Comparative Analysis Between Insulated Gel Bags and Direct Cooling for Temperature Management During Kidney Transplant Vascular Anastomosis
Abstract
1. Introduction
2. Patients and Methods
2.1. Immunosuppression Protocol
2.2. Statistical Analysis
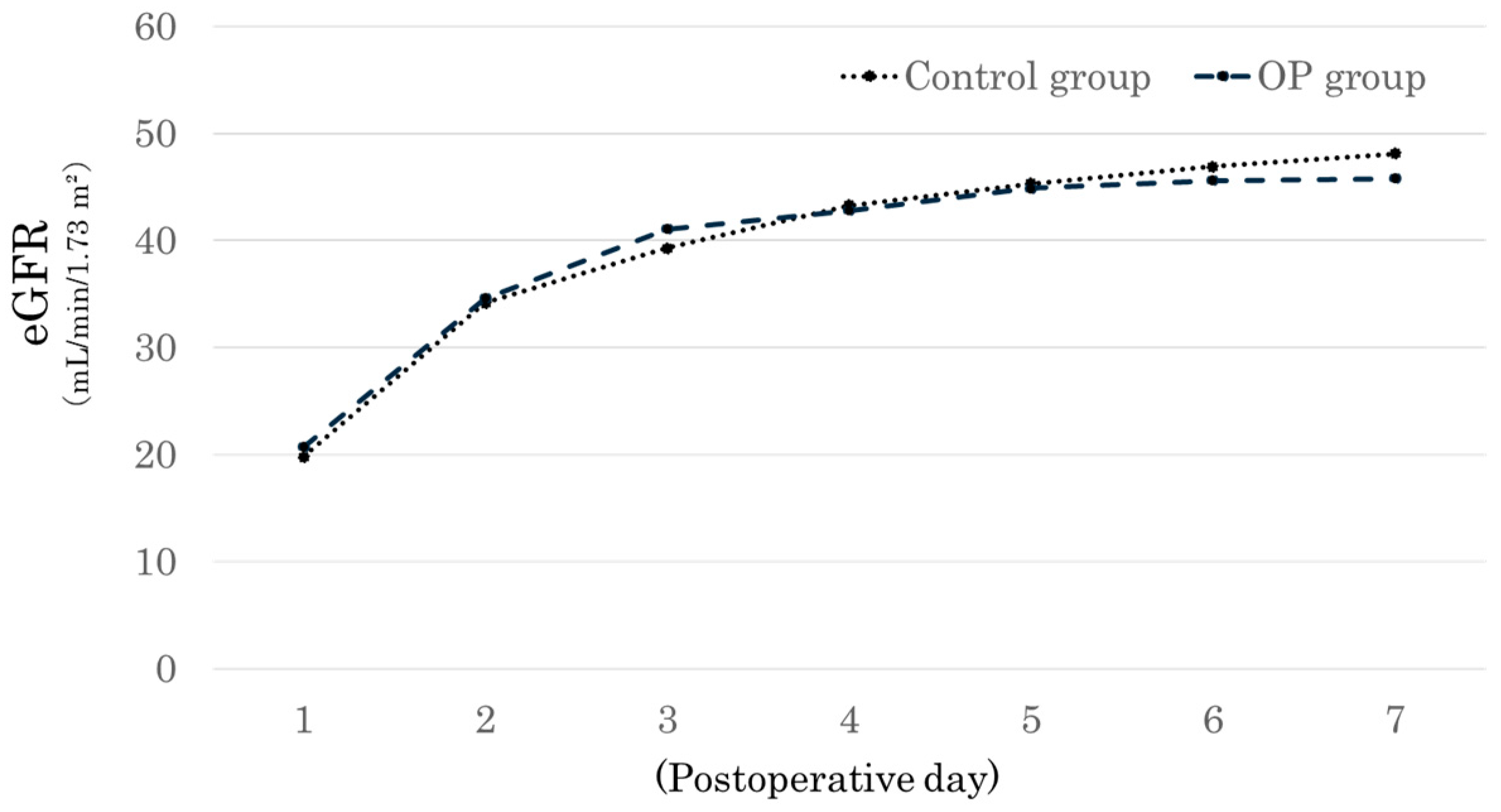
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| WIT | Warm ischemia time |
| CIT | Cold ischemia time |
| DGF | Delayed graft function |
| CNI | Calcineurin inhibitor |
| EVR | Everolimus |
| eGFR | Estimated glomerular filtration rate |
References
- Kuipers, T.G.; Hellegering, J.; El Moumni, M.; Krikke, C.; Haveman, J.W.; Berger, S.P.; Leuvenink, H.G.; Pol, R.A. Kidney temperature course during living organ procurement and transplantation. Transp. Int. 2017, 30, 162–169. [Google Scholar] [CrossRef]
- Tennankore, K.K.; Kim, S.J.; Alwayn, I.P.; Kiberd, B.A. Prolonged warm ischemia time is associated with graft failure and mortality after kidney transplantation. Kidney Int. 2016, 89, 648–658. [Google Scholar] [CrossRef] [PubMed]
- Brennan, T.V.; Freise, C.E.; Fuller, T.F.; Bostrom, A.; Tomlanovich, S.J.; Feng, S. Early graft function after living donor kidney transplantation predicts rejection but not outcomes. Am. J. Transplant. 2004, 4, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Urbanellis, P.; Mazilescu, L.; Kollmann, D.; Linares-Cervantes, I.; Kaths, J.M.; Ganesh, S.; Oquendo, F.; Sharma, M.; Goto, T.; Noguchi, Y.; et al. Prolonged warm ischemia time leads to severe renal dysfunction of donation-after-cardiac death kidney grafts. Sci. Rep. 2021, 11, 17930. [Google Scholar] [CrossRef]
- Hellegering, J.; Visser, J.; Kloke, H.J.; D’Ancona, F.C.; Hoitsma, A.J.; van der Vliet, J.A.; Warlé, M.C. Deleterious influence of prolonged warm ischemia in living donor kidney transplantation. Transplant. Proc. 2012, 44, 1222–1226. [Google Scholar] [CrossRef] [PubMed]
- Ferede, A.A.; Walsh, A.L.; Davis, N.F.; Smyth, G.; Mohan, P.; Power, R.; Forde, J.; O’Kelly, P.; Llittle, D. Warm Ischemia Time at Vascular Anastomosis is an Independent Predictor for Delayed Graft Function in Kidney Transplant Recipients. Exp. Clin. Transplant. 2020, 18, 13–18. [Google Scholar] [CrossRef]
- Heylen, L.; Pirenne, J.; Naesens, M.; Sprangers, B.; Jochmans, I. “Time is tissue”-A minireview on the importance of donor nephrectomy, donor hepatectomy, and implantation times in kidney and liver transplantation. Am. J. Transplant. 2021, 21, 2653–2661. [Google Scholar] [CrossRef]
- Feuillu, B.; Cormier, L.; Frimat, L.; Kessler, M.; Amrani, M.; Mangin, P.; Hubert, J. Kidney warming during transplantation. Transpl. Int. 2003, 16, 307–312. [Google Scholar] [CrossRef]
- Forsythe, J.L.; Dunnigan, P.M.; Proud, G.; Lennard, T.W.; Taylor, R.M. Reducing renal injury during transplantation. Br. J. Surg. 1989, 76, 999–1001. [Google Scholar] [CrossRef]
- Wickham, J.E. Regional renal hypothermia. Ann. R. Coll. Surg. Engl. 1971, 48, 99–113. [Google Scholar] [CrossRef]
- Hameed, A.M.; Yuen, L.; Pang, T.; Rogers, N.; Hawthorne, W.J.; Pleass, H.C. Techniques to Ameliorate the Impact of Second Warm Ischemic Time on Kidney Transplantation Outcomes. Transplant. Proc. 2018, 50, 3144–3151. [Google Scholar] [CrossRef] [PubMed]
- Meier, R.P.H.; Piller, V.; Hagen, M.E.; Joliat, C.; Buchs, J.B.; Nastasi, A.; Ruttimann, R.; Buchs, N.C.; Moll, S.; Vallee, J.P.; et al. Intra-Abdominal Cooling System Limits Ischemia-Reperfusion Injury During Robot-Assisted Renal Transplantation. Am. J. Transplant. 2018, 18, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Han, X.; Dagvadorj, B.U.; Zhao, Y.; Zhang, X.; Zhu, X.; Li, T.; Zhang, P.; Chen, Y.; Li, G.; et al. An Effective Cooling Device for Minimal-Incision Kidney Transplantation. Ann. Transplant. 2020, 25, e928773. [Google Scholar] [CrossRef]
- Ide, K.; Nakano, R.; Imaoka, Y.; Sakai, H.; Ono, K.; Tanimine, N.; Tahara, H.; Ohira, M.; Ueda, K.; Hirata, T.; et al. Protection From Second Warm Ischemic Injury Using a Thermal Barrier Bag in Kidney Transplantation. Transplant. Direct. 2023, 9, e1454. [Google Scholar] [CrossRef]
- Torai, S.; Kurauchi, K.; Kobayashi, E. Evaluating a New Device for Reducing Second Warm Ischemia During Organ Transplantation in a Porcine Model of Kidney, Heart, and Pancreas Transplantation. Transplant. Proc. 2023, 55, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, S.; Imai, E.; Horio, M.; Yasuda, Y.; Tomita, K.; Nitta, K.; Yamagata, K.; Tomino, Y.; Yokoyama, H.; Hishida, A. Revised equations for estimated GFR from serum creatinine in Japan. Am. J. Kidney Dis. 2009, 53, 982–992. [Google Scholar] [CrossRef] [PubMed]
- Abou Taka, M.; Dugbartey, G.J.; Sener, A. The Optimization of Renal Graft Preservation Temperature to Mitigate Cold Ischemia-Reperfusion Injury in Kidney Transplantation. Int. J. Mol. Sci. 2022, 24, 567. [Google Scholar] [CrossRef]
- Szostek, M.; Kosieradzki, M.; Chmura, A.; Pacholczyk, M.; Lagiewska, B.; Adadyński, L.; Păczek, L.; Lao, M.; Wałasewski, J.; Rowiński, W. Does “second warm ischemia time” play a role in kidney allograft function? Transplant. Proc. 1999, 31, 1037–1038. [Google Scholar] [CrossRef]
- Ward, J.P. Determination of the Optimum temperature for regional renal hypothermia during temporary renal ischaemia. Br. J. Urol. 1975, 47, 17–24. [Google Scholar] [CrossRef]
- Andras, I.; Piana, A.; Verri, P.; Telecan, T.; Gallioli, A.; Prudhomme, T.; Hevia, V.; Baboudjian, M.; Boissier, R.; Crisan, N.; et al. Systematic review of techniques and devices used to avoid warm ischemia time injury during kidney transplantation. World J. Urol. 2023, 41, 993–1003. [Google Scholar] [CrossRef]
- Dergham, A.; Witherspoon, L.; Power, L.; Nashed, J.Y.; Skinner, T.A.A. A Novel Cooling Device for Kidney Transplant Surgery. Surg. Innov. 2024, 31, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Heylen, L.; Naesens, M.; Jochmans, I.; Monbaliu, D.; Lerut, E.; Claes, K.; Heye, S.; Verhamme, P.; Coosemans, W.; Bammens, B.; et al. The effect of anastomosis time on outcome in recipients of kidneys donated after brain death: A cohort study. Am. J. Transplant. 2015, 15, 2900–2907. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.R.; Eggener, S.E. Warm ischemia less than 30 minutes is not necessarily safe during partial nephrectomy: Every minute matters. Urol. Oncol. 2011, 29, 826–828. [Google Scholar] [CrossRef]
- Malyszko, J.; Lukaszyk, E.; Glowinska, I.; Durlik, M. Biomarkers of delayed graft function as a form of acute kidney injury in kidney transplantation. Sci. Rep. 2015, 5, 11684. [Google Scholar] [CrossRef]
- Przybylowski, P.; Koc-Zorawska, E.; Malyszko, J.S.; Kozlowska, S.; Mysliwiec, M.; Malyszko, J. Liver fatty-acid-binding protein in heart and kidney allograft recipients in relation to kidney function. Transplant. Proc. 2011, 43, 3064–3067. [Google Scholar] [CrossRef]
- Bruyère, F.; Doumerc, N. Robotic kidney transplantation: Dream or future? Curr. Opin. Urol. 2018, 28, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Ganpule, A.; Patil, A.; Singh, A.; Desai, M.; Gill, I.; Sabnis, R.; Desai, M. Robotic-assisted kidney transplant: A single center experience with median follow-up of 2.8 years. World J. Urol. 2020, 38, 2651–2660. [Google Scholar] [CrossRef]
- Tzvetanov, I.G.; Spaggiari, M.; Tulla, K.A.; Di Bella, C.; Okoye, O.; Di Cocco, P.; Jeon, H.; Oberholzer, J.; Cristoforo Giulianotti, P.; Benedetti, E. Robotic kidney transplantation in the obese patient: 10-year experience from a single center. Am. J. Transplant. 2020, 20, 430–440. [Google Scholar] [CrossRef]

| Control (n = 16) | Organ Pocket (n = 33) | p-Value | |
|---|---|---|---|
| Recipient age | 44 (34.75–54) | 46 (40–54) | 0.798 |
| Recipient sex | 8/8 | 19/14 | 0.718 |
| Recipient BMI (kg/m2) | 22.6 (17.9–25.9) | 23.2 (23.2–26.4) | 0.466 |
| Harvested kidney volume (mL) | 130.8 (125.4–147.6) | 142.5 (123.4–163.7) | 0.43 |
| Donor age | 60.5 (54.5–62.5) | 55 (49–64) | 0.55 |
| Donor sex | 5/11 | 13/20 | 0.754 |
| Donor BMI (kg/m2) | 23.1 (21.3–24.2) | 22.2 (20.7–25.3) | 0.647 |
| Operation time (min) | 294.0 (250.75–332.75) | 290.5 (237.5–336.0) | 0.913 |
| CIT (min) | 22.5 (16.5–24.75) | 22.5 (19.75–27) | 0.298 |
| WIT (min) | 3 (2–3) | 3 (3–3) | 0.281 |
| SWIT (min) | 51 (45–54) | 55 (48.5–64.75) | 0.123 |
| ABO compatible | Compatible: 14 Incompatible: 2 | Compatible: 22 Incompatible: 11 | 0.174 |
| Number of renal arteries | One: 14 Two: 2 | One: 26 Two: 7 | 0.698 |
| Control (n = 16) | Organ Pocket (n = 33) | p-Value | |
|---|---|---|---|
| Postoperative period (month) | 11.5 (10–18) | 8 (4–24) | 0.515 |
| Best sCr | 0.94 (0.8–1.41) | 1.03 (0.92–1.3) | 0.533 |
| Best eGFR | 52.2 (45.6–69) | 50.6 (45.1–63.1) | 0.689 |
| Delayed graft function | 1 | 0 | 0.327 |
| L-FABP POD1 | 92.4 (59.8–138.5) | 88.1 (44.2–170) | 0.727 |
| L-FABP POD7 | 19.1 (4.3–60.9) | 29.3 (6.7–81) | 0.564 |
| L-FABP POD28 | 22.3 (5.7–22.3) | 6.8 (4.3–12) | 0.678 |
| Acute rejection | 3 | 2 | 0.163 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machida, Y.; Iwai, T.; Kabei, K.; Uchida, J. Comparative Analysis Between Insulated Gel Bags and Direct Cooling for Temperature Management During Kidney Transplant Vascular Anastomosis. J. Clin. Med. 2025, 14, 2368. https://doi.org/10.3390/jcm14072368
Machida Y, Iwai T, Kabei K, Uchida J. Comparative Analysis Between Insulated Gel Bags and Direct Cooling for Temperature Management During Kidney Transplant Vascular Anastomosis. Journal of Clinical Medicine. 2025; 14(7):2368. https://doi.org/10.3390/jcm14072368
Chicago/Turabian StyleMachida, Yuichi, Tomoaki Iwai, Kazuya Kabei, and Junji Uchida. 2025. "Comparative Analysis Between Insulated Gel Bags and Direct Cooling for Temperature Management During Kidney Transplant Vascular Anastomosis" Journal of Clinical Medicine 14, no. 7: 2368. https://doi.org/10.3390/jcm14072368
APA StyleMachida, Y., Iwai, T., Kabei, K., & Uchida, J. (2025). Comparative Analysis Between Insulated Gel Bags and Direct Cooling for Temperature Management During Kidney Transplant Vascular Anastomosis. Journal of Clinical Medicine, 14(7), 2368. https://doi.org/10.3390/jcm14072368






