Abstract
Background/Objectives: Transcatheter Aortic Valve Implantation (TAVI) has significantly improved the management of aortic valve disease, but post-TAVI infective endocarditis, occurring in 0.5–3.1% of cases, remains a serious complication. Due to a high mortality rate and technical challenges, surgical replacement of infected TAVI prosthetic valves is performed in only 11.4% of cases. Methods: This case describes a standardized surgical technique for the removal and replacement of self-expanding TAVI prosthetic valves in the case of infective endocarditis. Results: The proposed approach aims to facilitate valve explantation while minimizing surgical risks. Conclusions: We believe that this technique could be particularly beneficial for surgeons managing these complex cases, by reducing surgical complications and improving patient outcomes. Further studies are necessary to validate its long-term efficacy and applicability in broader clinical settings.
1. Introduction
Transcatheter aortic valve implantation (TAVI) was introduced in the early 2000s as an alternative to surgical aortic valve replacement [1]. It has revolutionized the management of aortic valve diseases, particularly in high-risk patients [2]. Despite its widespread adoption and favorable outcomes, infective endocarditis (IE) post-TAVI remains a rare but serious complication, occurring in 0.5% to 3.1% of cases [3,4], a rate comparable to that observed in conventional surgical valve replacement [5,6].
Endocarditis after cardiac surgery presents unique challenges, requiring a nuanced grasp of its temporal onset and causative microbial agents. Early-onset cases emerge shortly after surgery, constituting approximately 30% to 40% of all cases [7]. They are often associated with nosocomial pathogens such as Staphylococcus aureus and Enterococci. In contrast, late-onset endocarditis occurs months to years after implantation and is more frequently caused by less virulent organisms such as Viridans group streptococci and coagulase-negative staphylococci [8].
In post-TAVI infective endocarditis, Enterococci are the most commonly isolated pathogens, accounting for 25.9% of cases, followed by Staphylococcus aureus in 16.1% [9]. Although rare, post-TAVI infective endocarditis is associated with high mortality, reaching up to 39% [10]. It presents significant diagnostic and therapeutic challenges. Surgical replacement of infected TAVI prosthetic valves requires a meticulous approach due to the complexities of device removal, infection control, and subsequent valve replacement.
Post-TAVI infective endocarditis presents with varied clinical symptoms, ranging from nonspecific to acute manifestations such as fever, heart failure, or embolic stroke, particularly in elderly patients. Heart failure occurs in over 50% of cases, while approximately 20% of patients exhibit nonspecific symptoms, like malaise, weakness, or weight loss [5]. Unlike native valve endocarditis, high-grade fever and heart murmurs are less prevalent in post-TAVI cases. Echocardiographic diagnosis is particularly challenging due to the valve’s metallic components, which can cause reflectance and shadowing artifacts, limiting the detection of small vegetations [11].
With the increasing number of TAVI procedures performed worldwide, the incidence of this complication is expected to rise [12]. Although guidelines recommend early surgery for complicated cases, it is rarely performed.
This case report aims to provide a detailed description of our surgical approach for the replacement of a self-expanding TAVI prosthetic valve in the setting of infective endocarditis.
2. Materials and Methods
Case Report:
We present the case of a 75-year-old female patient, with multiple cardiovascular comorbidities, admitted to the hospital due to deterioration of her general condition. She presented with fever, asthenia, anorexia, and loss of mobility. Ten months prior to her admission, she underwent a TAVI with a self-expanding prosthetic valve (Navitor, 27 mm, Abbott Cardiovascular, Abbott Park, IL, USA). Blood cultures were positive for a multi-sensitive Enterococcus faecalis. Transesophageal echocardiography (TEE) demonstrated a 2 cm mobile mass on the aortic prosthesis leaflets, with a mean gradient of 49 mmHg and a normal left ventricle ejection fraction. Intravenous antibiotic therapy with amoxicillin-clavulanate plus ceftriaxone was initiated. One month later, TEE showed no change in the vegetation size or the mean transvalvular gradient (Figure 1a,b).
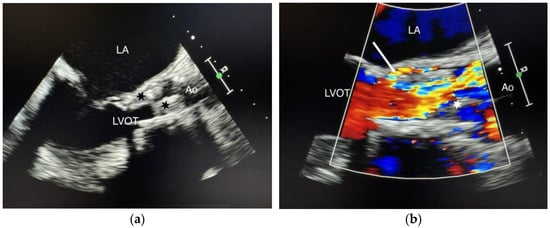
Figure 1.
(a) Standard TEE showing the vegetations. Cusp enlargement and vegetations (black stars); (b) Color Doppler TEE demonstrating paravalvular regurgitation. Vegetation (white star), paravalvular regurgitation (white arrow), LA (left atrium), LVOT (left ventricular outflow tract), Ao (aorta).
White blood cell count increased to 14,400/mm3 and CRP levels reached 108 mg/L. After evaluation by a multidisciplinary team including a cardiologist, an interventional cardiologist, an infectious disease specialist, and a cardiac surgeon, surgical valve replacement was decided.
3. Results
Technical Description:
After aortic cross-clamping, a hockey-stick incision was initiated just above the Navitor prosthesis (Figure 2a). The last cell row of the stent was gently dissected from the aortic wall and a silk stitch was passed through those cells and tied in order to crimp the distal end of it (Figure 2b).
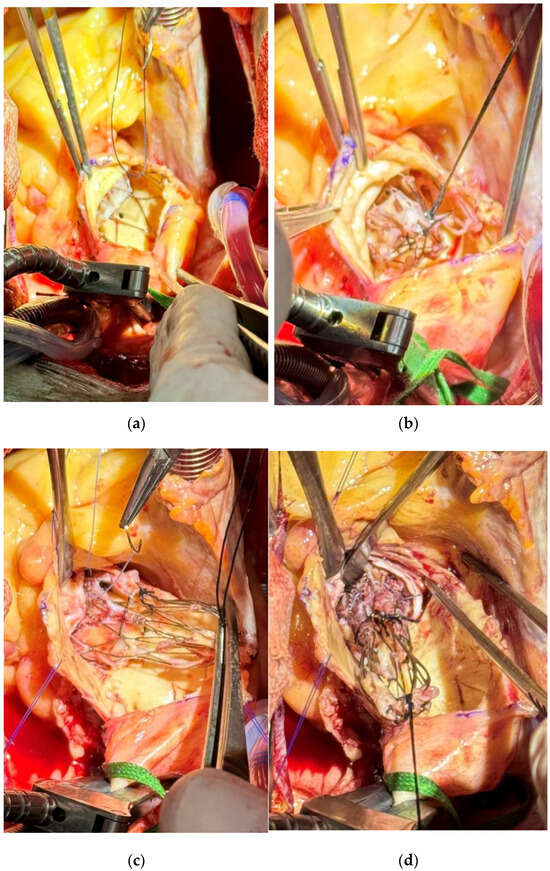
Figure 2.
(a) Initial exposure and first stitch on the last cell row of the implanted prosthesis after dissection of the aortic wall. (b) Crimping of the first silk suture on the upper part of the implanted prosthesis. (c) Placement of the third suture on the lower part of the implanted prosthesis. (d) Extraction of the infected prothesis valve with a careful detachment to minimize trauma to the aortic annulus.
The dissection was gradually prolonged proximally as well as the aortotomy. A second crimping stitch was passed through the second cell row (Figure 2c), allowing an easy mobilization of the stent and then easier proximal dissection. After a third similar stitch was added on the proximal cell row of the stent and the Navitor valve completely crimped, it was delicately detached from the aortic annulus without any damage (Figure 2d).
In Figure 3a, we can see the self-expanding valve after successful removal. A large vegetation obstructing the LVOT was found on the ventricular side of the cusps Figure 3b.
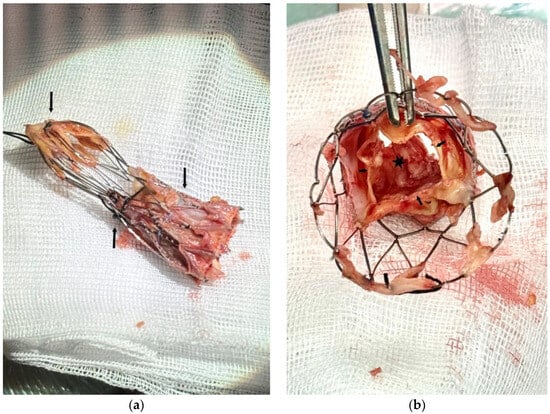
Figure 3.
(a) Self-expanding valve after successful removal with the three crimping stitches (arrows). (b) View from the aortic side of the valve. Leaflets free margin (small arrows). Obstructing vegetation on the ventricular aspect of the cusps (black star).
The bulky native cusps were removed and an annular enlargement using the Manouguian technique was used. An Edwards Inspiris, 21 mm, pericardial valve was implanted, and the patient was easily weaned from bypass.
The patient remained in the intensive care unit for 3 days and was discharged to a rehabilitation unit 10 days after surgery. On long-term follow-up, transthoracic echocardiography (TTE) showed no abnormalities.
4. Discussion
Our case highlights the surgical approach for the treatment of infective endocarditis after TAVI, a rare but serious complication.
Recent studies by Khan A. et al. [9] have emphasized the high mortality and complications associated with post-TAVI infective endocarditis. It is noteworthy that the majority of post-TAVI endocarditis cases are currently treated with antibiotic therapy. Surgical interventions remain rare, accounting for approximately 11.4% of cases [5].
Both TTE and TEE have reduced sensitivity and specificity in detecting prosthetic valve endocarditis (PVE) [13], which is particularly true for IE related to TAVI. A major challenge is the early and accurate diagnosis, particularly in patients with negative TTE and TEE but with persistent bacteremia after a TAVI (Mangner et al. [13]).
Recent data suggest that multimodal imaging techniques, such as 18F-FDG PET/CT, could improve the diagnostic sensitivity for IE, especially in cases where conventional echocardiographic results are inconclusive [14]. Incorporating these imaging modalities into the diagnostic algorithm could allow for earlier identification of patients requiring surgical intervention.
Surgical approaches remain poorly defined given the limited number of cases described in the literature. Our technique offers a simple and reproducible method for a step-by-step explantation of self-expanding TAVI valves.
Although surgery remains the cornerstone of treatment for carefully selected patients, promoting a multidisciplinary collaboration is crucial. Collaboration between cardiac surgeons, interventional cardiologists, infectious disease specialists, and radiologists could improve diagnosis, management, and prognosis in this at-risk population.
5. Conclusions
Post-TAVI endocarditis remains a complex condition with significant diagnostic and therapeutic challenges, particularly in high-risk patients. Our case demonstrates the successful surgical management of an infected self-expanding TAVI valve using a standardized approach, which could provide useful guidance for surgeons dealing with similar rare cases. However, this technique requires further validation. Future studies on a larger cohort are essential to assess its broader applicability and establish standardized surgical protocols.
Author Contributions
Conceptualization, S.D. and B.E.N.; methodology, S.D.; software, B.E.N.; validation, B.E.N.; formal analysis, S.D.; investigation, S.D. and K.H.; resources, S.D. and S.C.; data curation, B.E.N. and S.M.; writing—original draft preparation, S.D.; writing—review and editing, S.D. and B.E.N.; visualization, K.H.; supervision, B.E.N.; project administration, B.E.N.; funding acquisition, B.E.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of CHRAU: Chu Charleroi Chimay (CCB: B3252024000084, 18 December 2024).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient to publish this paper.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author(s).
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| TAVI | Transcatheter aortic valve implantation |
| IE | Infective endocarditis |
| TEE | Transesophageal echocardiography |
| LVOT | Left ventricular outflow tract |
| TTE | Transthoracic echocardiography |
| PVE | Prosthetic valve endocarditis |
| LA | Left atrium |
| AO | Aorta |
References
- Postolache, A.; Sperlongano, S.; Lancellotti, P. TAVI after More Than 20 Years. J. Clin. Med. 2023, 12, 5645. [Google Scholar] [CrossRef] [PubMed]
- Siontis, G.C.M.; Overtchouk, P.; Cahill, T.J.; Modine, T.; Prendergast, B.; Praz, F.; Pilgrim, T.; Petrinic, T.; Nikolakopoulou, A.; Salanti, G.; et al. Transcatheter aortic valve implantation vs. surgical aortic valve replacement for treatment of symptomatic severe aortic stenosis: An updated meta-analysis. Eur. Heart J. 2019, 40, 3143–3153. [Google Scholar] [CrossRef] [PubMed]
- Amat-Santos, I.J.; Ribeiro, H.B.; Urena, M.; Allende, R.; Houde, C.; Bédard, E.; Perron, J.; DeLarochellière, R.; Paradis, J.-M.; Dumont, E.; et al. Prosthetic valve endocarditis after transcatheter valve replacement: A systematic review. JACC Cardiovasc. Interv. 2015, 8, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Olsen, N.T.; De Backer, O.; Thyregod, H.G.; Vejlstrup, N.; Bundgaard, H.; Søndergaard, L.; Ihlemann, N. Prosthetic valve endocarditis after transcatheter aortic valve implantation. Circ. Cardiovasc. Interv. 2015, 8, e001939. [Google Scholar] [CrossRef] [PubMed]
- Tinica, G.; Tarus, A.; Enache, M.; Artene, B.; Rotaru, I.; Bacusca, A.; Burlacu, A. Infective endocarditis after TAVI: A meta-analysis and systematic review of epidemiology, risk factors and clinical consequences. Rev. Cardiovasc. Med. 2020, 21, 263–274. [Google Scholar] [PubMed]
- Ando, T.; Ashraf, S.; Villablanca, P.A.; Telila, T.A.; Takagi, H.; Grines, C.L.; Afonso, L.; Briasoulis, A. Meta-Analysis Comparing the Incidence of Infective Endocarditis Following Transcatheter Aortic Valve Implantation Versus Surgical Aortic Valve Replacement. Am. J. Cardiol. 2019, 123, 827–832. [Google Scholar] [PubMed]
- Vlessis, A.A.; Khaki, A.; Grunkemeier, G.L.; Li, H.H.; Starr, A. Risk, diagnosis and management of prosthetic valve endocarditis: A review. J. Heart Valve Dis. 1997, 6, 443–465. [Google Scholar] [PubMed]
- Ziegler, R.; Arnold, H.; Bertram, R.; Geißdörfer, W.; Pauschinger, M.; Fischlein, T.; Pollari, F.; Steinmann, J. Microbiologic diagnostics and pathogen spectrum in infective endocarditis of surgically treated patients: A five-year, retrospective, monocentric study. Infection 2023, 51, 1523–1530. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Aslam, A.; Satti, K.N.; Ashiq, S. Infective endocarditis post-transcatheter aortic valve implantation (TAVI), microbiological profile and clinical outcomes: A systematic review. PLoS ONE 2020, 15, e0225077. [Google Scholar] [CrossRef] [PubMed]
- Prasitlumkum, N.; Vutthikraivit, W.; Thangjui, S.; Leesutipornchai, T.; Kewcharoen, J.; Riangwiwat, T.; Dworkin, J. Epidemiology of infective endocarditis in transcatheter aortic valve replacement: Systemic review and meta-analysis. J. Cardiovasc. Med. 2020, 21, 790–801. [Google Scholar]
- Chourdakis, E.; Koniari, I.; Hahalis, G.; Kounis, N.G.; Hauptmann, K.E. Endocarditis after transcatheter aortic valve implantation: A current assessment. J. Geriatr. Cardiol. 2018, 15, 61–65. [Google Scholar] [PubMed]
- Ben-Shoshan, J.; Amit, S.; Finkelstein, A. Transcatheter Aortic Valve Implantation Infective Endocarditis: Current Data and Implications on Prophylaxis and Management. Curr. Pharm. Des. 2016, 22, 1959–1964. [Google Scholar] [CrossRef] [PubMed]
- Mangner, N.; Panagides, V.; del Val, D.; Abdel-Wahab, M.; Crusius, L.; Durand, E.; Ihlemann, N.; Urena, M.; Pellegrini, C.; Giannini, F.; et al. Incidence, clinical characteristics, and impact of absent echocardiographic signs in patients with infective endocarditis after transcatheter aortic valve implantation. Clin. Infect. Dis. 2022, 76, 1003–1012. [Google Scholar] [CrossRef]
- Pizzi, M.N.; Roque, A.; Fernández-Hidalgo, N.; Cuéllar-Calabria, H.; Ferreira-González, I.; Gonzàlez-Alujas, M.T.; Oristrell, G.; Gracia-Sánchez, L.; González, J.J.; Rodríguez-Palomares, J.; et al. Improving the Diagnosis of Infective Endocarditis in Prosthetic Valves and Intracardiac Devices with 18F-Fluordeoxyglucose Positron Emission Tomography/Computed Tomography Angiography: Initial Results at an Infective Endocarditis Referral Center. Circulation 2015, 132, 1113–1126. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).