Spontaneous Resolution of Ventricular Pre-Excitation During Childhood: A Retrospective Study
Abstract
1. Introduction
2. Methods
2.1. Risk Stratification Tools
2.2. Management
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Resolution of Ventricular Pre-Excitation
3.3. Risk Stratification
3.4. Chronic Drug Therapy
3.5. Catheter Ablation
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AF | Atrial fibrillation |
| AP | Accessory pathway |
| AV | Atrioventricular |
| CHD | Congenital heart disease |
| CI | Confidence interval |
| ECG | Electrocardiogram |
| EST | Exercise stress test |
| MAE | Major arrhythmic event |
| SCD | Sudden cardiac death |
| SD | Standard deviations |
| SPERRI | Shortest pre-excited RR interval |
| SVT | Supraventricular tachycardia |
| VP | Ventricular pre-excitation |
| WPW | Wolf–Parkinson–White |
References
- Hiss, R.G.; Lamb, L.E. Electrocardiographic findings in 122,043 individuals. Circulation 1962, 25, 947–961. [Google Scholar] [PubMed]
- Pærregaard, M.M.; Hartmann, J.; Sillesen, A.S.; Pihl, C.; Dannesbo, S.; Kock, T.O.; Pietersen, A.; Raja, A.A.; Iversen, K.K.; Bundgaard, H.; et al. The Wolff-Parkinson-White pattern in neonates: Results from a large population-based cohort study. Europace 2023, 25, euad165. [Google Scholar] [PubMed]
- Klein, G.J.; Bashore, T.M.; Sellers, T.D.; Pritchett, E.L.; Smith, W.M.; Gallagher, J.J. Ventricular fibrillation in the Wolff-Parkinson-White syndrome. N. Engl. J. Med. 1979, 301, 1080–1085. [Google Scholar] [CrossRef] [PubMed]
- Calkins, H. The 2019 ESC Guidelines for the Management of Patients with Supraventricular Tachycardia. Eur. Heart J. 2019, 40, 3812–3813. [Google Scholar] [CrossRef]
- Telishevska, M.; Hebe, J.; Paul, T.; Nürnberg, J.H.; Krause, U.; Gebauer, R.; Gass, M.; Balmer, C.; Berger, F.; Molatta, S.; et al. Catheter ablation in ASymptomatic PEDiatric patients with ventricular preexcitation: Results from the multicenter “CASPED” study. Clin. Res. Cardiol. 2019, 108, 683–690. [Google Scholar] [CrossRef]
- Philip Saul, J.; Kanter, R.J.; Abrams, D.; Asirvatham, S.; Bar-Cohen, Y.; Blaufox, A.D.; Cannon, B.; Clark, J.; Dick, M.; Freter, A.; et al. PACES/HRS expert consensus statement on the use of catheter ablation in children and patients with congenital heart disease: Developed in partnership with the Pediatric and Congenital Electrophysiology Society (PACES) and the Heart Rhythm Society (HRS). Endorsed by the governing bodies of PACES, HRS, the American Academy of Pediatrics (AAP), the American Heart Association (AHA), and the Association for European Pediatric and Congenital Cardiology (AEPC). Heart Rhythm. 2016, 13, e251–e289. [Google Scholar] [CrossRef]
- Munger, T.M.; Packer, D.L.; Hammill, S.C.; Feldman, B.J.; Bailey, K.R.; Ballard, D.J.; Holmes, D.R.; Gersh, B.J. A population study of the natural history of Wolff-Parkinson-White syndrome in Olmsted County, Minnesota, 1953–1989. Circulation 1993, 87, 866–873. [Google Scholar] [CrossRef]
- Deal, B.J.; Keane, J.F.; Gillette, P.C.; Garson, A. Wolff-Parkinson-White syndrome and supraventricular tachycardia during infancy: Management and follow-up. J. Am. Coll. Cardiol. 1985, 5, 130–135. [Google Scholar] [CrossRef]
- Santinelli, V.; Radinovic, A.; Manguso, F.; Vicedomini, G.; Gulletta, S.; Paglino, G.; Mazzone, P.; Ciconte, G.; Sacchi, S.; Sala, S.; et al. The natural history of asymptomatic ventricular pre-excitation a long-term prospective follow-up study of 184 asymptomatic children. J. Am. Coll. Cardiol. 2009, 53, 275–280. [Google Scholar] [CrossRef]
- Inoue, K.; Igarashi, H.; Fukushige, J.; Ohno, T.; Joh, K.; Hara, T. Long-term prospective study on the natural history of Wolff-Parkinson-White syndrome detected during a heart screening program at school. Acta Paediatr. 2000, 89, 542–545. [Google Scholar] [CrossRef]
- Goudevenos, J.A.; Katsouras, C.S.; Graekas, G.; Argiri, O.; Giogiakas, V.; Sideris, D.A. Ventricular pre-excitation in the general population: A study on the mode of presentation and clinical course. Heart 2000, 83, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Arruda, M.S.; McClelland, J.H.; Wang, X.; Beckman, K.J.; Widman, L.E.; Gonzalez, M.D.; Nakagawa, H.; Lazzara, R.; Jackman, W.M. Development and validation of an ECG algorithm for identifying accessory pathway ablation site in Wolff-Parkinson-White syndrome. J. Cardiovasc. Electrophysiol. 1998, 9, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.I.; Triedman, J.K.; Cannon, B.C.; Davis, A.M.; Drago, F.; Janousek, J.; Klein, G.J.; Law, I.H.; Morady, F.J.; Paul, T.; et al. PACES/HRS expert consensus statement on the management of the asymptomatic young patient with a Wolff-Parkinson-White (WPW, ventricular preexcitation) electrocardiographic pattern: Developed in partnership between the Pediatric and Congenital Electrophysiology Society (PACES) and the Heart Rhythm Society (HRS). Endorsed by the governing bodies of PACES, HRS, the American College of Cardiology Foundation (ACCF), the American Heart Association (AHA), the American Academy of Pediatrics (AAP), and the Canadian Heart Rhythm Society (CHRS). Heart Rhythm. 2012, 9, 1006–1024. [Google Scholar] [CrossRef] [PubMed]
- De Ponti, R.; Marazzi, R.; Doni, L.A.; Cremona, V.; Marazzato, J.; Salerno-Uriarte, J.A. Invasive electrophysiological evaluation and ablation in patients with asymptomatic ventricular pre-excitation persistent at exercise stress test. Europace 2015, 17, 946–952. [Google Scholar] [CrossRef][Green Version]
- Etheridge, S.P.; Escudero, C.A.; Blaufox, A.D.; Law, I.H.; Dechert-Crooks, B.E.; Stephenson, E.A.; Dubin, A.M.; Ceresnak, S.R.; Motonaga, K.S.; Skinner, J.R.; et al. Life-Threatening Event Risk in Children With Wolff-Parkinson-White Syndrome: A Multicenter International Study. JACC Clin. Electrophysiol. 2018, 4, 433–444. [Google Scholar] [CrossRef]
- Escudero, C.A.; Ceresnak, S.R.; Collins, K.K.; Pass, R.H.; Aziz, P.F.; Blaufox, A.D.; Ortega, M.C.; Cannon, B.C.; Cohen, M.I.; Dechert, B.E.; et al. Loss of ventricular preexcitation during noninvasive testing does not exclude high-risk accessory pathways: A multicenter study of WPW in children. Heart Rhythm. 2020, 17, 1729–1737. [Google Scholar] [CrossRef]
- Brugada, J.; Blom, N.; Sarquella-Brugada, G.; Blomstrom-Lundqvist, C.; Deanfield, J.; Janousek, J.; Abrams, D.; Bauersfeld, U.; Brugada, R.; Drago, F.; et al. Pharmacological and non-pharmacological therapy for arrhythmias in the pediatric population: EHRA and AEPC-Arrhythmia Working Group joint consensus statement. Europace 2013, 15, 1337–1382. [Google Scholar] [CrossRef]
- DeBaun, M.R.; Ghafuri, D.L.; Rodeghier, M.; Maitra, P.; Chaturvedi, S.; Kassim, A.; Ataga, K.I. Decreased median survival of adults with sickle cell disease after adjusting for left truncation bias: A pooled analysis. Blood 2019, 133, 615–617. [Google Scholar] [CrossRef]
- Cain, K.C.; Harlow, S.D.; Little, R.J.; Nan, B.; Yosef, M.; Taffe, J.R.; Elliott, M.R. Bias due to left truncation and left censoring in longitudinal studies of developmental and disease processes. Am. J. Epidemiol. 2011, 173, 1078–1084. [Google Scholar] [CrossRef]
- Cain, N.; Irving, C.; Webber, S.; Beerman, L.; Arora, G. Natural history of Wolff-Parkinson-White syndrome diagnosed in childhood. Am. J. Cardiol. 2013, 112, 961–965. [Google Scholar] [CrossRef]
- Perry, J.C.; Garson, A. Supraventricular tachycardia due to Wolff-Parkinson-White syndrome in children: Early disappearance and late recurrence. J. Am. Coll. Cardiol. 1990, 16, 1215–1220. [Google Scholar] [CrossRef] [PubMed]
- Benson, D.W.; Dunnigan, A.; Benditt, D.G. Follow-up evaluation of infant paroxysmal atrial tachycardia: Transesophageal study. Circulation 1987, 75, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Yammine, M.L.; Tamborrino, P.P.; Flore, F.; Di Mambro, C.; Pazzano, V.; Di Marzio, S.; Drago, F. Intermittent ventricular preexcitation in children: Not always a low-risk condition. Europace 2024, 26, euae250. [Google Scholar] [PubMed]
- Van Hare, G.F.; Javitz, H.; Carmelli, D.; Saul, J.P.; Tanel, R.E.; Fischbach, P.S.; Kanter, R.J.; Schaffer, M.; Dunnigan, A.; Colan, S.; et al. Prospective assessment after pediatric cardiac ablation: Demographics, medical profiles, and initial outcomes. J. Cardiovasc. Electrophysiol. 2004, 15, 759–770. [Google Scholar] [CrossRef]
- Jemtrén, A.; Saygi, S.; Åkerström, F.; Asaad, F.; Bourke, T.; Braunschweig, F.; Carnlöf, C.; Drca, N.; Insulander, P.; Kennebäck, G.; et al. Risk assessment in patients with symptomatic and asymptomatic pre-excitation. Europace 2024, 26, euae036. [Google Scholar] [CrossRef]

| Total (n = 153) | VP Persistence (n = 111) | VP Loss (n = 42) | p-Value | |
|---|---|---|---|---|
| Female sex (n,%) | 64 (41.8) | 44 (39.6) | 20 (47.6) | 0.372 |
| Age at diagnosis (years) | 4.9 (75 d–8.4) | 6 (1.3–9.2) | 61 d (24 d–4.3) | <0.001 |
| Follow-up time (days) | 4.9 (1.7–8) | 4.9 (1.6–7.2) | 1954.5 (5.4–8.6) | 0.550 |
| CHD | 8 (5.2) | 6 (5.4) | 2 (4.8) | 0.121 |
| Atrial septal defects (n,%) | 3 (1.8) | 1 (0.9) | 2 (4.8) | 0.122 |
| Ventricular septal defects (n,%) | 5 (3.3) | 5 (4.5) | 0 (0) | 0.163 |
| LV dyssynchrony (n,%) | 8 (5.2) | 6 (5.3) | 2 (4.8) | 0.897 |
| Symptoms (n,%) | 47 (30.7) | 39 (35.1) | 8 (19) | 0.054 |
| Palpitations | 32 (68.1) | 30 (76.8) | 2 (25) | |
| Chest pain | 1 (2.1) | 1 (2.6) | 0 (0) | |
| Dizziness | 1 (2.1) | 1 (2.6) | 0 (0) | |
| Vomiting | 3 (6.3) | 1 (2.6) | 2 (25) | |
| Poor oral intake | 1 (2.1) | 1 (2.6) | 0 (0) | |
| Slow growth | 1 (2.1) | 0 (0) | 1 (12.5) | |
| Heart failure | 7 (14.9) | 5 (12.8) | 2 (25) | |
| SVT (n,%) | 31 (20.3) | 23 (20.7) | 8 (19) | 0.818 |
| Variants | Univariable Crude HR (95% CI) | Crude p-Value | Multivariable Adjusted HR (95% CI) | Adjusted p-Value |
|---|---|---|---|---|
| Female sex | 1.22 (0.66–2–24) | 0.522 | ||
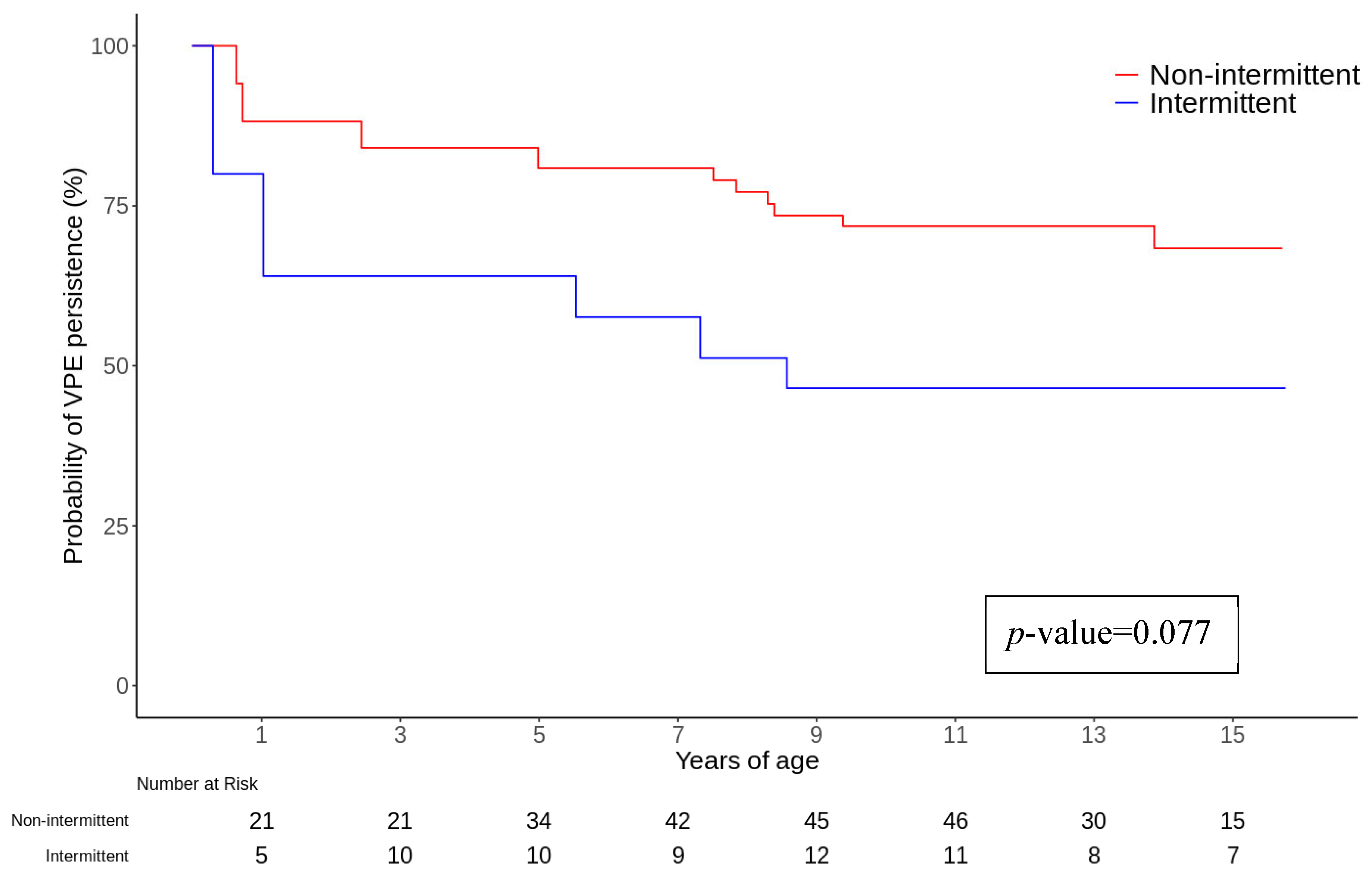
| Intermittent VP | 2.36 (0.91–6.12) | 0.077 | 3.45 (1.32–9.05) | 0.012 |
| Non-invasive “low risk” characteristics | 2.40 (0.79–7.26) | 0.122 | ||
| Presence of symptoms | 0.43 (0.20–0.93) | 0.032 | 0.15 (0.03–0.68) | 0.014 |
| SVT | 0.75 (0.35–1.64) | 0.473 | ||
| Chronic drug therapy | 0.82 (0.36–1.84) | 0.619 | ||
| Left free wall AP | 0.96 (0.43–2.15) | 0.922 | ||
| Left and right free wall AP | 0.93 (0.42–2.09) | 0.866 | ||
| Free walls + epicardial AP | 1.05 (0.51–2.17) | 0.896 |
| Total (n = 153) | VP Persistence (n = 111) | VP Loss (n = 42) | p-Value | |
|---|---|---|---|---|
| Non-invasive risk stratification (n, %) | 112 (73.2) | 93 (83.8) | 19 (45.2) | <0.001 |
| ECG Holter monitoring only (n, %) | 61 (39.9) | 48 (43.2) | 13 (30.9) | |
| Exercise test only (n, %) | 2 (1.3) | 2 (1.8) | 0 (0) | |
| Holter monitoring and ET (n, %) | 49 (32) | 43 (38.7) | 6 (14.3) | |
| “Low risk” (n, %) | 67 (43.8) | 52 (46.8) | 15 (35.7) | 0.057 |
| VP loss during exercise (n, %) | 45 (29.4) | 38 (34.2) | 7 (16.7) | 0.718 |
| Intermittent VP (n, %) | 22 (14.4) | 14 (12.6) | 8 (19) | 0.005 |
| AP localization | 0.262 | |||
| Right free wall (n, %) | 33 (21.6) | 26 (23.4) | 7 (16.7) | |
| Right posteroseptal (n, %) | 33 (21.6) | 27 (24.3) | 6 (14.3) | |
| Left posteroseptal (n, %) | 6 (3.9) | 6 (5.4) | 0 (0) | |
| Anteroseptal (n, %) | 19 (12.5) | 11 (9.9) | 8 (19.0) | |
| Left free wall (n, %) | 21 (13.6) | 17 (15.4) | 4 (9.5) | |
| Epicardial (n, %) | 12 (7.8) | 9 (8.1) | 3 (7.1) | |
| Indeterminate (n, %) | 29 (18.9) | 15 (13.5) | 14 (33.4) | |
| Chronic drug strategy tested | 27 (17.7) | 20 (18.0) | 7 (16.7) | 0.845 |
| None (n, %) | 126 (82.3) | 91 (82) | 35 (83.4) | |
| 1 strategy (n, %) | 20 (13.1) | 15 (13.5) | 5 (11.8) | |
| 2 strategies (n, %) | 6 (3.9) | 4 (3.6) | 2 (4.8) | |
| 3 strategies (n, %) | 1 (0.7) | 1 (0.9) | 0 (0) | |
| Treatment starting age (years) | 6.1 (27 d–11.1) | 3.9 (22 d–11.1) | 6.1 (1.1–11.1) | 0.083 |
| Invasive risk stratification (n, %) | 46 (30) | 41 (36.9) | 5 (11.9) | 0.002 |
| Transoesophageal EPS (n, %) | 9 (5.9) | 9 (8.2) | 0 (0) | 0.056 |
| Transvenous EPS (n, %) | 42 (27.5) | 37 (33.3) | 5 (11.9) | 0.080 |
| Ablation (n, %) | 30 (19.6) | 30 (27) | 0 (0) | |
| Ablation age (years) | 12.7 (11.5–14.5) | 12.7 (11.5–14.5) | ||
| Major procedural complications | 0 (0) | 0 (0) | ||
| Major arrhythmic events | 0 (0) | 0 (0) | 0 (0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanzo, A.; Seganti, A.; Demarchi, A.; Fino, R.S.; Raso, I.; Codazzi, A.C.; Petracci, B.; Bongiorno, A.; Rordorf, R.; Mannarino, S. Spontaneous Resolution of Ventricular Pre-Excitation During Childhood: A Retrospective Study. J. Clin. Med. 2025, 14, 2367. https://doi.org/10.3390/jcm14072367
Sanzo A, Seganti A, Demarchi A, Fino RS, Raso I, Codazzi AC, Petracci B, Bongiorno A, Rordorf R, Mannarino S. Spontaneous Resolution of Ventricular Pre-Excitation During Childhood: A Retrospective Study. Journal of Clinical Medicine. 2025; 14(7):2367. https://doi.org/10.3390/jcm14072367
Chicago/Turabian StyleSanzo, Antonio, Alessandro Seganti, Andrea Demarchi, Riccardo Simone Fino, Irene Raso, Alessia Claudia Codazzi, Barbara Petracci, Andrea Bongiorno, Roberto Rordorf, and Savina Mannarino. 2025. "Spontaneous Resolution of Ventricular Pre-Excitation During Childhood: A Retrospective Study" Journal of Clinical Medicine 14, no. 7: 2367. https://doi.org/10.3390/jcm14072367
APA StyleSanzo, A., Seganti, A., Demarchi, A., Fino, R. S., Raso, I., Codazzi, A. C., Petracci, B., Bongiorno, A., Rordorf, R., & Mannarino, S. (2025). Spontaneous Resolution of Ventricular Pre-Excitation During Childhood: A Retrospective Study. Journal of Clinical Medicine, 14(7), 2367. https://doi.org/10.3390/jcm14072367







