Brain Volume Measures in Adults with MOG-Antibody-Associated Disease: A Longitudinal Multicenter Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Enrollment
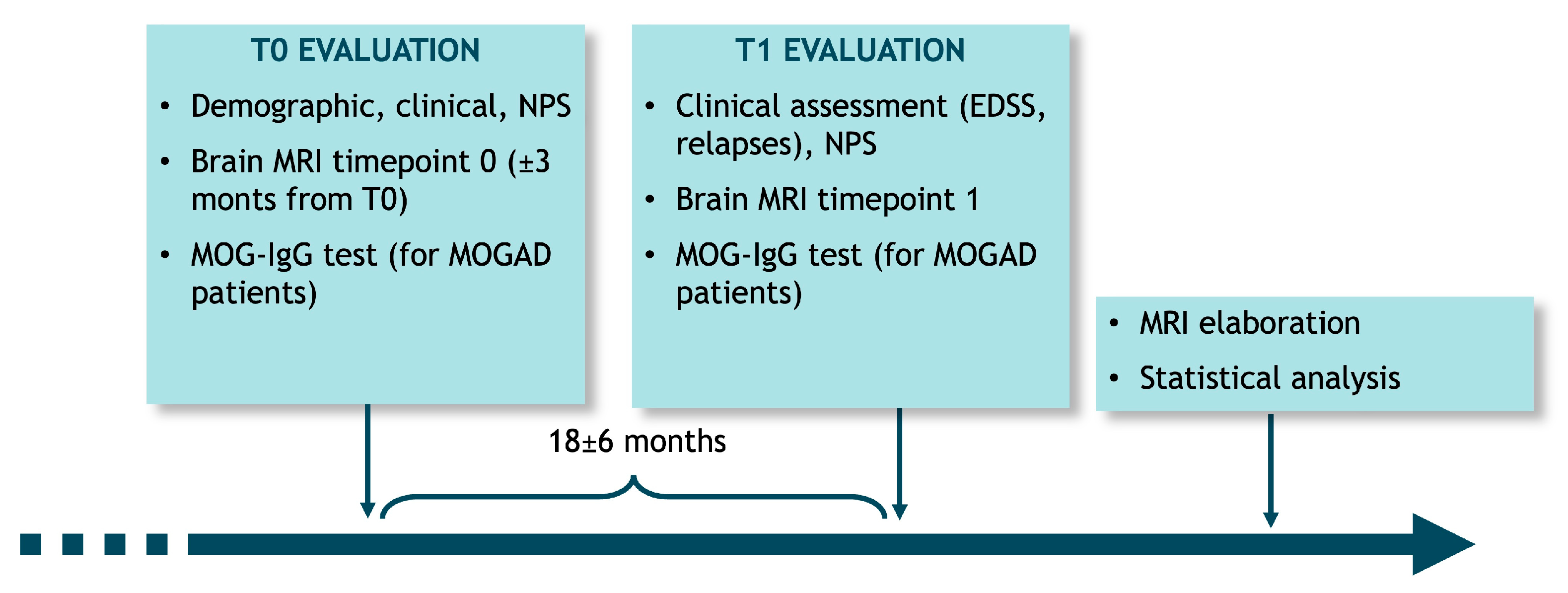
2.2. Study Design
2.3. Clinical Assessment
2.4. MRI Data Protocol
2.5. MOG-IgG Testing
2.6. Statistical Analysis
2.7. Patient and Public Statement
3. Results
3.1. Demographic and Clinical Features
3.2. Brain MRI Lesion Characteristics
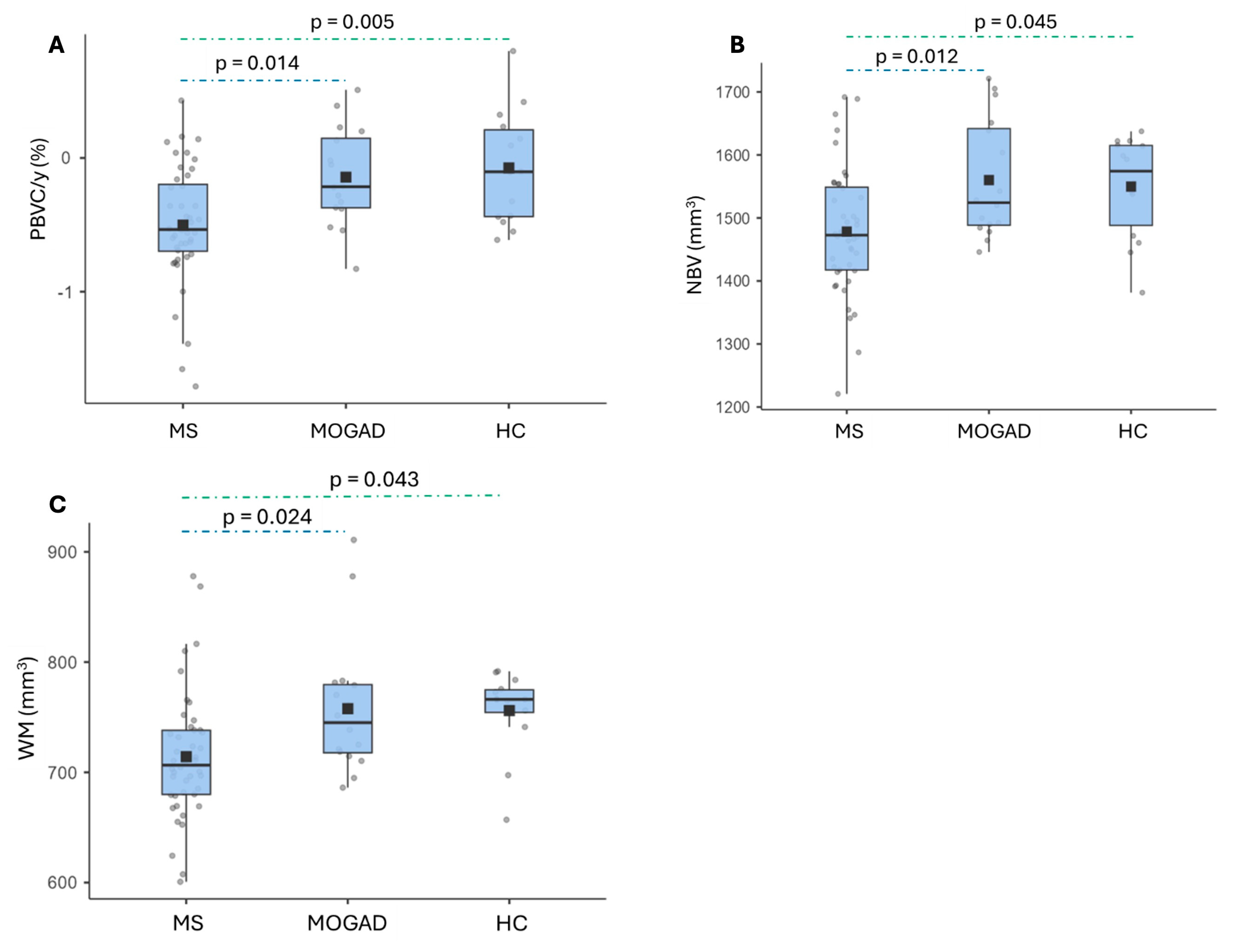
3.3. Brain Volume Measures
4. Discussion
4.1. Longitudinal Analysis of Brain Volumes
4.2. Brain MRI Lesion Distribution
4.3. Cross-Sectional Analysis of Brain Volumes
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADEM | Acute Disseminated Encephalomyelitis |
| ANOVA | Analysis of Variance |
| AQP4-Ab | Aquaporin-4 Antibody |
| CNS | Central Nervous System |
| CSF | Cerebrospinal Fluid |
| DIR | Double Inversion Recovery |
| EDSS | Expanded Disability Status Scale |
| GM | Gray Matter |
| HC | Healthy Control |
| IDE | Initial Demyelinating Event |
| MOG | Myelin Oligodendrocyte Glycoprotein |
| MOGAD | Myelin Oligodendrocyte Glycoprotein Antibody-Associated Disease |
| MRI | Magnetic Resonance Imaging |
| MS | Multiple Sclerosis |
| NBV | Normalized Brain Volume |
| NMOSD-AQP4 | Aquaporin-4 Antibody Seropositive Neuromyelitis Optica Spectrum Disorder |
| ON | Optic Neuritis |
| PBVC/y | Percentage Brain Volume Change per Year |
| RRMS | Relapsing-Remitting Multiple Sclerosis |
| T2LV | T2 Lesion Volume |
| TM | Transverse Myelitis |
| WM | White Matter |
References
- Jarius, S.; Ruprecht, K.; Kleiter, I.; Borisow, N.; Asgari, N.; Pitarokoili, K.; Pache, F.; Stich, O.; Beume, L.A.; Hümmert, M.W.; et al. MOG-IgG in NMO and related disorders: A multicenter study of 50 patients. Part 2: Epidemiology, clinical presentation, radiological and laboratory features, treatment responses, and long-term outcome. J. Neuroinflamm. 2016, 13, 1–45. [Google Scholar] [CrossRef]
- Foiadelli, T.; Gastaldi, M.; Scaranzin, S.; Franciotta, D.; Savasta, S. Seizures and myelin oligodendrocyte glycoprotein (MOG) antibodies: Two paradigmatic cases and a review of the literature. Mult. Scler. Relat. Disord. 2020, 41, 102011. [Google Scholar] [CrossRef] [PubMed]
- Peschl, P.; Bradl, M.; Höftberger, R.; Berger, T.; Reindl, M. Myelin oligodendrocyte glycoprotein: Deciphering a target in inflammatory demyelinating diseases. Front. Immunol. 2017, 8, 529. [Google Scholar] [CrossRef]
- Reindl, M.; Waters, P. Myelin oligodendrocyte glycoprotein antibodies in neurological disease. Nat. Rev. Neurol. 2019, 15, 89–102. [Google Scholar] [CrossRef]
- Orlandi, R.; Mariotto, S.; Ferrari, S.; Gobbin, F.; Sechi, E.; Capra, R.; Mancinelli, C.R.; Bombardi, R.; Zuliani, L.; Zoccarato, M.; et al. Diagnostic features of initial demyelinating events associated with serum MOG-IgG. J. Neuroimmunol. 2020, 344, 577260. [Google Scholar] [CrossRef]
- Contentti, E.C.; Okuda, D.T.; Rojas, J.I.; Chien, C.; Paul, F.; Alonso, R. MRI to differentiate multiple sclerosis, neuromyelitis optica, and myelin oligodendrocyte glycoprotein antibody disease. J. Neuroimaging 2023, 33, 688–702. [Google Scholar] [CrossRef]
- Banwell, B.; Bennett, J.L.; Marignier, R.; Kim, H.J.; Brilot, F.; Flanagan, E.P.; Ramanathan, S.; Waters, P.; Tenembaum, S.; Graves, J.S.; et al. Diagnosis of myelin oligodendrocyte glycoprotein antibody-associated disease: International MOGAD Panel proposed criteria. Lancet Neurol. 2023, 22, 268–282. [Google Scholar] [CrossRef]
- Cortese, R.; Carrasco, F.P.; Tur, C.; Bianchi, A.; Brownlee, W.; De Angelis, F.; De La Paz, I.; Grussu, F.; Haider, L.; Jacob, A.; et al. Differentiating Multiple Sclerosis From AQP4-Neuromyelitis Optica Spectrum Disorder and MOG-Antibody Disease With Imaging. Neurology 2023, 100, e308–e323. [Google Scholar] [CrossRef]
- Rocca, M.A.; Battaglini, M.; Benedict, R.H.; de Stefano, N.; Geurts, J.J.; Henry, R.G.; Horsfield, M.A.; Jenkinson, M.; Pagani, E.; Filippi, M. Brain MRI atrophy quantification in MS. Neurology 2017, 88, 403–413. [Google Scholar] [CrossRef]
- Smith, S.M.; Zhanga, Y.; Jenkinsona, M.; Chenab, J.; Matthews, P.; Federicoc, A.; De Stefano, N. Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. NeuroImage 2002, 17, 479–489. [Google Scholar] [CrossRef]
- Smith, S.M.; Jenkinson, M.; Woolrich, M.W.; Beckmann, C.F.; Behrens, T.E.; Johansen-Berg, H.; Bannister, P.R.; De Luca, M.; Drobnjak, I.; Flitney, D.E.; et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage 2004, 23, S208–S219. [Google Scholar] [CrossRef] [PubMed]
- Yushkevich, P.A.; Piven, J.; Hazlett, H.C.; Smith, R.G.; Ho, S.; Gee, J.C.; Gerig, G. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. NeuroImage 2006, 31, 1116–1128. [Google Scholar] [CrossRef] [PubMed]
- Manjón, J.V.; Coupé, P. volBrain: An Online MRI Brain Volumetry System. Front. Neurosci. 2016, 10, 30. [Google Scholar] [CrossRef]
- Waters, P.J.; Komorowski, L.; Woodhall, M.; Lederer, S.; Majed, M.; Fryer, J.; Mills, J.; Flanagan, E.P.; Irani, S.R.; Kunchok, A.C.; et al. A multicenter comparison of MOG-IgG cell-based assays. Neurology 2019, 92, e1250–e1255. [Google Scholar] [CrossRef]
- Mariotto, S.; Gajofatto, A.; Batzu, L.; Delogu, R.; Sechi, G.; Leoni, S.; Pirastru, M.I.; Bonetti, B.; Zanoni, M.; Alberti, D.; et al. Relevance of antibodies to myelin oligodendrocyte glycoprotein in CSF of seronegative cases. Neurology 2019, 93, e1867–e1872. [Google Scholar] [CrossRef]
- Mariotto, S.; Ferrari, S.; Monaco, S.; Benedetti, M.D.; Schanda, K.; Alberti, D.; Farinazzo, A.; Capra, R.; Mancinelli, C.; De Rossi, N.; et al. Clinical spectrum and IgG subclass analysis of anti-myelin oligodendrocyte glycoprotein antibody-associated syndromes: A multicenter study. J. Neurol. 2017, 264, 2420–2430. [Google Scholar] [CrossRef]
- Mader, S.; Gredler, V.; Schanda, K.; Rostasy, K.; Dujmovic, I.; Pfaller, K.; Lutterotti, A.; Jarius, S.; Di Pauli, F.; Kuenz, B.; et al. Complement activating antibodies to myelin oligodendrocyte glycoprotein in neuromyelitis optica and related disorders. J. Neuroinflamm. 2011, 8, 184. [Google Scholar] [CrossRef]
- Di Pauli, F.; Mader, S.; Rostasy, K.; Schanda, K.; Bajer-Kornek, B.; Ehling, R.; Deisenhammer, F.; Reindl, M.; Berger, T. Temporal dynamics of anti-MOG antibodies in CNS demyelinating diseases. Clin. Immunol. 2011, 138, 247–254. [Google Scholar] [CrossRef]
- De Stefano, N.; Stromillo, M.L.; Giorgio, A.; Bartolozzi, M.L.; Battaglini, M.; Baldini, M.; Portaccio, E.; Amato, M.P.; Sormani, M.P. Establishing pathological cut-offs of brain atrophy rates in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2016, 87, 93–99. [Google Scholar] [CrossRef]
- De Stefano, N.; Giorgio, A.; Battaglini, M.; Rovaris, M.; Sormani, M.; Barkhof, F.; Korteweg, T.; Enzinger, C.; Fazekas, F.; Calabrese, M.; et al. Assessing brain atrophy rates in a large population of untreated multiple sclerosis subtypes. Neurology 2010, 74, 1868–1876. [Google Scholar] [CrossRef]
- Orlandi, R.; Mariotto, S.; Gobbin, F.; Mancinelli, C.R.; Rossi, F.; Zoccarato, M.; Zuliani, L.; Ferraro, D.; Turatti, M.; Gajofatto, A. Brain volume measures in adults with MOG-antibody associated disease: A longitudinal multicentre study. J. Neurol. Sci. 2021, 429, 118108. [Google Scholar] [CrossRef]
- Lotan, I.; Billiet, T.; Ribbens, A.; Van Hecke, W.; Huang, B.; Kister, I.; Lotan, E. Volumetric brain changes in MOGAD: A cross-sectional and longitudinal comparative analysis. Mult. Scler. Relat. Disord. 2022, 69, 104436. [Google Scholar] [CrossRef] [PubMed]
- Battaglini, M.; Gentile, G.; Luchetti, L.; Giorgio, A.; Vrenken, H.; Barkhof, F.; Cover, K.S.; Bakshi, R.; Chu, R.; Sormani, M.P.; et al. Lifespan normative data on rates of brain volume changes. Neurobiol. Aging 2019, 81, 30–37. [Google Scholar] [CrossRef]
- Liu, Y.; Duan, Y.; Huang, J.; Ren, Z.; Liu, Z.; Dong, H.; Weiler, F.; Hahn, H.K.; Shi, F.-D.; Butzkueven, H.; et al. Different patterns of longitudinal brain and spinal cord changes and their associations with disability progression in NMO and MS. Eur. Radiol. 2017, 28, 96–103. [Google Scholar] [CrossRef]
- Jurynczyk, M.; Messina, S.; Woodhall, M.R.; Raza, N.; Everett, R.; Roca-Fernandez, A.; Tackley, G.; Hamid, S.; Sheard, A.; Reynolds, G.; et al. Clinical presentation and prognosis in MOG-antibody disease: A UK study. Brain 2017, 140, 3128–3138. [Google Scholar] [CrossRef]
- Jarius, S.; Paul, F.; Aktas, O.; Asgari, N.; Dale, R.C.; de Seze, J.; Franciotta, D.; Fujihara, K.; Jacob, A.; Kim, H.J.; et al. MOG encephalomyelitis: International recommendations on diagnosis and antibody testing. J. Neuroinflamm. 2018, 15, 1–10. [Google Scholar] [CrossRef]
- Camera, V.; Holm-Mercer, L.; Ali, A.A.H.; Messina, S.; Horvat, T.; Kuker, W.; Leite, M.I.; Palace, J. Frequency of New Silent MRI Lesions in Myelin Oligodendrocyte Glycoprotein Antibody Disease and Aquaporin-4 Antibody Neuromyelitis Optica Spectrum Disorder. JAMA Netw. Open 2021, 4, e2137833. [Google Scholar] [CrossRef]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Filippi, M.; Bar-Or, A.; Piehl, F.; Preziosa, P.; Solari, A.; Vukusic, S.; Rocca, A.M. Multiple sclerosis. Nat. Rev. Dis. Prim. 2018, 4, 43. [Google Scholar] [CrossRef]
- Jarius, S.; Pellkofer, H.; Siebert, N.; Korporal-Kuhnke, M.; Hümmert, M.W.; Ringelstein, M.; Rommer, P.S.; Ayzenberg, I.; Ruprecht, K.; Klotz, L.; et al. Cerebrospinal fluid findings in patients with myelin oligodendrocyte glycoprotein (MOG) antibodies. Part 1: Results from 163 lumbar punctures in 100 adult patients. J. Neuroinflamm. 2020, 17, 1–26. [Google Scholar] [CrossRef]
- Mariotto, S.; Gastaldi, M.; Grazian, L.; Mancinelli, C.; Capra, R.; Marignier, R.; Alberti, D.; Zanzoni, S.; Schanda, K.; Franciotta, D.; et al. NfL levels predominantly increase at disease onset in MOG-Abs-associated disorders. Mult. Scler. Relat. Disord. 2021, 50, 102833. [Google Scholar] [CrossRef] [PubMed]
- Höftberger, R.; Guo, Y.; Flanagan, E.P.; Lopez-Chiriboga, A.S.; Endmayr, V.; Hochmeister, S.; Joldic, D.; Pittock, S.J.; Tillema, J.M.; Gorman, M.; et al. The pathology of central nervous system inflammatory demyelinating disease accompanying myelin oligodendrocyte glycoprotein autoantibody. Acta Neuropathol. 2020, 139, 875–892. [Google Scholar] [CrossRef]
- Cobo-Calvo, A.; Ruiz, A.; Maillart, E.; Audoin, B.; Zephir, H.; Bourre, B.; Ciron, J.; Collongues, N.; Brassat, D.; Cotton, F.; et al. Clinical spectrum and prognostic value of CNS MOG autoimmunity in adults: The MOGADOR study. Neurology 2018, 90, e1858–e1869. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, S.; Prelog, K.; Barnes, E.H.; Tantsis, E.M.; Reddel, S.W.; Henderson, A.P.; Vucic, S.; Gorman, M.P.; A Benson, L.; Alper, G.; et al. Radiological differentiation of optic neuritis with myelin oligodendrocyte glycoprotein antibodies, aquaporin-4 antibodies, and multiple sclerosis. Mult. Scler. J. 2016, 22, 470–482. [Google Scholar] [CrossRef]
- Zivadinov, R. Role of Neuroimaging in Multiple Sclerosis. In Multiple Sclerosis; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar] [CrossRef]
- Juryńczyk, M.; Tackley, G.; Kong, Y.; Geraldes, R.; Matthews, L.; Woodhall, M.; Waters, P.; Kuker, W.; Craner, M.; Weir, A.; et al. Brain lesion distribution criteria distinguish MS from AQP4-antibody NMOSD and MOG-antibody disease. J. Neurol. Neurosurg. Psychiatry 2017, 88, 132–136. [Google Scholar] [CrossRef]
- Schmidt, F.A.; Chien, C.; Kuchling, J.; Bellmann-Strobl, J.; Ruprecht, K.; Siebert, N.; Asseyer, S.; Jarius, S.; Brandt, A.U.; Scheel, M.; et al. Differences in Advanced Magnetic Resonance Imaging in MOG-IgG and AQP4-IgG Seropositive Neuromyelitis Optica Spectrum Disorders: A Comparative Study. Front. Neurol. 2020, 11, 499910. [Google Scholar] [CrossRef]
- Messina, S.; Mariano, R.; Roca-Fernandez, A.; Cavey, A.; Jurynczyk, M.; Leite, M.I.; Calabrese, M.; Jenkinson, M.; Palace, J. Contrasting the brain imaging features of MOG-antibody disease, with AQP4-antibody NMOSD and multiple sclerosis. Mult. Scler. J. 2022, 28, 217–227. [Google Scholar] [CrossRef]
- Duan, Y.; Zhuo, Z.; Li, H.; Tian, D.-C.; Li, Y.; Yang, L.; Gao, C.; Zhang, T.; Zhang, X.; Shi, F.-D.; et al. Brain structural alterations in MOG antibody diseases: A comparative study with AQP4 seropositive NMOSD and MS. J. Neurol. Neurosurg. Psychiatry 2021, 92, 709–716. [Google Scholar] [CrossRef]


| MOGAD (n = 16) | MS (n = 44) | HC (n = 14) | p Value | |
|---|---|---|---|---|
| Median age at T0—years (range) | 36 (21–69) | 36 (23–69) | 36 (21–71) | 0.935 |
| Median age at onset—years (range) | 33 (21–68) | 31 (16–47) | 0.482 | |
| Sex—n (%) | 0.902 | |||
| F | 9 (56.2) | 26 (59.1) | 9 (64.2) | |
| M | 7 (43.8) | 18 (40.9) | 5 (35.8) | |
| Clinical onset—n (%) | 0.167 | |||
| Optic neuritis | 6 (37.5) | 6 (13.6) | ||
| Monolateral | 6 (37.5) | 5 (11.4) | ||
| Bilateral | 0 (0.0) | 1 (2.2) | ||
| Myelitis | 5 (31.3) | 24 (54.5) | ||
| Brainstem | 2 (12.5) | 9 (20.5) | ||
| Encephalopathy | 1 (6.3) | 4 (9.1) | ||
| Multifocal | 2 (12.4) | 1 (2.3) | ||
| MOGAD clinical course—n (%) | ||||
| Monophasic | 11 (68.8) | |||
| Relapsing | 5 (31.2) | |||
| Disease duration at T0—median months (range) | 8 (0–65) | 60 (0–418) | <0.001 | |
| EDSS—median (range) | ||||
| T0 | 2.0 (0–6) | 1.5 (0–4.5) | 0.793 | |
| T1 | 0.0 (0–5.5) | 1.5 (0–4.0) | 0.050 | |
| Previous relapses—median (range) | 0 (0–3) | 1 (0–12) | 0.008 | |
| Relapsed patients during follow-up—n (%) | 2 (33.3%) | 8 (18.2%) | 0.384 | |
| Interval between MRI scans—months (range) | 12 (12–17) | 13 (12–18) | 24 (24) | 0.595 |
| Brain MRI lesions at T0—n of patients (%) | ||||
| Cortical | 0.007 | |||
| 0 | 11 (68.8) | 16 (36.3) | ||
| 1–2 | 0 (0.0) | 9 (20.5) | ||
| ≥3 | 0 (0.0) | 14 (31.8) | ||
| n.a. | 5 (31.2) | 5 (11.4) | ||
| Juxtacortical | <0.001 | |||
| 0 | 10 (62.4) | 5 (11.4) | ||
| 1–2 | 3 (18.8) | 4 (9.1) | ||
| ≥3 | 3 (18.8) | 35 (79.5) | ||
| Periventricular | <0.001 | |||
| 0 | 8 (50.0) | 1 (2.3) | ||
| 1–2 | 6 (37.6) | 2 (4.6) | ||
| ≥3 | 2 (12.4) | 41 (93.1) | ||
| Thalamus | 0.141 | |||
| 0 | 16 (100.0) | 32 (72.7) | ||
| 1–2 | 0 (0.00) | 10 (22.7) | ||
| ≥3 | 0 (0.00) | 2 (4.6) | ||
| Corpus callosum | <0.001 | |||
| 0 | 16 (100.0) | 16 (36.4) | ||
| 1–2 | 0 (0.0) | 16 (36.4) | ||
| ≥3 | 0 (0.0) | 12 (27.2) | ||
| Basal ganglia | 0.415 | |||
| 0 | 15 (93.8) | 35 (79.5) | ||
| 1–2 | 1 (6.2) | 9 (20.5) | ||
| ≥3 | 0 (0.0) | 0 (0.0) | ||
| Brainstem | 0.001 | |||
| 0 | 13 (81.3) | 11 (25.0) | ||
| 1–2 | 3 (18.7) | 29 (69.9) | ||
| ≥3 | 0 (0.0) | 4 (9.1) | ||
| Cerebellar | 0.127 | |||
| 0 | 14 (87.5) | 24 (54.5) | ||
| 1–2 | 2 (12.5) | 18 (40.9) | ||
| ≥3 | 0 (0.0) | 2 (4.6) | ||
| Tumefactive lesions | 0.024 | |||
| 0 | 13 (81.2) | 43 (97.7) | ||
| ≥1 | 3 (18.8) | 1 (2.3) | ||
| Blurred margins | 0.020 | |||
| 0 | 12 (75.0) | 42 (95.5) | ||
| ≥1 | 4 (25.0) | 2 (4.5) | ||
| Contrast enhancement | 0.329 | |||
| 0 | 13 (81.0) | 26 (59.2) | ||
| ≥1 | 2 (12.5) | 6 (13.5) | ||
| n.a. | 1 (6.5) | 12 (27.3) | ||
| New T2 lesions | 0.178 | |||
| 0 | 14 (87.5) | 31 (70.5) | ||
| ≥1 | 2 (12.5) | 13 (29.5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orlandi, R.; Mariotto, S.; Gobbin, F.; Rossi, F.; Camera, V.; Calabrese, M.; Calabria, F.; Gajofatto, A. Brain Volume Measures in Adults with MOG-Antibody-Associated Disease: A Longitudinal Multicenter Study. J. Clin. Med. 2025, 14, 2445. https://doi.org/10.3390/jcm14072445
Orlandi R, Mariotto S, Gobbin F, Rossi F, Camera V, Calabrese M, Calabria F, Gajofatto A. Brain Volume Measures in Adults with MOG-Antibody-Associated Disease: A Longitudinal Multicenter Study. Journal of Clinical Medicine. 2025; 14(7):2445. https://doi.org/10.3390/jcm14072445
Chicago/Turabian StyleOrlandi, Riccardo, Sara Mariotto, Francesca Gobbin, Francesca Rossi, Valentina Camera, Massimiliano Calabrese, Francesca Calabria, and Alberto Gajofatto. 2025. "Brain Volume Measures in Adults with MOG-Antibody-Associated Disease: A Longitudinal Multicenter Study" Journal of Clinical Medicine 14, no. 7: 2445. https://doi.org/10.3390/jcm14072445
APA StyleOrlandi, R., Mariotto, S., Gobbin, F., Rossi, F., Camera, V., Calabrese, M., Calabria, F., & Gajofatto, A. (2025). Brain Volume Measures in Adults with MOG-Antibody-Associated Disease: A Longitudinal Multicenter Study. Journal of Clinical Medicine, 14(7), 2445. https://doi.org/10.3390/jcm14072445







