. 2001, 37, 445–450. [Google Scholar]
- Argueta-Figueroa, L.; Arzate-Sosa, G.; Mendieta-Zeron, H. Anesthetic efficacy of articaine for inferior alveolar nerve blocks in patients with symptomatic versus asymptomatic irreversible pulpitis. Gen. Dent. 2012, 60, e39–e43. [Google Scholar] [PubMed]
- Geismar, K.; Stoltze, K.; Sigurd, B.; Gyntelberg, F.; Holmstrup, P. Periodontal disease and coronary heart disease. J. Periodontol. 2006, 77, 1547–1554. [Google Scholar]
- Desvarieux, M.; Demmer, R.T.; Jacobs, D.R., Jr.; Rundek, T.; Boden-Albala, B.; Sacco, R.L.; Papapanou, P.N. Periodontal bacteria and hypertension: He oral infections and vascular disease epidemiology study (NV). J. Hypertens. 2010, 28, 1413–1421. [Google Scholar]
- Roth, G.A.; Forouzanfar, M.H.; Moran, A.E.; Barber, R.; Nguyen, G.; Feigin, V.L.; Naghavi, M.; Mensah, G.A.; Murray, C.J. Demographic and epidemiologic drivers of global cardiovascular mortality. N. Engl. J. Med. 2015, 372, 1333–1341. [Google Scholar] [CrossRef]
- Bui, F.Q.; Almeida-da-Silva, C.L.C.; Huynh, B.; Trinh, A.; Liu, J.; Woodward, J.; Asadi, H.; Ojcius, D.M. Association between periodontal pathogens and systemic disease. Biomed. J. 2019, 42, 27–35. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, N.-H.; Lee, G.-Y.; Park, S.-K.; Kim, Y.-J.; Lee, M.-Y.; Kim, C.-B. Provision of oral hygiene services as a potential method for preventing periodontal disease and control hypertension and diabetes in a community health centre in Korea. Health Soc. Care Community 2018, 26, e378–e385. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yuan, X.; Zheng, Q.; Mo, F.; Zhu, S.; Shen, T.; Yang, W.; Chen, Q. The association of periodontal disease and oral health with hypertension, NHANES 2009–2018. BMC Public Health 2023, 23, 1122. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sumayin Ngamdu, K.; Mallawaarachchi, I.; Dunipace, E.A.; Chuang, L.H.; Jafri, S.H.; Shah, N.R.; Jeong, Y.N.; Morrison, A.R.; Bhatt, D.L. Association Between Periodontal Disease and Cardiovascular Disease (from the NHANES). Am. J. Cardiol. 2022, 178, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Carra, M.C.; Fessi, S.; Detzen, L.; Darnaud, C.; Julia, C.; Hercberg, S.; Touvier, M.; Andreeva, V.A.; Bouchard, P. Self-reported periodontal health and incident hypertension: Longitudinal evidence from the NutriNet-Santé e-cohort. J. Hypertens. 2021, 39, 2422–2430. [Google Scholar] [CrossRef]
- Kawabata, Y.; Ekuni, D.; Miyai, H.; Kataoka, K.; Yamane, M.; Mizutani, S.; Irie, K.; Azuma, T.; Tomofuji, T.; Iwasaki, Y.; et al. Relationship Between Prehypertension/Hypertension and Periodontal Disease: A Prospective Cohort Study. Am. J.Hypertens. 2016, 29, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Rivas-Tumanyan, S.; Spiegelman, D.; Curhan, G.C.; Forman, J.P.; Joshipura, K.J. Periodontal disease and incidence of hypertension in the health professionals follow-up study. Am. J. Hypertens. 2012, 25, 770–776. [Google Scholar] [CrossRef]
- Tsai, K.-Z.; Su, F.-Y.; Cheng, W.-C.; Huang, R.-Y.; Lin, Y.-P.; Lin, G.-M. Associations between metabolic biomarkers and localized stage II/III periodontitis in young adults: The CHIEF oral health study. J. Clin. Periodontol. 2021, 48, 1549–1558. [Google Scholar] [CrossRef]
- Schulze-Späte, U.; Mizani, I.; Salaverry, K.R.; Chang, J.; Wu, C.; Jones, M.; Kennel, P.J.; Brunjes, D.L.; Choo, T.H.; Kato, T.S.; et al. Periodontitis and bone metabolism in patients with advanced heart failure and after heart transplantation. ESC Heart Fail. 2017, 4, 169–177. [Google Scholar] [CrossRef]
- Fröhlich, H.; Herrmann, K.; Franke, J.; Karimi, A.; Täger, T.; Cebola, R.; Katus, H.A.; Zugck, C.; Frankenstein, L. Periodontitis in Chronic Heart Failure. Tex. Heart Inst. J. 2016, 43, 297–304. [Google Scholar] [CrossRef]
- Wood, N.; Johnson, R.B. The relationship between tomato intake and congestive heart failure risk in periodontitis subjects. J. Clin. Periodontol. 2004, 31, 574–580. [Google Scholar] [CrossRef]
- Chen, D.Y.; Lin, C.H.; Chen, Y.M.; Chen, H.H. Risk of atrial fibrillation or flutter associated with periodontitis: A nationwide, population-based cohort study. PLoS ONE 2016, 11, e0165601. [Google Scholar] [CrossRef]
- Hassan, A.; Lip, G.Y.H.; Harris, R.V. Atrial fibrillation and cardiac arrhythmia associated with acute dental infection: A systematic literature review and case report. Int. J. Clin. Pract. 2021, 75, e13875. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Redd, K.; Trivedi, T.; Moss, K.; Alonso, A.; Soliman, E.Z.; Magnani, J.W.; Chen, L.Y.; Gottesman, R.F.; Rosamond, W.; et al. Periodontal Disease, Atrial Fibrillation and Stroke. Am. Heart J. 2021, 235, 36–43. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miyauchi, S.; Tokuyama, T.; Shintani, T.; Nishi, H.; Hamamoto, Y.; Ouhara, K.; Furusho, H.; Miyauchi, M.; Komatsuzawa, H.; Nakano, Y. Periodontitis and the outcome of atrial fibrillation ablation: Porphyromonas gingivalis is related to atrial fibrillation recurrence. J. Cardiovasc. Electrophysiol. 2021, 32, 1240–1250. [Google Scholar] [CrossRef] [PubMed]
- Segovia, M.; Ganzinelli, S.; Reina, S.; Borda, E.; Sterin-Borda, L. Role of anti-β1 adrenergic antibodies from patients with periodontitis in cardiac dysfunction. J. Oral Pathol. Med. 2012, 41, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Ohki, T.; Itabashi, Y.; Kohno, T.; Yoshizawa, A.; Nishikubo, S.; Watanabe, S.; Yamane, G.; Ishihara, K. Detection of periodontal bacteria in thrombi of patients with acute myocardial infarction by polymerase chain reaction. Am. Heart J. 2012, 163, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Ninomiya, M.; Hashimoto, M.; Yamanouchi, K.; Fukumura, Y.; Nagata, T.; Naruishi, K. Relationship of oral conditions to the incidence of infective endocarditis in periodontitis patients with valvular heart disease: A cross-sectional study. Clin. Oral Investig. 2020, 24, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Yang, H.J.; Zhang, Y.; Li, X.E.; Jia, Y.R.; Wang, C.M. Prediction of loss to follow-up in long-term supportive periodontal therapy in patients with chronic periodontitis. PLoS ONE 2018, 13, e0192221. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Amerio, E.; Mainas, G.; Petrova, D.; Giner Tarrida, L.; Nart, J.; Monje, A. Compliance with supportive periodontal/peri-implant therapy: A systematic review. J. Clin. Periodontol. 2020, 47, 81–100. [Google Scholar] [CrossRef] [PubMed]


| Parameter (Nr., %) | Total | No Periodontitis | With Periodontitis | p |
|---|---|---|---|---|
| N | 166 | 63 (38%) | 103 (62%) | - |
| Age | ||||
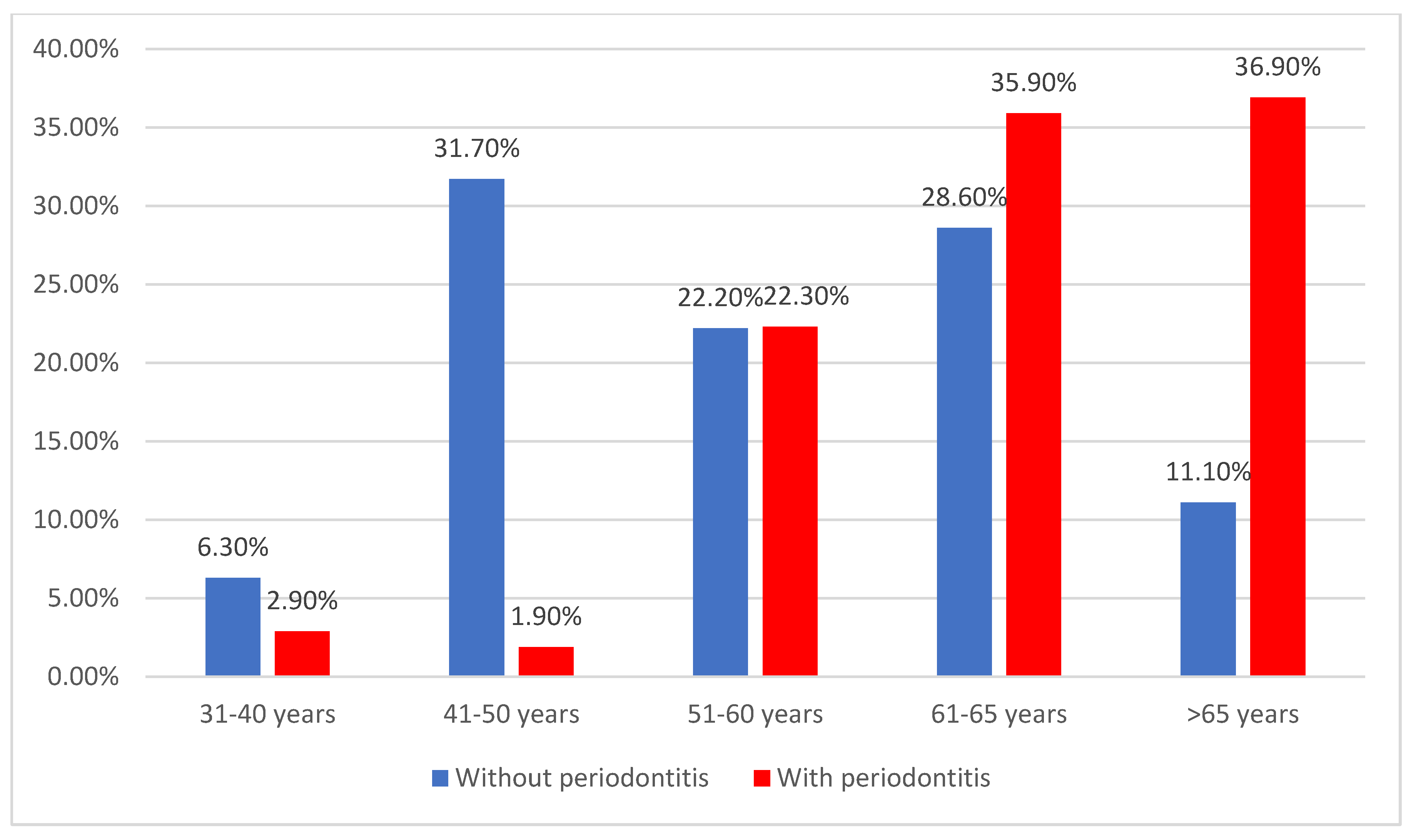
| 31–40 years | 7 (4.2%) | 4 (6.3%) | 3 (2.9%) | <0.001 * |
| 41–50 years | 22 (13.3%) | 20 (31.7%) | 2 (1.9%) | |
| 51–60 years | 37 (22.3%) | 14 (22.2%) | 23 (22.3%) | |
| 61–65 years | 55 (33.1%) | 18 (28.6%) | 37 (35.9%) | |
| >65 years | 45 (27.1%) | 7 (11.1%) | 38 (36.9%) | |
| Gender (Male) | 86 (51.8%) | 26 (41.3%) | 60 (58.3%) | 0.038 * |
| Background | ||||
| Rural | 78 (47%) | 15 (23.8%) | 63 (61.2%) | <0.001 * |
| Urban | 88 (53%) | 48 (76.2%) | 40 (38.8%) | |
| ACS | ||||
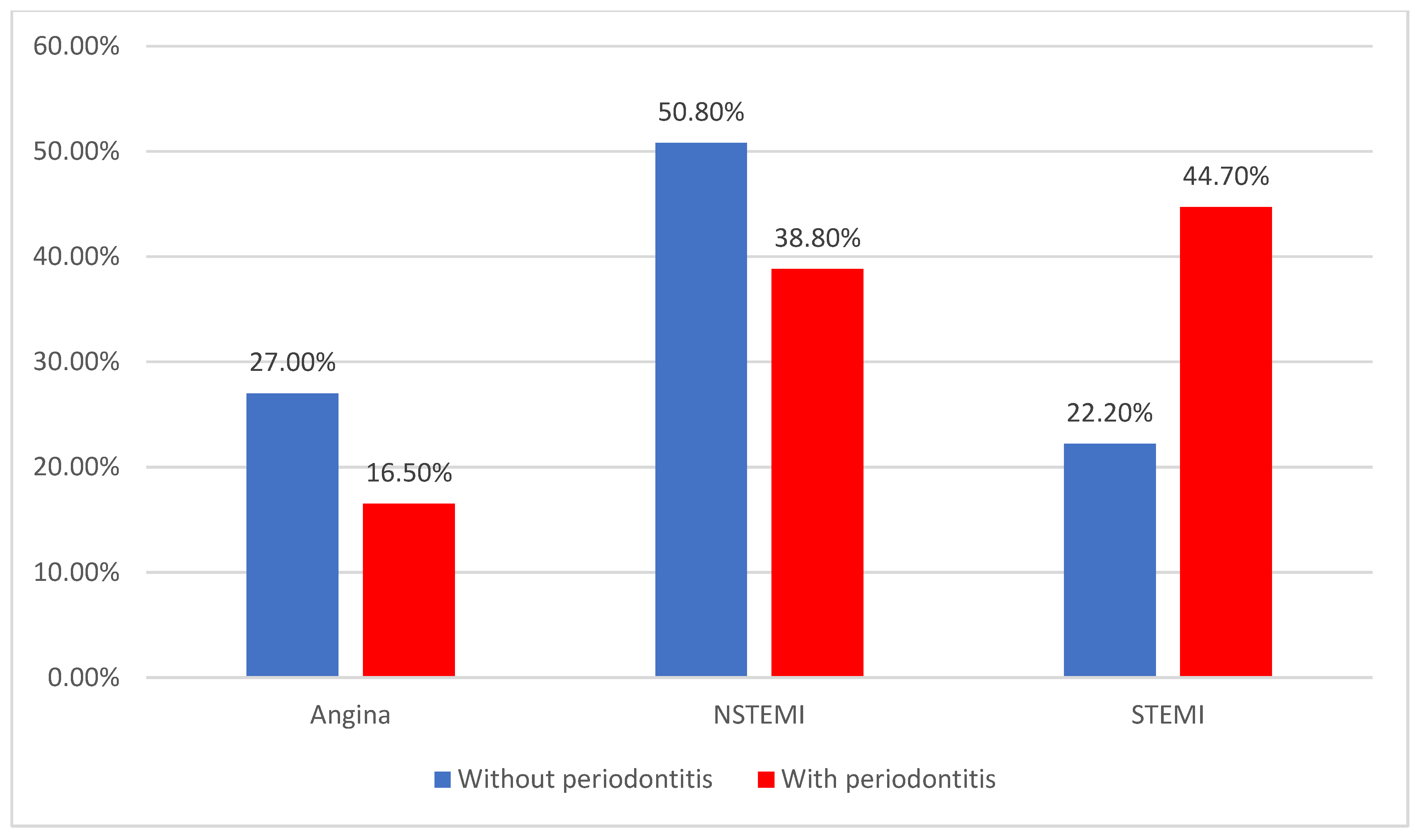
| Angina | 34 (20.5%) | 17 (27%) | 17 (16.5%) | 0.011 * |
| N-STEMI | 72 (43.4%) | 32 (50.8%) | 40 (38.8%) | |
| STEMI | 60 (36.1%) | 14 (22.2%) | 46 (44.7%) | |
| Risk factors | ||||
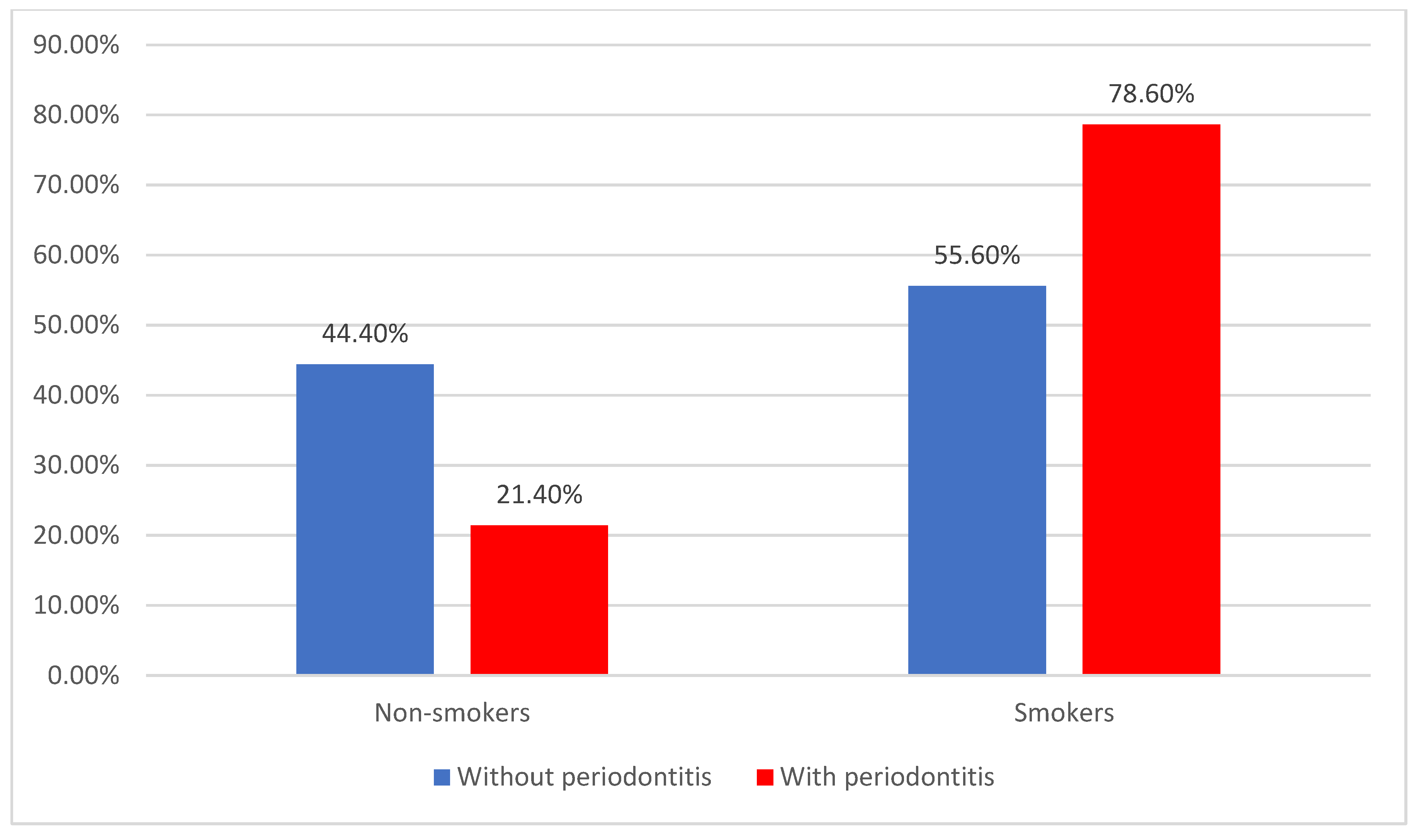
| Smokers | 116 (69.9%) | 35 (55.6%) | 81 (78.6%) | 0.003 * |
| Systemic diseases | 153 (92.2%) | 55 (87.3%) | 98 (95.1%) | 0.080 * |
| Systemic disease—type | ||||
| DM | 79 (51.6%) | 27 (49.1%) | 52 (53.1%) | 0.468 * |
| Autoimmune | 32 (20.9%) | 13 (23.6%) | 19 (19.4%) | |
| Obesity | 34 (22.2%) | 14 (25.5%) | 20 (20.4%) | |
| Other | 8 (5.2%) | 1 (1.8%) | 7 (7.1%) | |
| Coronary artery disease | ||||
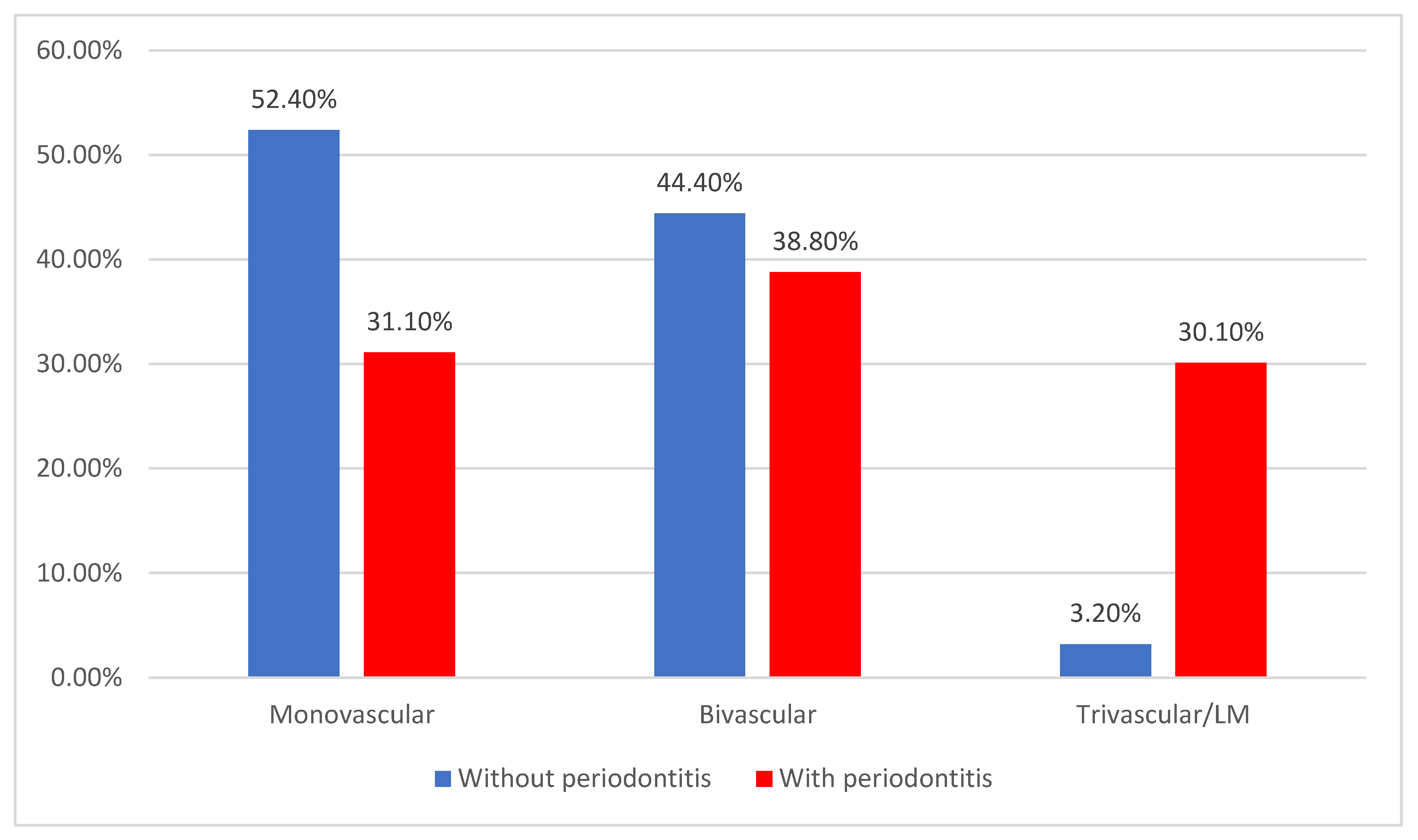
| Single-vessel disease | 65 (39.2%) | 33 (52.4%) | 32 (31.1%) | <0.001 * |
| Two-vessel disease | 68 (41%) | 28 (44.4%) | 40 (38.8%) | |
| Three-vessel/LM disease | 33 (19.9%) | 2 (3.2%) | 31 (30.1%) | |
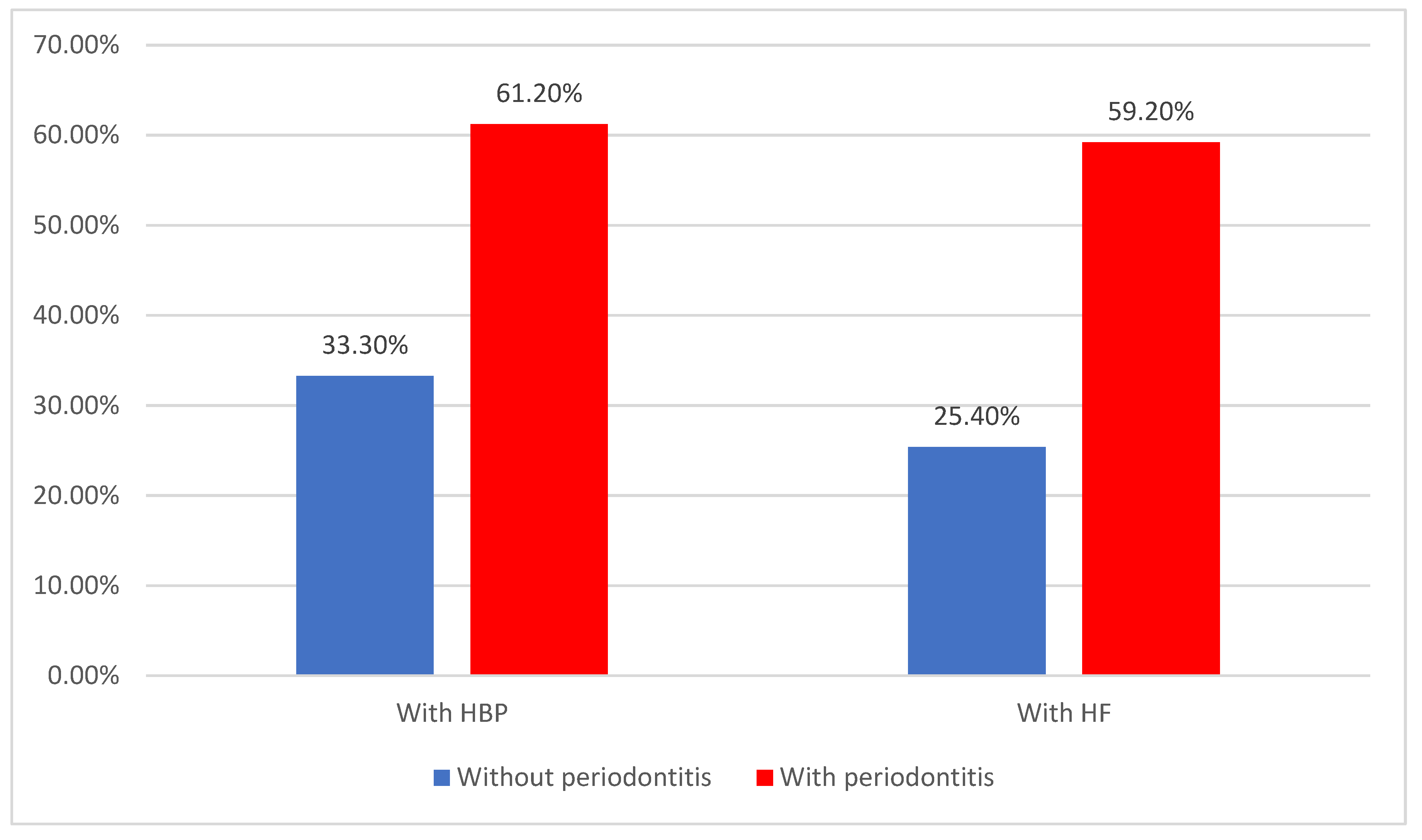
| HBP | 84 (50.6%) | 21 (33.3%) | 63 (61.2%) | 0.001 * |
| HF | 77 (46.4%) | 16 (25.4%) | 61 (59.2%) | <0.001 * |
| Congenital HD | 14 (8.4%) | 5 (7.9%) | 9 (8.7%) | 1.000 * |
| Valvulopathy | 23 (13.9%) | 6 (9.5%) | 17 (16.5%) | 0.254 * |
| Other CVD | 28 (16.9%) | 12 (19%) | 16 (15.5%) | 0.670 * |
| Other CVD—type | ||||
| Endocarditis | 9 (32.1%) | 3 (25%) | 6 (37.5%) | 0.296 * |
| Pericarditis | 2 (7.1%) | 2 (16.7%) | 0 (0%) | |
| Arrythmias | 17 (60.7%) | 7 (58.3%) | 10 (62.5%) | |
| Dyslipidemia | 141 (84.9%) | 55 (87.3%) | 86 (83.5%) | 0.656 * |
| Dental risk factors | ||||
| Poor hygiene | 113 (68.1%) | 38 (60.3%) | 75 (72.8%) | 0.122 * |
| Misalignment | 53 (31.9%) | 25 (39.7%) | 28 (27.2%) | |
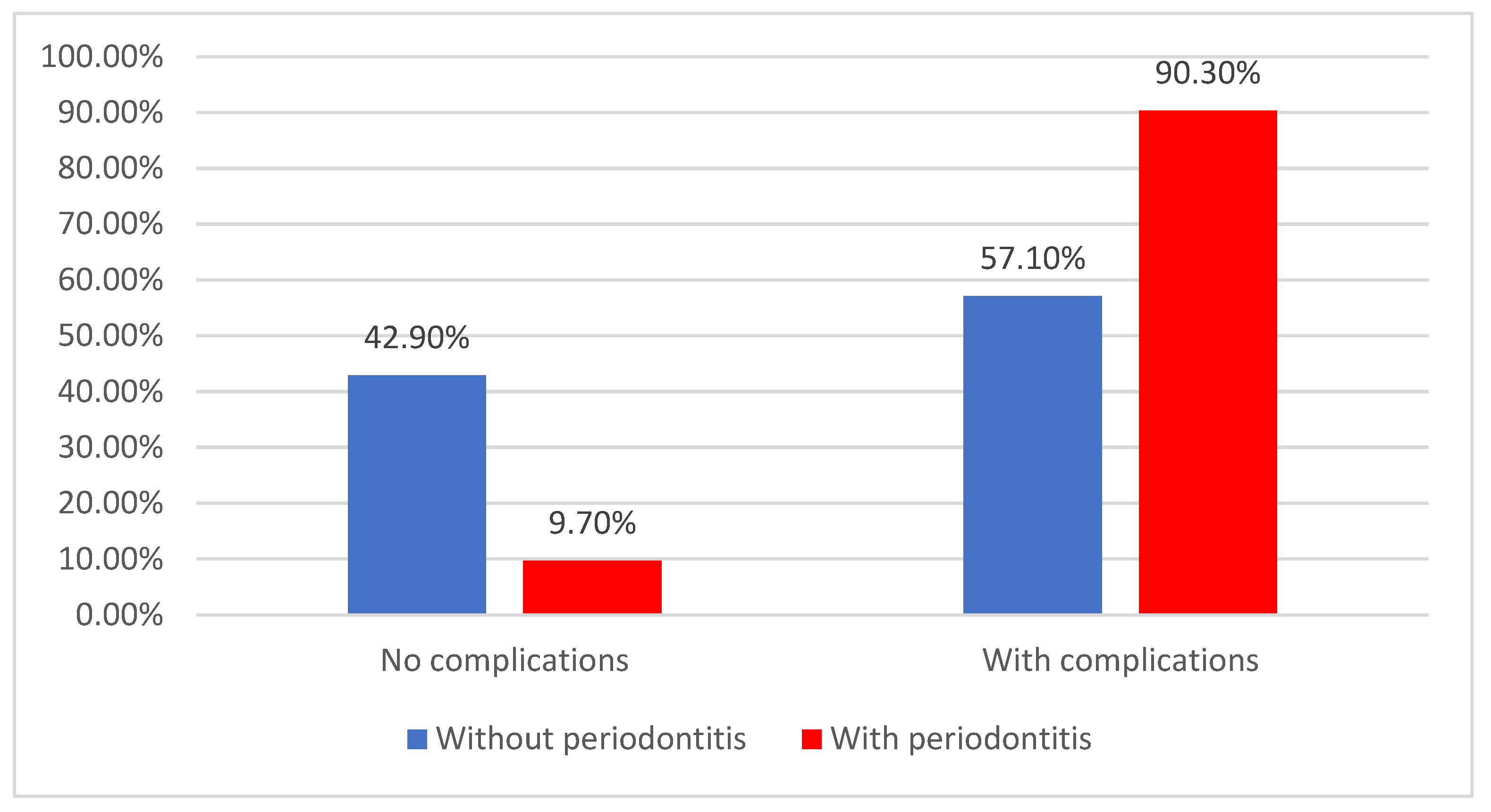
| CVD complications | 129 (77.7%) | 36 (57.1%) | 93 (90.3%) | <0.001 * |
| CVD complications—type | ||||
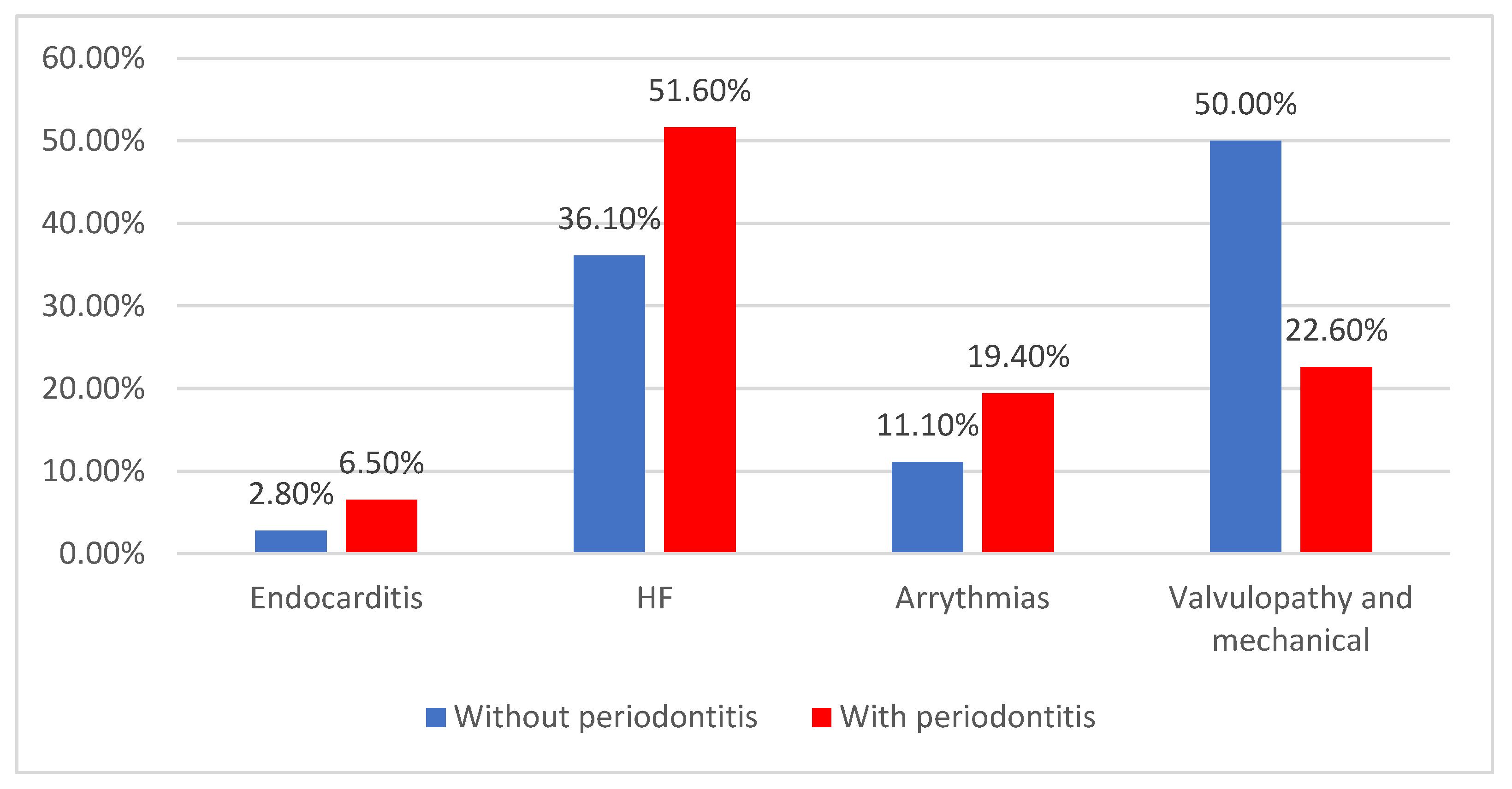
| Endocarditis | 7 (5.4%) | 1 (2.8%) | 6 (6.5%) | 0.030 * |
| HF | 61 (47.3%) | 13 (36.1%) | 48 (51.6%) | |
| Arrythmias | 22 (17.1%) | 4 (11.1%) | 18 (19.4%) | |
| Valvulopathy and mechanical | 39 (30.2%) | 18 (50%) | 21 (22.6%) | |
| Dental complications | ||||
| Dental mobility | 33 (19.9%) | 11 (17.5%) | 22 (21.4%) | 0.505 * |
| Gingival bleeding | 26 (15.7%) | 13 (20.6%) | 13 (12.6%) | |
| Gingival retraction | 21 (12.7%) | 10 (15.9%) | 11 (10.7%) | |
| Pain | 33 (19.9%) | 11 (17.5%) | 22 (21.4%) | |
| Teeth loss | 53 (31.9%) | 18 (28.6%) | 35 (34%) | |
| Treatment | ||||
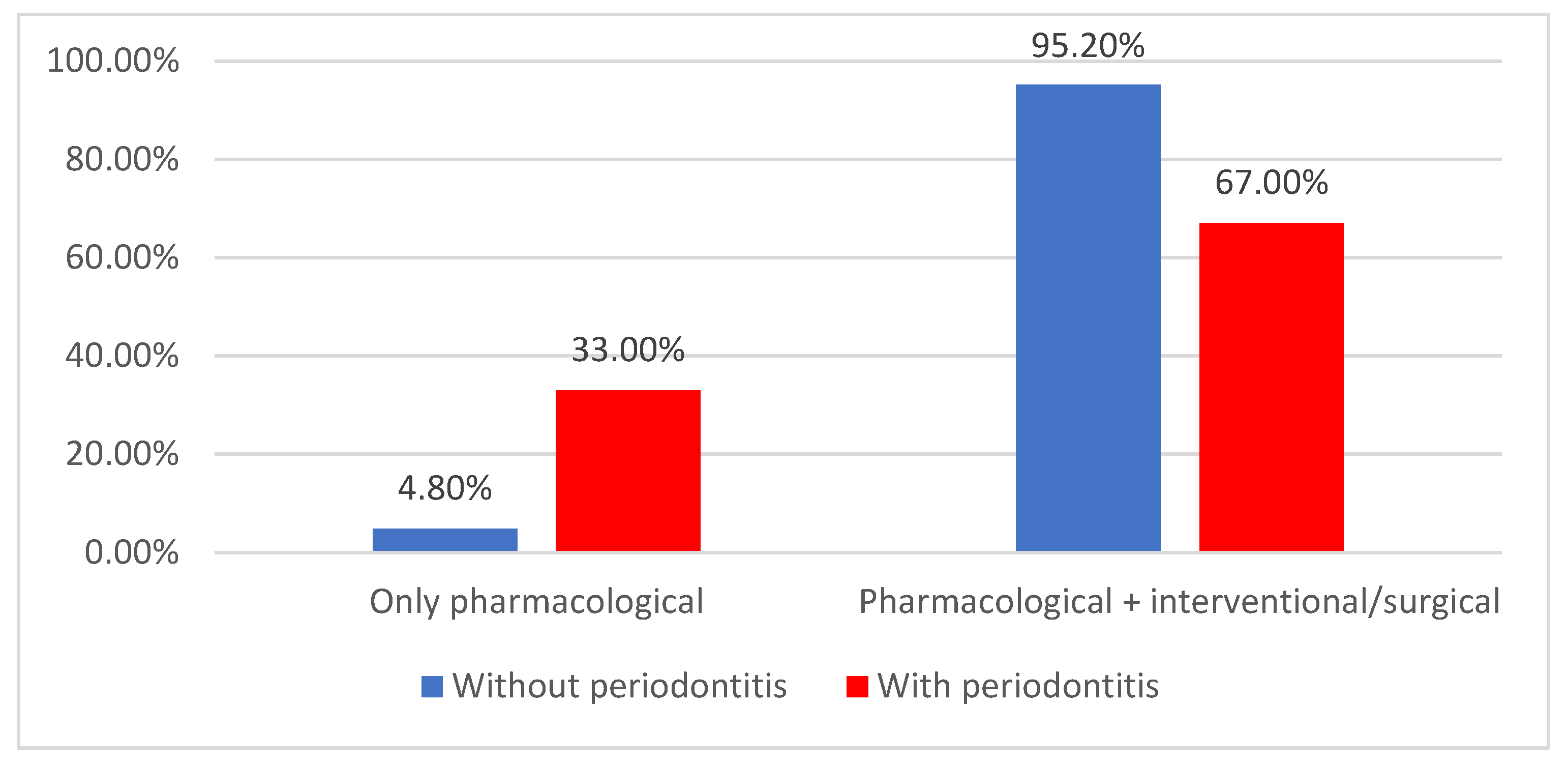
| Pharmacological | 37 (22.3%) | 3 (4.8%) | 34 (33%) | <0.001 * |
| Pharmacological + interventional/surgical | 129 (77.7%) | 60 (95.2%) | 69 (67%) | |
| Parameter/Model * | OR (95% C.I.) | p |
|---|---|---|
| Periodontitis | 2.294 (1.056–4.983) | 0.036 |
| Gender (Male) | 1.473 (0.750–2.893) | 0.261 |
| Smoking | 1.376 (0.641–2.956) | 0.413 |
| Systemic diseases | 0.884 (0.245–3.184) | 0.850 |
| Heart failure | 1.347 (0.675–2.684) | 0.398 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rus, M.; Negruțiu, B.M.; Sava, C.N.; Pasca, G.; Andronie-Cioara, F.L.; Crisan, S.; Popescu, M.-I.; Staniș, C.E.; Judea Pusta, C. The Association Between Periodontal Disease and Acute Coronary Syndrome—A Clinical Analysis. J. Clin. Med. 2025, 14, 2447. https://doi.org/10.3390/jcm14072447
Rus M, Negruțiu BM, Sava CN, Pasca G, Andronie-Cioara FL, Crisan S, Popescu M-I, Staniș CE, Judea Pusta C. The Association Between Periodontal Disease and Acute Coronary Syndrome—A Clinical Analysis. Journal of Clinical Medicine. 2025; 14(7):2447. https://doi.org/10.3390/jcm14072447
Chicago/Turabian StyleRus, Marius, Bianca Maria Negruțiu, Cristian Nicolae Sava, Georgeta Pasca, Felicia Liana Andronie-Cioara, Simina Crisan, Mircea-Ioachim Popescu, Claudia Elena Staniș, and Claudia Judea Pusta. 2025. "The Association Between Periodontal Disease and Acute Coronary Syndrome—A Clinical Analysis" Journal of Clinical Medicine 14, no. 7: 2447. https://doi.org/10.3390/jcm14072447
APA StyleRus, M., Negruțiu, B. M., Sava, C. N., Pasca, G., Andronie-Cioara, F. L., Crisan, S., Popescu, M.-I., Staniș, C. E., & Judea Pusta, C. (2025). The Association Between Periodontal Disease and Acute Coronary Syndrome—A Clinical Analysis. Journal of Clinical Medicine, 14(7), 2447. https://doi.org/10.3390/jcm14072447






