Precision Psychiatry for Obsessive-Compulsive Disorder: Clinical Applications of Deep Learning Architectures
Abstract
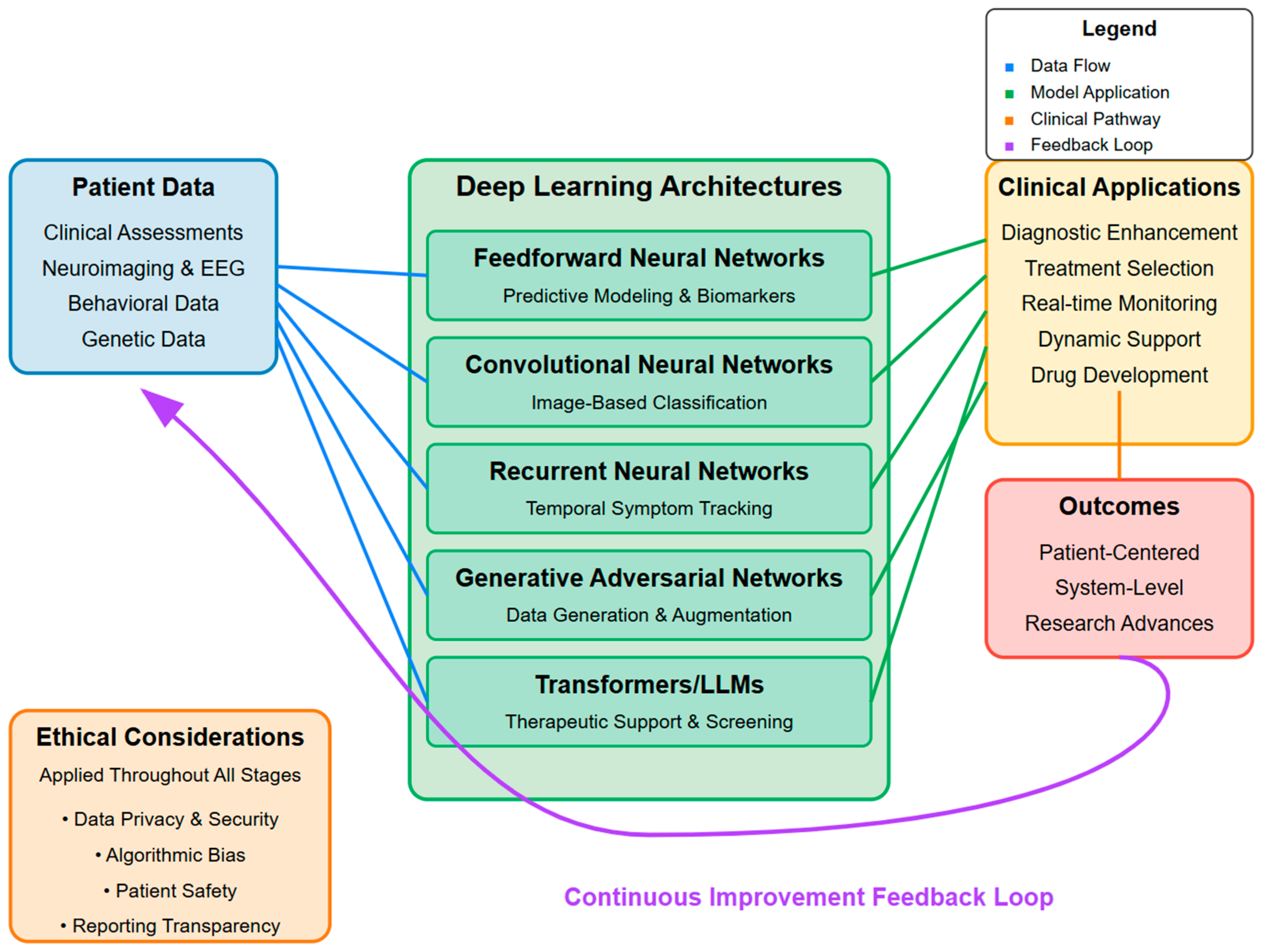
1. Introduction
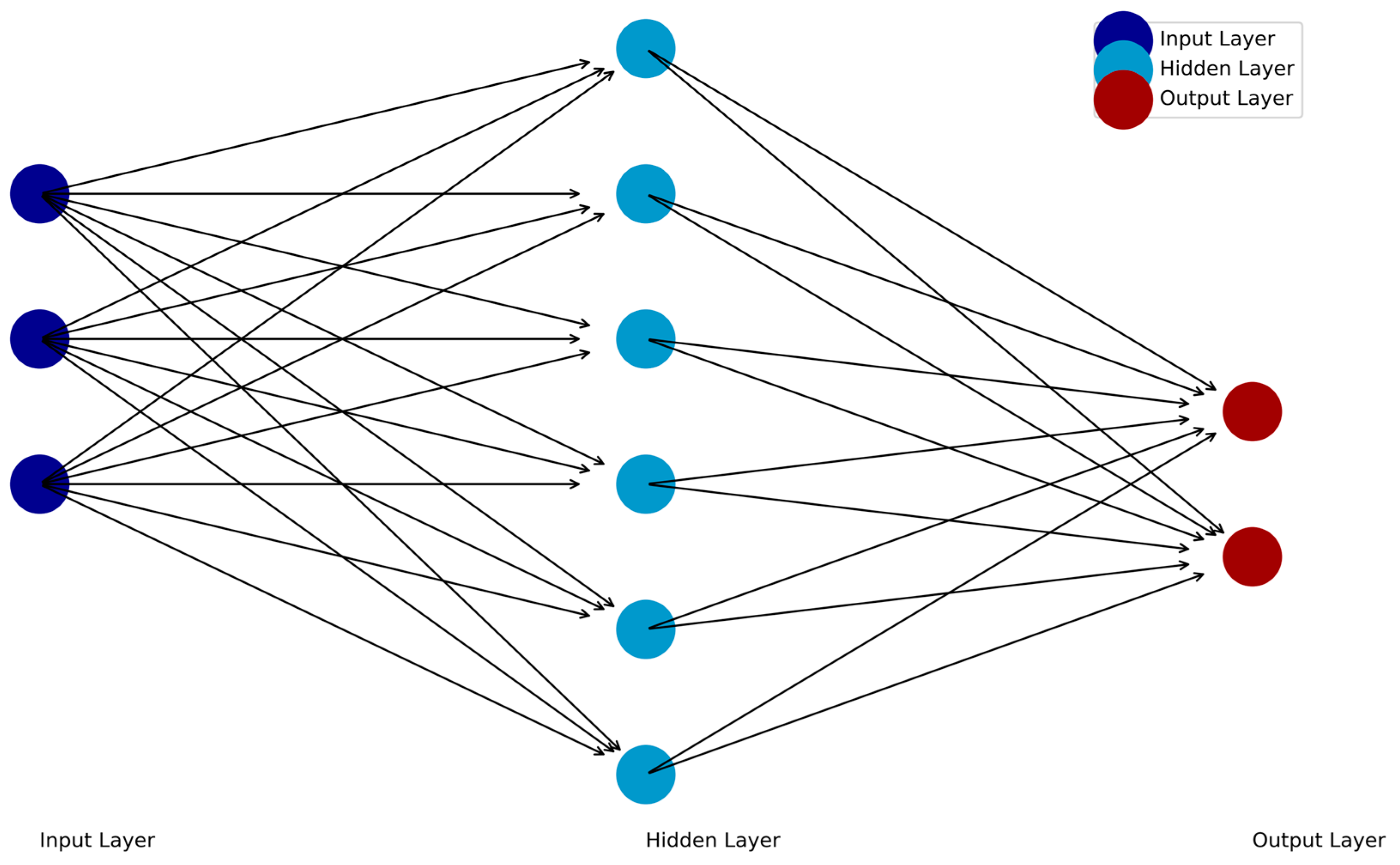
2. Predictive Modeling and Biomarker Identification with Feedforward Neural Networks
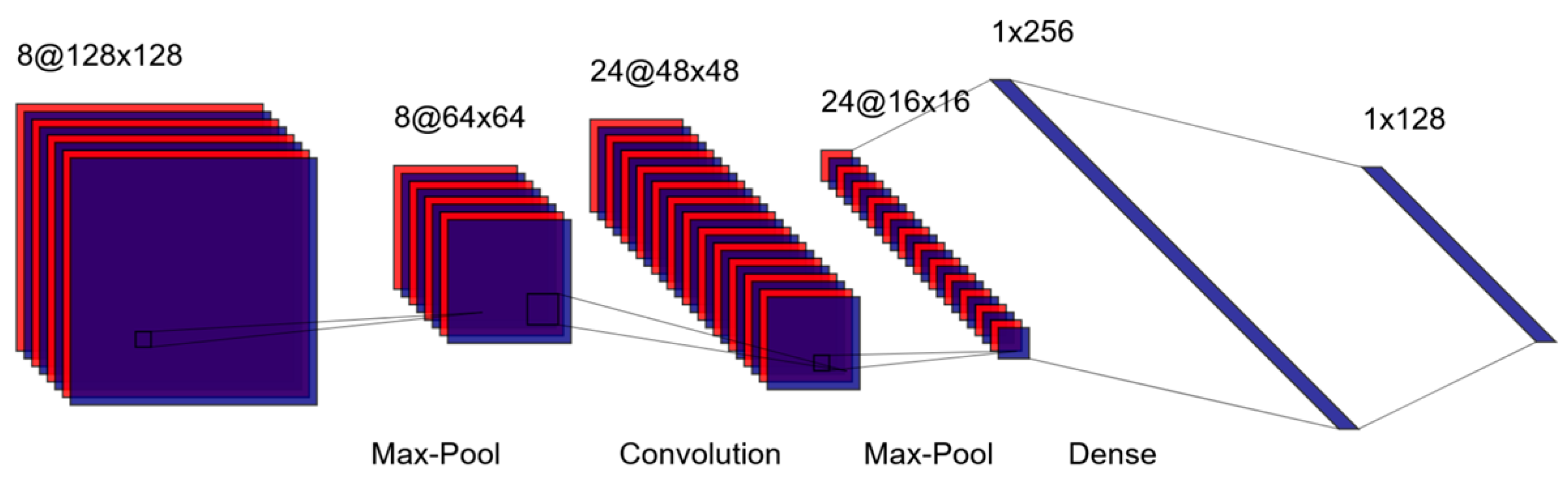
3. Image-Based Classification: Convolutional Neural Networks
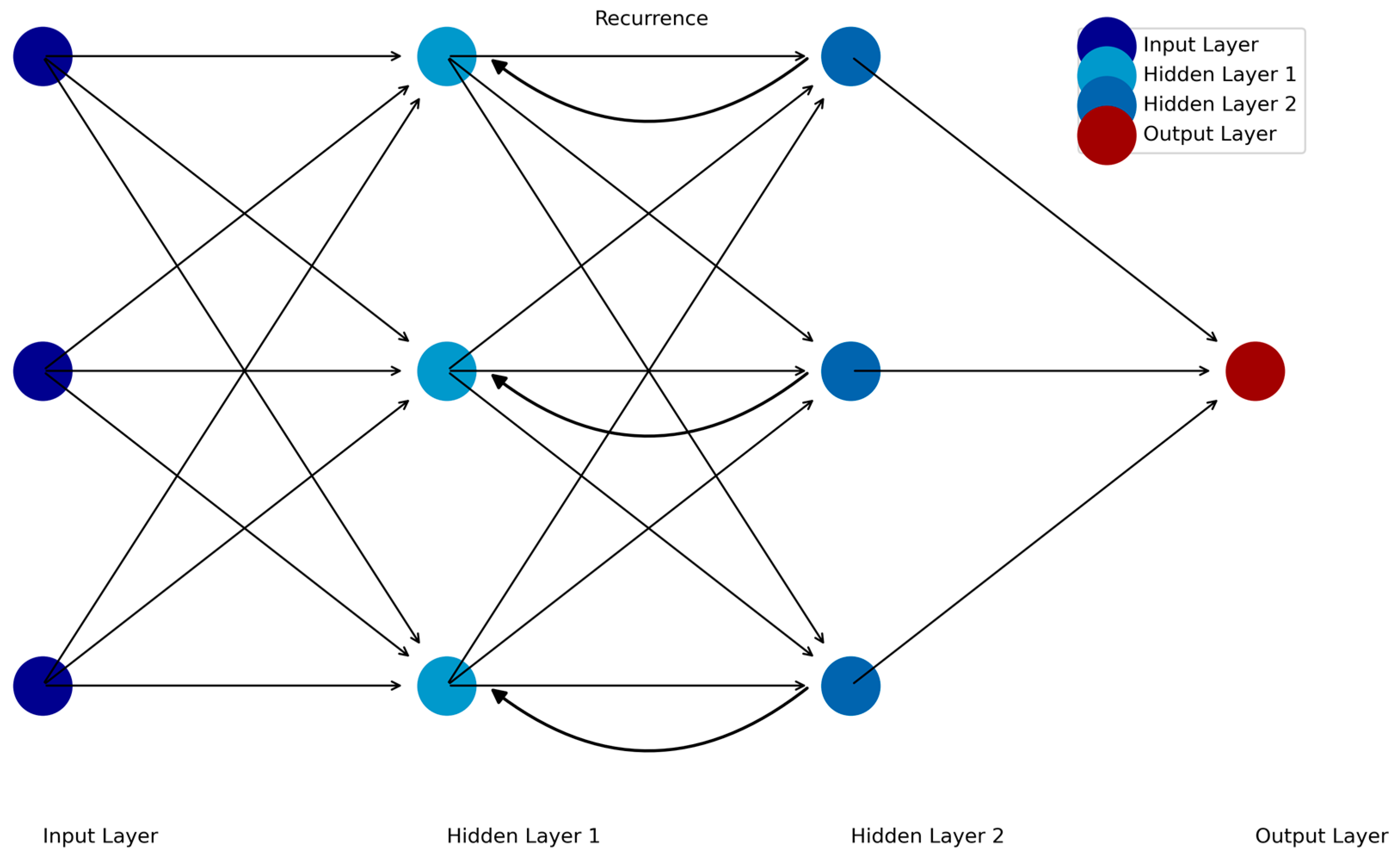
4. Temporal Tracking of Symptoms: Recurrent Neural Networks
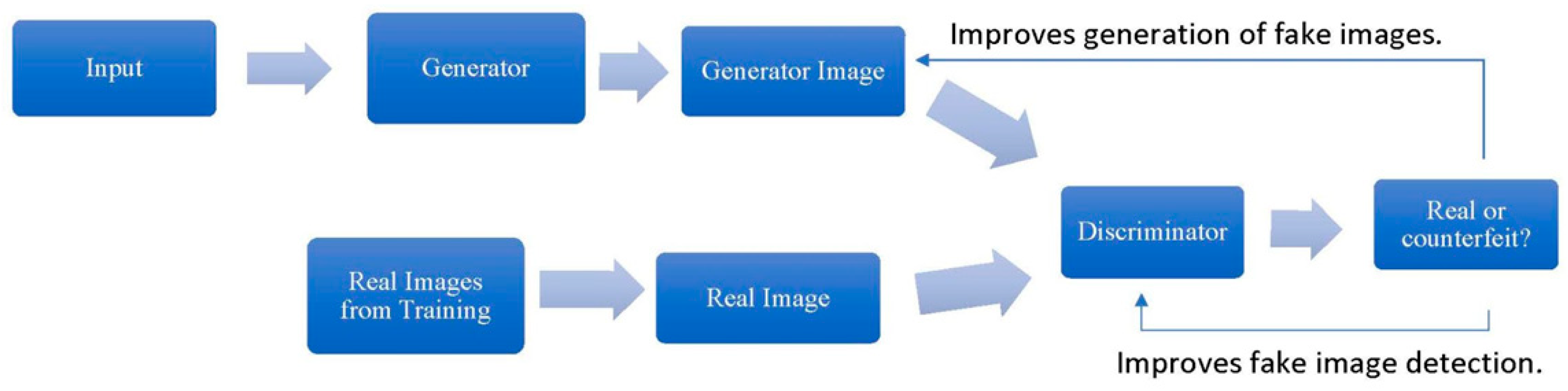
5. Data Generation and Augmentation: Generative Adversarial Networks
6. Automated Screening and Dynamic Therapeutic Support: Transformers
7. Evaluating Model Performance
7.1. Clinical Evaluation
7.2. Model Evaluation
8. AI-Driven Personalization: Clinical Implementation
8.1. Biomarker Identification
8.2. Digital Therapeutics and Real-Time Interventions
9. Challenges and Future Directions
9.1. General Limitations of AI in OCD
9.1.1. Uncertain Comparative Effectiveness
9.1.2. Cost and Resource Constraints
9.1.3. Limited Patient Perspectives Data
9.1.4. Reproducibility
9.1.5. Interpretability
9.2. Ethical Limitations
9.2.1. OCD Research
9.2.2. OCD Clinical Care
10. Toward Precision OCD Care
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| OCD | Obsessive-compulsive disorder |
| ERP | Exposure and response prevention |
| SSRIs | Serotonin reuptake inhibitors |
| DL | Deep learning |
| AI | Artificial intelligence |
| FFNNs | Feedforward neural networks |
| qEEG | Quantitative electroencephalography |
| TMS | Transcranial magnetic stimulation |
| CNNs | Convolutional neural networks |
| fMRI | Functional magnetic resonance imaging |
| RNNs | Recurrent neural networks |
| EMA | Ecological momentary assessment |
| LSTM | Long short-term memory |
| GANs | Generative adversarial networks |
| LLMs | Large language models |
| CSTC | Cortical-striatal-thalamic-cortical |
| VRET | Virtual reality exposure therapy |
References
- Stahnke, B. A Systematic Review of Misdiagnosis in Those with Obsessive-Compulsive Disorder. J. Affect. Disord. Rep. 2021, 6, 100231. [Google Scholar]
- Lochner, C.; Stein, D.J. Does Work on Obsessive–Compulsive Spectrum Disorders Contribute to Understanding the Heterogeneity of Obsessive–Compulsive Disorder? Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2006, 30, 353–361. [Google Scholar] [CrossRef]
- Cervin, M.; Miguel, E.C.; Güler, A.S.; Ferrão, Y.A.; Erdoğdu, A.B.; Lazaro, L.; Gökçe, S.; Geller, D.A.; Yulaf, Y.; Başgül, Ş.S. Towards a Definitive Symptom Structure of Obsessive-Compulsive Disorder: A Factor and Network Analysis of 87 Distinct Symptoms in 1366 Individuals. Psychol. Med. 2021, 52, 3267–3279. [Google Scholar] [PubMed]
- Lebowitz, E.R.; Panza, K.E.; Su, J.; Bloch, M.H. Family Accommodation in Obsessive–Compulsive Disorder. Expert Rev. Neurother. 2012, 12, 229–238. [Google Scholar] [CrossRef]
- Stein, D.J.; Costa, D.L.C.; Lochner, C.; Miguel, E.C.; Reddy, Y.C.J.; Shavitt, R.G.; van den Heuvel, O.A.; Simpson, H.B. Obsessive–Compulsive Disorder. Nat. Rev. Dis. Primers 2019, 5, 52. [Google Scholar] [CrossRef]
- Ong, C.W.; Clyde, J.W.; Bluett, E.J.; Levin, M.E.; Twohig, M.P. Dropout Rates in Exposure with Response Prevention for Obsessive-Compulsive Disorder: What Do the Data Really Say? J. Anxiety Disord. 2016, 40, 8–17. [Google Scholar]
- Frank, H.E.; Cain, G.; Freeman, J.; Benito, K.G.; O’Connor, E.; Kemp, J.; Kim, B. Parent-Identified Barriers to Accessing Exposure Therapy: A Qualitative Study Using Process Mapping. Front. Psychiatry 2023, 14, 1068255. [Google Scholar]
- O’Neill, J.; Feusner, J. Cognitive-Behavioral Therapy for Obsessive–Compulsive Disorder: Access to Treatment, Prediction of Long-Term Outcome with Neuroimaging. PRBM 2015, 8, 211–223. [Google Scholar] [CrossRef]
- Bloch, M.H.; McGuire, J.; Landeros-Weisenberger, A.; Leckman, J.F.; Pittenger, C. Meta-Analysis of the Dose-Response Relationship of SSRI in Obsessive-Compulsive Disorder. Mol. Psychiatry 2010, 15, 850–855. [Google Scholar] [CrossRef]
- Martiadis, V.; Pessina, E.; Martini, A.; Raffone, F.; Cattaneo, C.I.; De Berardis, D.; Pampaloni, I. Serotonin Reuptake Inhibitors Augmentation with Cariprazine in Patients with Treatment-Resistant Obsessive-Compulsive Disorder: A Retrospective Observational Study. CNS Spectr. 2024, 29, 296–299. [Google Scholar]
- Martiadis, V.; Pessina, E.; Martini, A.; Raffone, F.; Besana, F.; Olivola, M.; Cattaneo, C.I. Brexpiprazole Augmentation in Treatment-Resistant OCD: Safety and Efficacy in an Italian Sample. Psychiatr. Danub. 2024, 36, 396–401. [Google Scholar]
- Koran, L.M.; Hanna, G.L.; Hollander, E.; Nestadt, G.; Simpson, H.B.; American Psychiatric Association. Practice Guideline for the Treatment of Patients with Obsessive-Compulsive Disorder. Am. J. Psychiatry 2007, 164, 5–53. [Google Scholar] [PubMed]
- Bandelow, B.; Sher, L.; Bunevicius, R.; Hollander, E.; Kasper, S.; Zohar, J.; Möller, H.-J.; WFSBP Task Force on Mental Disorders in Primary Care; WFSBP Task Force on Anxiety Disorders, OCD and PTSD. Guidelines for the Pharmacological Treatment of Anxiety Disorders, Obsessive–Compulsive Disorder and Posttraumatic Stress Disorder in Primary Care. Int. J. Psychiatry Clin. Pract. 2012, 16, 77–84. [Google Scholar] [PubMed]
- National Institute for Health and Care Excellence. Obsessive-Compulsive Disorder and Body Dysmorphic Disorder: Treatment Guidance. Available online: https://www.nice.org.uk/guidance/cg31 (accessed on 30 March 2025).
- Di Stefano, V.; D’Angelo, M.; Monaco, F.; Vignapiano, A.; Martiadis, V.; Barone, E.; Fornaro, M.; Steardo, L.; Solmi, M.; Manchia, M. Decoding Schizophrenia: How AI-Enhanced fMRI Unlocks New Pathways for Precision Psychiatry. Brain Sci. 2024, 14, 1196. [Google Scholar] [CrossRef]
- Smucny, J.; Davidson, I.; Carter, C.S. Comparing Machine and Deep learning-based Algorithms for Prediction of Clinical Improvement in Psychosis with Functional Magnetic Resonance Imaging. Hum. Brain Mapp. 2021, 42, 1197–1205. [Google Scholar] [CrossRef]
- Monaco, F.; Vignapiano, A.; Piacente, M.; Pagano, C.; Mancuso, C.; Steardo, L., Jr.; Marenna, A.; Farina, F.; Petrillo, G.; Leo, S.; et al. An Advanced Artificial Intelligence Platform for a Personalised Treatment of Eating Disorders. Front. Psychiatry 2024, 15, 1414439. [Google Scholar]
- Lin, E.; Lin, C.-H.; Lane, H.-Y. Machine Learning and Deep Learning for the Pharmacogenomics of Antidepressant Treatments. Clin. Psychopharmacol. Neurosci. 2021, 19, 557. [Google Scholar]
- Zhao, Y.; Fu, Z.; Barnett, E.J.; Wang, N.; Zhang, K.; Gao, X.; Zheng, X.; Tian, J.; Zhang, H.; Ding, X. Genome Data Based Deep Learning Identified New Genes Predicting Pharmacological Treatment Response of Attention Deficit Hyperactivity Disorder. Transl. Psychiatry 2025, 15, 46. [Google Scholar]
- Askland, K.D.; Garnaat, S.; Sibrava, N.J.; Boisseau, C.L.; Strong, D.; Mancebo, M.; Greenberg, B.; Rasmussen, S.; Eisen, J. Prediction of Remission in Obsessive Compulsive Disorder Using a Novel Machine Learning Strategy. Int. J. Methods Psychiatr. Res. 2015, 24, 156–169. [Google Scholar] [CrossRef]
- Koppe, G.; Meyer-Lindenberg, A.; Durstewitz, D. Deep Learning for Small and Big Data in Psychiatry. Neuropsychopharmacology 2021, 46, 176–190. [Google Scholar] [CrossRef]
- Su, C.; Xu, Z.; Pathak, J.; Wang, F. Deep Learning in Mental Health Outcome Research: A Scoping Review. Transl. Psychiatry 2020, 10, 116. [Google Scholar] [CrossRef] [PubMed]
- Durstewitz, D.; Koppe, G.; Meyer-Lindenberg, A. Deep Neural Networks in Psychiatry. Mol. Psychiatry 2019, 24, 1583–1598. [Google Scholar] [CrossRef]
- Avberšek, L.K.; Repovš, G. Deep Learning in Neuroimaging Data Analysis: Applications, Challenges, and Solutions. Front. Neuroimaging 2022, 1, 981642. [Google Scholar] [CrossRef] [PubMed]
- Valverde, J.M.; Imani, V.; Abdollahzadeh, A.; De Feo, R.; Prakash, M.; Ciszek, R.; Tohka, J. Transfer Learning in Magnetic Resonance Brain Imaging: A Systematic Review. J. Imaging 2021, 7, 66. [Google Scholar] [CrossRef] [PubMed]
- Askr, H.; Elgeldawi, E.; Aboul Ella, H.; Elshaier, Y.A.M.M.; Gomaa, M.M.; Hassanien, A.E. Deep Learning in Drug Discovery: An Integrative Review and Future Challenges. Artif. Intell. Rev. 2023, 56, 5975–6037. [Google Scholar] [CrossRef]
- Salomoni, G.; Grassi, M.; Mosini, P.; Riva, P.; Cavedini, P.; Bellodi, L. Artificial Neural Network Model for the Prediction of Obsessive-Compulsive Disorder Treatment Response. J. Clin. Psychopharmacol. 2009, 29, 343. [Google Scholar] [CrossRef]
- Metin, S.Z.; Balli Altuglu, T.; Metin, B.; Erguzel, T.T.; Yigit, S.; Arıkan, M.K.; Tarhan, K.N. Use of EEG for Predicting Treatment Response to Transcranial Magnetic Stimulation in Obsessive Compulsive Disorder. Clin. EEG Neurosci. 2020, 51, 139–145. [Google Scholar] [CrossRef]
- Zaboski, B.A.; Wilens, A.; McNamara, J.P.; Muller, G.N. Predicting OCD Severity from Religiosity and Personality: A Machine Learning and Neural Network Approach. J. Mood Anxiety Disord. 2024, 8, 100089. [Google Scholar] [CrossRef]
- Zhang, A.; Lipton, Z.C.; Li, M.; Smola, A.J. Dive Into Deep Learning; Cambridge University Press: Cambridge, UK, 2022. [Google Scholar]
- LeCun, Y.; Bottou, L.; Bengio, Y.; Haffner, P. Gradient-Based Learning Applied to Document Recognition. Proc. IEEE 1998, 86, 2278–2324. [Google Scholar] [CrossRef]
- Goodfellow, I.J.; Pouget-Abadie, J.; Mirza, M.; Xu, B.; Warde-Farley, D.; Ozair, S.; Courville, A.; Bengio, Y. Generative Adversarial Networks. arXiv 2014, arXiv:1406.2661. [Google Scholar] [CrossRef]
- Kalmady, S.V.; Paul, A.K.; Narayanaswamy, J.C.; Agrawal, R.; Shivakumar, V.; Greenshaw, A.J.; Dursun, S.M.; Greiner, R.; Venkatasubramanian, G.; Reddy, Y.C.J. Prediction of Obsessive-Compulsive Disorder: Importance of Neurobiology-Aided Feature Design and Cross-Diagnosis Transfer Learning. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2022, 7, 735–746. [Google Scholar] [CrossRef] [PubMed]
- Krohn, J.; Beyleveld, G.; Bassens, A. Deep Learning Illustrated; Addison-Wesley Professional: Linköping, Sweden, 2019. [Google Scholar]
- Rashed, A.E.E.; Atwa, A.E.M.; Ahmed, A.; Badawy, M.; Elhosseini, M.A.; Bahgat, W.M. Facial Image Analysis for Automated Suicide Risk Detection with Deep Neural Networks. Artif. Intell. Rev. 2024, 57, 274. [Google Scholar] [CrossRef]
- Aggarwal, C.C. Neural Networks and Deep Learning: A Textbook; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Mishra, S.; Seth, S.; Jain, S.; Pant, V.; Parikh, J.; Chugh, N.; Puri, Y. An Emotionally Intelligent Haptic System–An Efficient Solution for Anxiety Detection and Mitigation. Comput. Methods Programs Biomed. 2025, 260, 108590. [Google Scholar]
- Kirsten, K.; Pfitzner, B.; Löper, L.; Arnrich, B. Sensor-Based Obsessive-Compulsive Disorder Detection With Personalised Federated Learning. In Proceedings of the 2021 20th IEEE International Conference on Machine Learning and Applications (ICMLA), Virtual, 13–15 December 2021; pp. 333–339. [Google Scholar]
- Pan, J.; Lei, B.; Wang, S.; Wang, B.; Liu, Y.; Shen, Y. DecGAN: Decoupling Generative Adversarial Network Detecting Abnormal Neural Circuits for Alzheimer’s Disease. IEEE Trans. Artif. Intelligence 2024, 5, 5050–5063. [Google Scholar]
- Tripathi, S.; Augustin, A.I.; Dunlop, A.; Sukumaran, R.; Dheer, S.; Zavalny, A.; Haslam, O.; Austin, T.; Donchez, J.; Tripathi, P.K.; et al. Recent Advances and Application of Generative Adversarial Networks in Drug Discovery, Development, and Targeting. Artif. Intell. Life Sci. 2022, 2, 100045. [Google Scholar] [CrossRef]
- Nair, R.; Mohan, D.D.; Frank, S.; Setlur, S.; Govindaraju, V.; Ramanathan, M. Generative Adversarial Networks for Modelling Clinical Biomarker Profiles with Race/Ethnicity. Br. J. Clin. Pharmacol. 2023, 89, 1588–1600. [Google Scholar] [CrossRef]
- Vaswani, A.; Shazeer, N.; Parmar, N.; Uszkoreit, J.; Jones, L.; Gomez, A.N.; Kaiser, Ł.; Polosukhin, I. Attention Is All You Need. In Proceedings of the 31st Conference on Neural Information Processing Systems (NIPS 2017), Long Beach, CA, USA, 4–9 December 2017; pp. 6000–6010. [Google Scholar]
- Cao, Y.; Li, S.; Liu, Y.; Yan, Z.; Dai, Y.; Yu, P.S.; Sun, L. A Comprehensive Survey of AI-Generated Content (AIGC): A History of Generative AI from GAN to ChatGPT. arXiv 2023, arXiv:2303.04226. [Google Scholar]
- Wu, T.; He, S.; Liu, J.; Sun, S.; Liu, K.; Han, Q.-L.; Tang, Y. A Brief Overview of ChatGPT: The History, Status Quo and Potential Future Development. IEEE/CAA J. Autom. Sin. 2023, 10, 1122–1136. [Google Scholar]
- Kim, J.; Leonte, K.G.; Chen, M.L.; Torous, J.B.; Linos, E.; Pinto, A.; Rodriguez, C.I. Large Language Models Outperform Mental and Medical Health Care Professionals in Identifying Obsessive-Compulsive Disorder. NPJ Digit. Med. 2024, 7, 193. [Google Scholar] [CrossRef]
- Levkovich, I. Harnessing Large Language Models for Identification and Treatment of Obsessive-Compulsive Disorder. Preprints 2024, 2024060857. [Google Scholar] [CrossRef]
- Park, S.H.; Kressel, H.Y. Connecting Technological Innovation in Artificial Intelligence to Real-World Medical Practice through Rigorous Clinical Validation: What Peer-Reviewed Medical Journals Could Do. J. Korean Med. Sci. 2018, 33, e152. [Google Scholar] [CrossRef] [PubMed]
- Nagendran, M.; Chen, Y.; Lovejoy, C.A.; Gordon, A.C.; Komorowski, M.; Harvey, H.; Topol, E.J.; Ioannidis, J.P.A.; Collins, G.S.; Maruthappu, M. Artificial Intelligence versus Clinicians: Systematic Review of Design, Reporting Standards, and Claims of Deep Learning Studies. BMJ 2020, 368, m689. [Google Scholar] [CrossRef]
- Ramspek, C.L.; Jager, K.J.; Dekker, F.W.; Zoccali, C.; van Diepen, M. External Validation of Prognostic Models: What, Why, How, When and Where? Clin. Kidney J. 2021, 14, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Belkin, M.; Hsu, D.; Ma, S.; Mandal, S. Reconciling Modern Machine-Learning Practice and the Classical Bias–Variance Trade-Off. Proc. Natl. Acad. Sci. USA 2019, 116, 15849–15854. [Google Scholar] [CrossRef] [PubMed]
- Dar, Y.; Muthukumar, V.; Baraniuk, R.G. A Farewell to the Bias-Variance Tradeoff? An Overview of the Theory of Overparameterized Machine Learning. arXiv 2021, arXiv:2109.02355. [Google Scholar]
- Yang, Z.; Yu, Y.; You, C.; Steinhardt, J.; Ma, Y. Rethinking Bias-Variance Trade-off for Generalization of Neural Networks. In Proceedings of the 37th International Conference on Machine Learning, PMLR, Online, 21 November 2020; pp. 10767–10777. [Google Scholar]
- Naidu, G.; Zuva, T.; Sibanda, E.M. A Review of Evaluation Metrics in Machine Learning Algorithms. In Artificial Intelligence Application in Networks and Systems; Silhavy, R., Silhavy, P., Eds.; Lecture Notes in Networks and Systems; Springer International Publishing: Cham, Switzerland, 2023; Volume 724, pp. 15–25. ISBN 978-3-031-35313-0. [Google Scholar]
- Altuğlu, T.B.; Metin, B.; Tülay, E.E.; Tan, O.; Sayar, G.H.; Taş, C.; Arikan, K.; Tarhan, N. Prediction of Treatment Resistance in Obsessive Compulsive Disorder Patients Based on EEG Complexity as a Biomarker. Clin. Neurophysiol. 2020, 131, 716–724. [Google Scholar] [CrossRef]
- Li, X.; Kang, Q.; Gu, H. A Comprehensive Review for Machine Learning on Neuroimaging in Obsessive-Compulsive Disorder. Front. Hum. Neurosci. 2023, 17, 1280512. [Google Scholar] [CrossRef]
- Zaboski, B.A.; Gilbert, A.; Hamblin, R.; Andrews, J.; Ramos, A.; Nadeau, J.M.; Storch, E.A. Quality of Life in Children and Adolescents with Obsessive-Compulsive Disorder: The Pediatric Quality of Life Enjoyment and Satisfaction Questionnaire (PQ-LES-Q). Bull. Menn. Clin. 2019, 83, 377–397. [Google Scholar] [CrossRef]
- BioPharm International First Drug Created via AI to Enter Clinical Trials, Signaling New Era for Pharma. Available online: https://www.biopharminternational.com/view/first-drug-created-ai-enter-clinical-trials-signaling-new-era-pharma (accessed on 12 February 2025).
- Sumitomo Dainippon Pharma Co., Ltd. Sumitomo Dainippon Pharma and Exscientia Joint Development New Drug Candidate Created Using Artificial Intelligence (AI) Begins Clinical Study. Available online: https://www.sumitomo-pharma.com/news/20200130.html (accessed on 12 February 2025).
- Burki, T. A New Paradigm for Drug Development. Lancet Digit. Health 2020, 2, e226–e227. [Google Scholar] [CrossRef]
- Farzan, M.; Ebrahimi, H.; Pourali, M.; Sabeti, F. Artificial Intelligence-Powered Cognitive Behavioral Therapy Chatbots, a Systematic Review. Iran. J. Psychiatry 2025, 20, 102–110. [Google Scholar] [CrossRef]
- Jiang, M.; Zhao, Q.; Li, J.; Wang, F.; He, T.; Cheng, X.; Yang, B.X.; Ho, G.W.K.; Fu, G. A Generic Review of Integrating Artificial Intelligence in Cognitive Behavioral Therapy 2024. arXiv 2024, arXiv:2407.19422. [Google Scholar]
- Obremski, D.; Wienrich, C. Autonomous VR Exposure Therapy for Anxiety Disorders Using Conversational AI—Ethical and Technical Implications and Preliminary Results. J. Comput. Commun. 2024, 12, 111–138. [Google Scholar] [CrossRef]
- Yin, J.; Ngiam, K.Y.; Teo, H.H. Role of Artificial Intelligence Applications in Real-Life Clinical Practice: Systematic Review. J. Med. Internet Res. 2021, 23, e25759. [Google Scholar] [CrossRef]
- Keel, S.; Lee, P.Y.; Scheetz, J.; Li, Z.; Kotowicz, M.A.; MacIsaac, R.J.; He, M. Feasibility and Patient Acceptability of a Novel Artificial Intelligence-Based Screening Model for Diabetic Retinopathy at Endocrinology Outpatient Services: A Pilot Study. Sci. Rep. 2018, 8, 4330. [Google Scholar]
- Rigoux, L.; Stephan, K.E.; Petzschner, F.H. Beliefs, Compulsive Behavior and Reduced Confidence in Control. PLoS Comput. Biol. 2024, 20, e1012207. [Google Scholar]
- Brandes, O.; Stern, A.; Doron, G. “I Just Can’t Trust My Partner”: Evaluating Associations between Untrustworthiness Obsessions, Relationship Obsessions and Couples Violence. J. Obs. Compuls. Relat. Disord. 2020, 24, 100500. [Google Scholar] [CrossRef]
- Moore, A.; Wheaton, M.G.; Rodriguez, C.I.; Raila, H.; Shen, H. Compulsively Seeking Certainty: Clarifying the Association between Intolerance of Uncertainty and Compulsion Severity in OCD. J. Emot. Psychopathol. 2023, 1, 262–272. [Google Scholar]
- Breiman, L. Statistical Modeling: The Two Cultures (with Comments and a Rejoinder by the Author). Stat. Sci. 2001, 16, 199–231. [Google Scholar] [CrossRef]
- Chandler, C.; Foltz, P.W.; Elvevåg, B. Improving the Applicability of AI for Psychiatric Applications through Human-in-the-Loop Methodologies. Schizophr. Bull. 2022, 48, 949–957. [Google Scholar] [CrossRef]
- International OCD Foundation|Special Interest Groups. Available online: https://iocdf.org/special-interest-groups/ (accessed on 28 May 2024).
- Golden, A.; Aboujaoude, E. Describing the Framework for AI Tool Assessment in Mental Health and Applying It to a Generative AI Obsessive-Compulsive Disorder Platform: Tutorial. JMIR Form. Res. 2024, 8, e62963. [Google Scholar]
- Hua, Y.; Liu, F.; Yang, K.; Li, Z.; Na, H.; Sheu, Y.; Zhou, P.; Moran, L.V.; Ananiadou, S.; CliDon, D.A.; et al. Large Language Models in Mental Health Care. arXiv 2024, arXiv:2401.02984. [Google Scholar]
- Vagwala, M.K.; Asher, R. Conversational Artificial Intelligence and Distortions of the Psychotherapeutic Frame: Issues of Boundaries, Responsibility, and Industry Interests. Am. J. Bioeth. 2023, 23, 28–30. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaboski, B.A.; Bednarek, L. Precision Psychiatry for Obsessive-Compulsive Disorder: Clinical Applications of Deep Learning Architectures. J. Clin. Med. 2025, 14, 2442. https://doi.org/10.3390/jcm14072442
Zaboski BA, Bednarek L. Precision Psychiatry for Obsessive-Compulsive Disorder: Clinical Applications of Deep Learning Architectures. Journal of Clinical Medicine. 2025; 14(7):2442. https://doi.org/10.3390/jcm14072442
Chicago/Turabian StyleZaboski, Brian A., and Lora Bednarek. 2025. "Precision Psychiatry for Obsessive-Compulsive Disorder: Clinical Applications of Deep Learning Architectures" Journal of Clinical Medicine 14, no. 7: 2442. https://doi.org/10.3390/jcm14072442
APA StyleZaboski, B. A., & Bednarek, L. (2025). Precision Psychiatry for Obsessive-Compulsive Disorder: Clinical Applications of Deep Learning Architectures. Journal of Clinical Medicine, 14(7), 2442. https://doi.org/10.3390/jcm14072442





