Percutaneous Left Atrial Appendage Closure: Supporting Evidence, Limitations and Future Directions
Abstract
1. Introduction
2. Supporting Evidence
3. Procedural Optimization: What Are the Main Determinants?
3.1. Preprocedural Planning
3.2. Procedural Imaging: Moving Towards a Mini-Invasive Approach Without General Anesthesia?
3.3. Procedural Volume and Its Importance in LAAO Outcomes
4. Device-Related Thrombus: From an Innocent Bystander to a Clinical Dilemma
4.1. Which Patients Are at Higher Risk of Developing DRT?
4.2. A Modifiable Risk Factor: Device Implantation Depth
4.3. DRT Detection: Are TEE and CT Equivalent?
4.4. The Controversial Aspects of Antithrombotic Therapy for DRT Prevention
5. Peridevice Leaks (PDL): From Detection to Recent Insights into Clinical Implications
6. Conclusions
Funding
Conflicts of Interest
Abbreviations and Acronyms
| LAA | Left Atrial Appendage |
| DRT | Device-Related Thrombus |
| PDL | Peri-Device Leak |
| LUPV | Left Upper Pulmonary Vein |
| PR | Pulmonary Ridge |
| TEE | Transesophageal Echocardiography |
| CT | Computed Tomography |
| HAT | Hypo-attenuated Thickening |
| AF | Atrial Fibrillation |
| SE | Systemic Embolization |
| TIA | Transient Ischemic Attack |
References
- Marini, C.; De Santis, F.; Sacco, S.; Russo, T.; Olivieri, L.; Totaro, R.; Carolei, A. Contribution of Atrial Fibrillation to Incidence and Outcome of Ischemic Stroke: Results From a Population-Based Study. Stroke 2005, 36, 1115–1119. [Google Scholar] [CrossRef] [PubMed]
- Carnicelli, A.P.; Hong, H.; Connolly, S.J.; Eikelboom, J.; Giugliano, R.P.; Morrow, D.A.; Patel, M.R.; Wallentin, L.; Alexander, J.H.; Bahit, M.C.; et al. Direct Oral Anticoagulants Versus Warfarin in Patients with Atrial Fibrillation: Patient-Level Network Meta-Analyses of Randomized Clinical Trials with Interaction Testing by Age and Sex. Circulation 2022, 145, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Wiebe, J.; Ndrepepa, G.; Kufner, S.; Lahmann, A.L.; Xhepa, E.; Kuna, C.; Voll, F.; Gosetti, R.; Laugwitz, K.; Joner, M.; et al. Early Aspirin Discontinuation after Coronary Stenting: A Systematic Review and Meta-Analysis. JAHA 2021, 10, e018304. [Google Scholar] [CrossRef] [PubMed]
- Pastormerlo, L.E.; De Caterina, A.R.; Esposito, A.; Korsholm, K.; Berti, S. State-of-the-Art of Transcatheter Left Atrial Appendage Occlusion. JCM 2024, 13, 939. [Google Scholar] [CrossRef]
- Osman, M.; Busu, T.; Osman, K.; Khan, S.U.; Daniels, M.; Holmes, D.R.; Alkhouli, M. Short-Term Antiplatelet Versus Anticoagulant Therapy after Left Atrial Appendage Occlusion. JACC Clin. Electrophysiol. 2020, 6, 494–506. [Google Scholar] [CrossRef]
- Holmes, D.R.; Korsholm, K.; Rodés-Cabau, J.; Saw, J.; Berti, S.; Alkhouli, M.A. Left atrial appendage occlusion. EuroIntervention 2023, 18, e1038–e1065. [Google Scholar] [CrossRef]
- Landmesser, U.; Skurk, C.; Tzikas, A.; Falk, V.; Reddy, V.Y.; Windecker, S. Left atrial appendage closure for stroke prevention in atrial fibrillation: Current status and perspectives. Eur. Heart J. 2024, 45, 2914–2932. [Google Scholar] [CrossRef]
- Holmes, D.R.; Kar, S.; Price, M.J.; Whisenant, B.; Sievert, H.; Doshi, S.K.; Huber, K.; Reddy, V.Y. Prospective Randomized Evaluation of the Watchman Left Atrial Appendage Closure Device in Patients with Atrial Fibrillation Versus Long-Term Warfarin Therapy. J. Am. Coll. Cardiol. 2014, 64, 1–12. [Google Scholar] [CrossRef]
- Camm, A.J. Leap or lag: Left atrial appendage closure and guidelines. Europace 2023, 25, euad067. [Google Scholar] [CrossRef]
- Reddy, V.Y.; Sievert, H.; Halperin, J.; Doshi, S.K.; Buchbinder, M.; Neuzil, P.; Huber, K.; Whisenant, B.; Kar, S.; Swarup, V.; et al. Percutaneous Left Atrial Appendage Closure vs Warfarin for Atrial Fibrillation: A Randomized Clinical Trial. JAMA 2014, 312, 1988–1998. [Google Scholar] [CrossRef]
- Ruff, C.T.; Giugliano, R.P.; Braunwald, E.; Hoffman, E.B.; Deenadayalu, N.; Ezekowitz, M.D.; Camm, A.J.; Weitz, J.I.; Lewis, B.S.; Parkhomenko, A.; et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: A meta-analysis of randomised trials. Lancet 2014, 383, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Ilkhanoff, L. Anticoagulants for atrial fibrillation: From warfarin and DOACs to the promise of factor XI inhibitors. Front. Cardiovasc. Med. 2024, 11, 1352734. [Google Scholar] [CrossRef] [PubMed]
- Chow, D.H.; Bieliauskas, G.; Sawaya, F.J.; Millan-Iturbe, O.; Kofoed, K.F.; Søndergaard, L.; De Backer, O. A comparative study of different imaging modalities for successful percutaneous left atrial appendage closure. Open Heart 2017, 4, e000627. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, M.; Li, W.; Ruan, Z.; Zhu, L.; Gao, R.; Zhao, J. A Randomized Trial of Preoperative Planning of Left Atrial Appendage Occlusion Using Cardiac Computed Tomography Angiography. Surg. Innov. 2023, 30, 303–313. [Google Scholar] [CrossRef]
- Galea, R.; Aminian, A.; Meneveau, N.; De Marco, F.; Heg, D.; Anselme, F.; Gräni, C.; Huber, A.T.; Teiger, E.; Iriart, X.; et al. Impact of Preprocedural Computed Tomography on Left Atrial Appendage Closure Success: A Swiss-Apero Trial Subanalysis. JACC Cardiovasc. Interv. 2023, 16, 1332–1343. [Google Scholar] [CrossRef] [PubMed]
- Offhaus, A.; Linss, L.; Roehl, P.; Sakriss, C.; Pertschy, U.; Schwenzky, A.; Ebelt, H. CT-Based Preplanning Allows Abstaining from Intraprocedural TEE during Interventional Closure of the LAA in Patients with Atrial Fibrillation. JCM 2023, 12, 4019. [Google Scholar] [CrossRef]
- Hagendorff, A.; Stöbe, S.; Helfen, A.; Knebel, F.; Altiok, E.; Beckmann, S.; Bekfani, T.; Binder, T.; Ewers, A.; Hamadanchi, A.; et al. Echocardiographic assessment of left atrial appendage morphology and function—An expert proposal by the German Working Group of Cardiovascular Ultrasound. Clin. Res. Cardiol. 2024, 114, 25–40. [Google Scholar] [CrossRef]
- Korsholm, K.; Berti, S.; Iriart, X.; Saw, J.; Wang, D.D.; Cochet, H.; Chow, D.; Clemente, A.; De Backer, O.; Jensen, J.M.; et al. Expert Recommendations on Cardiac Computed Tomography for Planning Transcatheter Left Atrial Appendage Occlusion. JACC Cardiovasc. Interv. 2020, 13, 277–292. [Google Scholar] [CrossRef]
- Clarke, J.R.D.; Higgins, A.Y.; Wang, Y.; Faridi, K.F.; Curtis, J.A.; Freeman, J.V.; Friedman, D.J. Impact of Preprocedure Imaging for Left Atrial Appendage Occlusion. JACC Cardiovasc. Interv. 2023, 16, 1317–1328. [Google Scholar] [CrossRef]
- De Backer, O.; Iriart, X.; Kefer, J.; Nielsen-Kudsk, J.E.; Aminian, A.; Rosseel, L.; Kofoed, K.F.; Odenstedt, J.; Berti, S.; Saw, J.; et al. Impact of Computational Modeling on Transcatheter Left Atrial Appendage Closure Efficiency and Outcomes. JACC Cardiovasc. Interv. 2023, 16, 655–666. [Google Scholar] [CrossRef]
- Balla, S.; Alkhouli, M. Moore’s Law and the Quest for Minimalist LAAO. JACC Cardiovasc. Interv. 2023, 16, 1899–1901. [Google Scholar] [CrossRef] [PubMed]
- Ferro, E.G.; Alkhouli, M.; Nair, D.G.; Kapadia, S.R.; Hsu, J.C.; Gibson, D.N.; Freeman, J.V.; Price, M.J.; Roy, K.; Allocco, D.J.; et al. Intracardiac vs Transesophageal Echocardiography for Left Atrial Appendage Occlusion With Watchman FLX in the U.S. JACC Clin. Electrophysiol. 2023, 9, 2587–2599. [Google Scholar] [CrossRef] [PubMed]
- Landmesser, U.; Schmidt, B.; Nielsen-Kudsk, J.E.; Lam, S.C.C.; Park, J.-W.; Tarantini, G.; Cruz-Gonzalez, I.; Geist, V.; Della Bella, P.; Colombo, A.; et al. Left atrial appendage occlusion with the AMPLATZER Amulet device: Periprocedural and early clinical/echocardiographic data from a global prospective observational study. EuroIntervention 2017, 13, 867–876. [Google Scholar] [CrossRef] [PubMed]
- Samaras, A.; Papazoglou, A.S.; Balomenakis, C.; Bekiaridou, A.; Moysidis, D.V.; Patsiou, V.; Orfanidis, A.; Giannakoulas, G.; Kassimis, G.; Fragakis, N.; et al. Residual leaks following percutaneous left atrial appendage occlusion and outcomes: A meta-analysis. Eur. Heart J. 2024, 45, 214–229. [Google Scholar] [CrossRef]
- Garot, P.; Iriart, X.; Aminian, A.; Kefer, J.; Freixa, X.; Cruz-Gonzalez, I.; Berti, S.; Rosseel, L.; Ibrahim, R.; Korsholm, K.; et al. Value of FEops HEARTguide patient-specific computational simulations in the planning of left atrial appendage closure with the Amplatzer Amulet closure device: Rationale and design of the PREDICT-LAA study. Open Heart 2020, 7, e001326. [Google Scholar] [CrossRef]
- Harb, S.C.; Cohen, J.A.; Krishnaswamy, A.; Kapadia, S.R.; Miyasaka, R.L. Targeting the Future: Three-Dimensional Imaging for Precise Guidance of the Transseptal Puncture. Struct. Heart 2024, 9, 100340. [Google Scholar] [CrossRef]
- Nielsen-Kudsk, J.E.; Berti, S.; De Backer, O.; Aguirre, D.; Fassini, G.; Cruz-Gonzalez, I.; Grassi, G.; Tondo, C. Use of Intracardiac Compared With Transesophageal Echocardiography for Left Atrial Appendage Occlusion in the Amulet Observational Study. JACC Cardiovasc. Interv. 2019, 12, 1030–1039. [Google Scholar] [CrossRef]
- Aminian, A.; Leduc, N.; Freixa, X.; Waans, M.J.; Ben Yedder, M.; Maarse, M.; Sanchis, L.; Cepas-Guillen, P.; Cruz-González, I.; Blanco-Fernandez, F.; et al. Left Atrial Appendage Occlusion Under Miniaturized Transesophageal Echocardiographic Guidance and Conscious Sedation. JACC Cardiovasc. Interv. 2023, 16, 1889–1898. [Google Scholar] [CrossRef]
- Liu, Q.; You, L.; Yang, J.; Zhang, Y.; Wu, J.; Yin, H.; Zhang, Y.; Xie, R. Clinical Results and Safety of Intracardiac Echocardiography Guidance for Combined Catheter Ablation and Left Atrial Appendage Occlusion. Rev. Cardiovasc. Med. 2024, 25, 192. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Li, F.; Zhang, J.; Zhang, L.; Liu, H.-H.; Zhao, N.; Yang, F.; Kong, Q.; Zhou, Y.-T.; Qian, L.-L.; et al. A comparable efficacy and safety between intracardiac echocardiography and transesophageal echocardiography for percutaneous left atrial appendage occlusion. Front. Cardiovasc. Med. 2023, 10, 1194771. [Google Scholar] [CrossRef]
- Della Rocca, D.G.; Magnocavallo, M.; Gianni, C.; Mohanty, S.; Al-Ahmad, A.; Bassiouny, M.; Denora, M.; La Fazia, V.M.; Lavalle, C.; Gallinghouse, G.J.; et al. Three-dimensional intracardiac echocardiography for left atrial appendage sizing and percutaneous occlusion guidance. Europace 2023, 26, euae010. [Google Scholar] [CrossRef] [PubMed]
- Gidney, B.; Della Rocca, D.G.; Horton, R.; Hoffman, J.; Valderrábano, M.; Natale, A.; Garg, J.; Bhardwaj, R.; Doshi, S. Step-by-step recommendations utilizing four-dimensional intracardiac echocardiography in left atrial appendage procedures. Cardiovasc. Electrophysiol. 2024, 35, 1601–1613. [Google Scholar] [CrossRef] [PubMed]
- Pison, L.; Potpara, T.S.; Chen, J.; Larsen, T.B.; Bongiorni, M.G.; Blomström-Lundqvist, C.; Proclemer, A.; Dagres, N.; Estner, H.; Hernandez-Madrid, A.; et al. Left atrial appendage closure-indications, techniques, and outcomes: Results of the European Heart Rhythm Association Survey. Europace 2015, 17, 642–646. [Google Scholar] [CrossRef] [PubMed]
- Korsholm, K.; Iriart, X.; Saw, J.; Wang, D.D.; Berti, S.; Galea, R.; Freixa, X.; Arzamendi, D.; De Backer, O.; Kramer, A.; et al. Position Statement on Cardiac Computed Tomography Following Left Atrial Appendage Occlusion. JACC Cardiovasc. Interv. 2024, 17, 1747–1764. [Google Scholar] [CrossRef]
- Alkhouli, M.; Alarouri, H.; Kramer, A.; Korsholm, K.; Collins, J.; De Backer, O.; Hatoum, H.; Nielsen-Kudsk, J.E. Device-Related Thrombus after Left Atrial Appendage Occlusion. JACC Cardiovasc. Interv. 2023, 16, 2695–2707. [Google Scholar] [CrossRef]
- Dukkipati, S.R.; Kar, S.; Holmes, D.R.; Doshi, S.K.; Swarup, V.; Gibson, D.N.; Maini, B.; Gordon, N.T.; Main, M.L.; Reddy, V.Y. Device-Related Thrombus after Left Atrial Appendage Closure: Incidence, Predictors, and Outcomes. Circulation 2018, 138, 874–885. [Google Scholar] [CrossRef]
- Sedaghat, A.; Vij, V.; Al-Kassou, B.; Gloekler, S.; Galea, R.; Fürholz, M.; Meier, B.; Valgimigli, M.; O’hara, G.; Arzamendi, D.; et al. Device-Related Thrombus after Left Atrial Appendage Closure: Data on Thrombus Characteristics, Treatment Strategies, and Clinical Outcomes From the EUROC-DRT-Registry. Circ. Cardiovasc. Interv. 2021, 14, e010195. [Google Scholar] [CrossRef]
- Mill, J.; Agudelo, V.; Hion Li, C.; Noailly, J.; Freixa, X.; Camara, O.; Arzamendi, D. Patient-specific flow simulation analysis to predict device-related thrombosis in left atrial appendage occluders. REC Interv. Cardiol. 2022, 3, 278–285. [Google Scholar] [CrossRef]
- Mesnier, J.; Simard, T.; Jung, R.G.; Lehenbauer, K.R.; Piayda, K.; Pracon, R.; Jackson, G.G.; Flores-Umanzor, E.; Faroux, L.; Korsholm, K.; et al. Persistent and Recurrent Device-Related Thrombus after Left Atrial Appendage Closure. JACC Cardiovasc. Interv. 2023, 16, 2722–2732. [Google Scholar] [CrossRef]
- Main, M.L.; Fan, D.; Reddy, V.Y.; Holmes, D.R.; Gordon, N.T.; Coggins, T.R.; House, J.A.; Liao, L.; Rabineau, D.; Latus, G.G.; et al. Assessment of Device-Related Thrombus and Associated Clinical Outcomes with the WATCHMAN Left Atrial Appendage Closure Device for Embolic Protection in Patients with Atrial Fibrillation (from the PROTECT-AF Trial). Am. J. Cardiol. 2016, 117, 1127–1134. [Google Scholar] [CrossRef]
- Pracon, R.; Bangalore, S.; Dzielinska, Z.; Konka, M.; Kepka, C.; Kruk, M.; Kaczmarska-Dyrda, E.; Petryka-Mazurkiewicz, J.; Bujak, S.; Solecki, M.; et al. Device Thrombosis after Percutaneous Left Atrial Appendage Occlusion Is Related to Patient and Procedural Characteristics but Not to Duration of Postimplantation Dual Antiplatelet Therapy. Circ. Cardiovasc. Interv. 2018, 11, e005997. [Google Scholar] [CrossRef] [PubMed]
- Aminian, A.; Schmidt, B.; Mazzone, P.; Berti, S.; Fischer, S.; Montorfano, M.; Lam, S.C.C.; Lund, J.; Asch, F.M.; Gage, R.; et al. Incidence, Characterization, and Clinical Impact of Device-Related Thrombus Following Left Atrial Appendage Occlusion in the Prospective Global AMPLATZER Amulet Observational Study. JACC Cardiovasc. Interv. 2019, 12, 1003–1014. [Google Scholar] [CrossRef] [PubMed]
- Simard, T.; Jung, R.G.; Lehenbauer, K.; Piayda, K.; Pracoń, R.; Jackson, G.G.; Flores-Umanzor, E.; Faroux, L.; Korsholm, K.; Chun, J.K.; et al. Predictors of Device-Related Thrombus Following Percutaneous Left Atrial Appendage Occlusion. J. Am. Coll. Cardiol. 2021, 78, 297–313. [Google Scholar] [CrossRef]
- Schmidt, B.; Nielsen-Kudsk, J.E.; Ellis, C.R.; Thaler, D.; Sabir, S.A.; Gambhir, A.; Landmesser, U.; Shah, N.; Gray, W.; Swarup, V.; et al. Incidence, Predictors, and Clinical Outcomes of Device-Related Thrombus in the Amulet IDE Trial. JACC Clin. Electrophysiol. 2023, 9, 96–107. [Google Scholar] [CrossRef]
- Vij, V.; Piayda, K.; Nelles, D.; Gloekler, S.; Galea, R.; Fürholz, M.; Meier, B.; Valgimigli, M.; O’hara, G.; Arzamendi, D.; et al. Clinical and echocardiographic risk factors for device-related thrombus after left atrial appendage closure: An analysis from the multicenter EUROC-DRT registry. Clin. Res. Cardiol. 2022, 111, 1276–1285. [Google Scholar] [CrossRef]
- Cepas-Guillén, P.; Flores-Umanzor, E.; Leduc, N.; Bajoras, V.; Perrin, N.; Farjat-Pasos, J.; McInerney, A.; Lafond, A.; Millán, X.; Zendjebil, S.; et al. Impact of Device Implant Depth after Left Atrial Appendage Occlusion. JACC Cardiovasc. Interv. 2023, 16, 2139–2149. [Google Scholar] [CrossRef]
- Freixa, X.; Cepas-Guillen, P.; Flores-Umanzor, E.; Regueiro, A.; Sanchis, L.; Fernandez-Valledor, A.; Brugaletta, S.; Carretero, M.J.; Vidal, B.; Masotti, M.; et al. Pulmonary ridge coverage and device-related thrombosis after left atrial appendage occlusion. EuroIntervention 2021, 16, e1288–e1294. [Google Scholar] [CrossRef]
- Asmarats, L.; Cruz-González, I.; Nombela-Franco, L.; Arzamendi, D.; Peral, V.; Nietlispach, F.; Latib, A.; Maffeo, D.; González-Ferreiro, R.; Rodríguez-Gabella, T.; et al. Recurrence of Device-Related Thrombus after Percutaneous Left Atrial Appendage Closure. Circulation 2019, 140, 1441–1443. [Google Scholar] [CrossRef]
- Moussa, I. Predicting Behavior of Device-Related Thrombus after Left Atrial Appendage Occlusion. JACC Cardiovasc. Interv. 2023, 16, 2733–2735. [Google Scholar] [CrossRef]
- Mesnier, J.; Cepas-Guillén, P.; Freixa, X.; Flores-Umanzor, E.; Trinh, K.H.; O’hara, G.; Rodés-Cabau, J. Antithrombotic Management after Left Atrial Appendage Closure: Current Evidence and Future Perspectives. Circ. Cardiovasc. Interv. 2023, 16, e012812. [Google Scholar] [CrossRef]
- Turagam, M.K.; Kawamura, I.; Neuzil, P.; Nair, D.; Doshi, S.; Valderrabano, M.; Hala, P.; Della Rocca, D.; Gibson, D.; Funasako, M.; et al. Severity of Ischemic Stroke after Left Atrial Appendage Closure vs Nonwarfarin Oral Anticoagulants. JACC Clin. Electrophysiol. 2024, 10, 270–283. [Google Scholar] [CrossRef] [PubMed]
- Freixa, X.; Cruz-González, I.; Cepas-Guillén, P.; Millán, X.; Antúnez-Muiños, P.; Flores-Umanzor, E.; Asmarats, L.; Regueiro, A.; López-Tejero, S.; Li, C.-H.P. Low-Dose Direct Oral Anticoagulation vs Dual Antiplatelet Therapy after Left Atrial Appendage Occlusion: The ADALA Randomized Clinical Trial. JAMA Cardiol. 2024, 9, 922–926. [Google Scholar] [PubMed]
- Asmarats, L.; O’Hara, G.; Champagne, J.; Paradis, J.-M.; Bernier, M.; O’connor, K.; Beaudoin, J.; Junquera, L.; Del Val, D.; Muntané-Carol, G.; et al. Short-Term Oral Anticoagulation versus Antiplatelet Therapy Following Transcatheter Left Atrial Appendage Closure. Circ. Cardiovasc. Interv. 2020, 13, e009039. [Google Scholar] [CrossRef] [PubMed]
- Alkhouli, M.; Du, C.; Killu, A.; Simard, T.; Noseworthy, P.A.; Friedman, P.A.; Curtis, J.P.; Freeman, J.V.; Holmes, D.R. Clinical Impact of Residual Leaks Following Left Atrial Appendage Occlusion. JACC Clin. Electrophysiol. 2022, 8, 766–778. [Google Scholar] [CrossRef]
- Freeman, J.V.; Varosy, P.; Price, M.J.; Slotwiner, D.; Kusumoto, F.M.; Rammohan, C.; Kavinsky, C.J.; Turi, Z.G.; Akar, J.; Koutras, C.; et al. The NCDR Left Atrial Appendage Occlusion Registry. J. Am. Coll. Cardiol. 2020, 75, 1503–1518. [Google Scholar] [CrossRef]
- Freixa, X.; Aminian, A.; Tzikas, A.; Saw, J.; Nielsen-Kudsk, J.-E.; Ghanem, A.; Schmidt, B.; Hildick-Smith, D. Left atrial appendage occlusion with the Amplatzer Amulet: Update on device sizing. J. Interv. Card. Electrophysiol. 2020, 59, 71–78. [Google Scholar] [CrossRef]
- Alkhouli, M.; Busu, T.; Shah, K.; Osman, M.; Alqahtani, F.; Raybuck, B. Incidence and Clinical Impact of Device-Related Thrombus Following Percutaneous Left Atrial Appendage Occlusion. JACC Clin. Electrophysiol. 2018, 4, 1629–1637. [Google Scholar] [CrossRef]
- Carvalho, P.E.P.; Gewehr, D.M.; Miyawaki, I.A.; Nogueira, A.; Felix, N.; Garot, P.; Darmon, A.; Mazzone, P.; Preda, A.; Nascimento, B.R.; et al. Network Meta-Analysis of Initial Antithrombotic Regimens after Left Atrial Appendage Occlusion. J. Am. Coll. Cardiol. 2023, 82, 1765–1773. [Google Scholar] [CrossRef]
- Della Rocca, D.G.; Magnocavallo, M.; Di Biase, L.; Mohanty, S.; Trivedi, C.; Tarantino, N.; Gianni, C.; Lavalle, C.; Van Niekerk, C.J.; Romero, J.; et al. Half-Dose Direct Oral Anticoagulation Versus Standard Antithrombotic Therapy After Left Atrial Appendage Occlusion. JACC Cardiovasc. Interv. 2021, 14, 2353–2364. [Google Scholar] [CrossRef]
- Bergmann, M.W.; Ince, H.; Kische, S.; Schmitz, T.; Meincke, F.; Schmidt, B.; Foley, D.; Betts, T.R.; Grygier, M.; Protopopov, A.V.; et al. Real-world safety and efficacy of WATCHMAN LAA closure at one year in patients on dual antiplatelet therapy: Results of the DAPT subgroup from the EWOLUTION all-comers study. EuroIntervention 2018, 13, 2003–2011. [Google Scholar] [CrossRef]
- Clemente, A.; Avogliero, F.; Berti, S.; Paradossi, U.; Jamagidze, G.; Rezzaghi, M.; Della Latta, D.; Chiappino, D. Multimodality imaging in preoperative assessment of left atrial appendage transcatheter occlusion with the Amplatzer Cardiac Plug. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 1276–1287. [Google Scholar] [CrossRef] [PubMed]
- Eng, M.H.; Wang, D.D.; Greenbaum, A.B.; Gheewala, N.; Kupsky, D.; Aka, T.; Song, T.; Kendall, B.J.; Wyman, J.; Myers, E.; et al. Prospective, randomized comparison of 3-dimensional computed tomography guidance versus TEE data for left atrial appendage occlusion (PRO3DLAAO). Catheter. Cardiovasc. Interv. 2018, 92, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Berti, S.; Pastormerlo, L.E.; Korsholm, K.; Saw, J.; Alkhouli, M.; Costa, M.P.; Odenstedt, J.; Packer, E.J.; Tondo, C.; Santoro, G.; et al. Intracardiac echocardiography for guidance of transcatheter left atrial appendage occlusion: An expert consensus document. Catheter. Cardiovasc. Interv. 2021, 98, 815–825. [Google Scholar] [CrossRef]
- So, C.; Kang, G.; Villablanca, P.A.; Ignatius, A.; Asghar, S.; Dhillon, D.; Lee, J.C.; Khan, A.; Singh, G.; Frisoli, T.M.; et al. Additive Value of Preprocedural Computed Tomography Planning Versus Stand-Alone Transesophageal Echocardiogram Guidance to Left Atrial Appendage Occlusion: Comparison of Real-World Practice. JAHA 2021, 10, e020615. [Google Scholar] [CrossRef] [PubMed]

| Trial | Patients (Randomisation) | Main Inclusion Criteria | Primary Endpoint |
|---|---|---|---|
| Patients with high bleeding risk, LAAO vs. best medical care | |||
| CLOSURE AF (NCT028301521) | 1000 (1:1) | Non-valvular AF (CHA2DS2VASc ≥ 2) and high risk for bleeding:
| Survival free of events (combined endpoint: stroke, SE, bleeding, CV or unexplained death > 2 years) |
| COMPARE LAAO (NCT04676880) | 609 (2:1) | Non-valvular AF (CHA2DS2-VASc score ≥ 2), non-eligible for long-term OAC | Time to first occurrence of stroke; Time to combined endpoint: stroke, TIA, or SE; Peri-procedural complications |
| Patients without high bleeding risk, LAAO vs. DOAC | |||
| CHAMPION AF (NCT04394546) | 3000 (1:1) | Non-valvular AF (CHA2DS2VASc score ≥ 2), eligible for long-term OAC | Non-inferiority for combined endpoint: stroke, CV death < 36 months; superiority non-procedural bleeding < 36 months; non-inferiority for combined endpoint: ischaemic stroke, SE < 60 months |
| CATALYST (NCT04226547) | 2650 (1:1) | Non-valvular AF (CHA2DS2-VASc score ≥ 3), eligible for long-term OAC | Non-inferiority of combined endpoint: ischaemic stroke, SE and CV mortality at 2 years; superiority: clinically relevant bleeding, excluding procedure-related bleeding at 2 years; non-inferiority of combined endpoint: ischaemic stroke, SE at 3 years |
| Post-intracerebral bleeding status, LAAO vs. best medical care | |||
| CLEARANCE (NCT05063409) | 500 (1:1) | Non-valvular AF (CHA2DS2VASc score ≥ 2), status post-intracerebral bleeding > 6 weeks | Combined endpoint: stroke, SE, bleeding or CV/unexplained death (2-year FU) |
| STROKECLOSE (NCT02830152) | 750 (2:1) | Non-valvular AF (CHA2DS2VASc score ≥ 2), status post-intracerebral bleeding > 4 weeks but <6 months before randomization | Combined endpoint: stroke, SE, bleeding or death (5-year FU) |
| Post-ischaemic stroke/TIA status, LAAO vs. DOAC | |||
| OCCLUSION AF (NCT03642509) | 750 (1:1) | Non-valvular AF (CHA2DS2VASc score ≥ 2), eligible for long-term OAC. S/P stroke/TIA within 6 months before randomization | Combined endpoint: stroke, SE, bleeding, or death (5-year FU) |
| ELAPSE (NCT05976685) | 482 (1:1) | Recent (≤3 months) symptomatic stroke., active and ongoing OAC therapy at stroke onset not stopped/paused for >48 h due to any reason, Active or planned long-term therapy with DOAC | Composite of recurrent ischemic stroke, systemic embolism, or cardiovascular death (whatever comes first). |
| LAAO and terminal renal insufficiency | |||
| LAA-Kidney (NCT05204212) | 430 (1:1) | Non-valvular AF (CHA2DS2VASc ≥ 2), end-stage chronic kidney disease (GFR < 15 mL/min/1.73 m2) | Combined endpoint of stroke, SE, CV/unexplained death and major bleeding (≥3 BARC) through 18 FU months |
| LAAO plus OAC vs. OAC alone | |||
| LAAOS-4 (NCT059636989) | 4000 (1:1) | AF in patients with a history of ischemic stroke or SE CHA2DS2-VASc ≥ 4. OAC therapy for at least 3 months | Ischemic stroke/SE |
| First Author (Year) | Study Design (Patients) | Patient-Related Predictors | Procedural/Post-Procedural Predictors |
|---|---|---|---|
| Kaneko et al. [41] (2017) | Single-centre analysis (78) | CHA2DS2-VASc score * | device implantation depth * |
| Dukkipati et al. [36] (2018) | Ad hoc analysis of PROTECT-AF and PREVAIL trial (1739) | LAA orifice width *, permanent AF *, prior stroke/TIA *, LV dysfunction *, vascular disease * | |
| Pracon et al. [41] (2018) | Single-centre analysis (99) | LV dysfunction, previous VTE | Device size, device implantation depth |
| Fauchier et al. [37] (2018) | Multicentre registry (France) (469) | Older age *, prior stroke/TIA * | No DAPT or OAC at discharge * |
| Aminian et al. [42] (2019) | Prospective global Amulet registry (1088) | LAA orifice width * | |
| Simard et al. [43] (2021) | Global DRT registry (711) | Hypercoagulopathy *, permanent AF *, renal insufficiency * | Pericardial effusion *, device implantation depth * |
| Schmidt et al. [44] (2022) | Ad hoc analysis of Amulet IDE trial (1788) | Older age *, female sex *, AF the time of the procedure * | |
| Vij et al. [45] (2022) | Multicentre registry EUROC-DRT (537) | Older age *, prior stroke/TIA *, SEC * | |
| Freixa et al. [46] (2023) | Multicentre registry (n1317) | Device implantation depth *, no or single APT post-LAAO * |
| TEE | CT | |
|---|---|---|
| DEFINITION/METHODS |
|
|
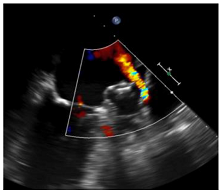 | 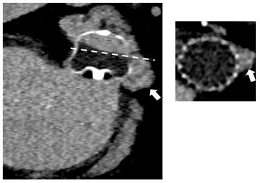 | |
| LIMITATIONS |
|
|
| INCIDENCE * | PDL: 26% [24] | LAA Patency: 55% [24] PDL: 57% [24] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imperatore, G.; Lochy, S.; Ben Yedder, M.; Galea, R.; Aminian, A. Percutaneous Left Atrial Appendage Closure: Supporting Evidence, Limitations and Future Directions. J. Clin. Med. 2025, 14, 2300. https://doi.org/10.3390/jcm14072300
Imperatore G, Lochy S, Ben Yedder M, Galea R, Aminian A. Percutaneous Left Atrial Appendage Closure: Supporting Evidence, Limitations and Future Directions. Journal of Clinical Medicine. 2025; 14(7):2300. https://doi.org/10.3390/jcm14072300
Chicago/Turabian StyleImperatore, Giuseppe, Stijn Lochy, Mohamed Ben Yedder, Roberto Galea, and Adel Aminian. 2025. "Percutaneous Left Atrial Appendage Closure: Supporting Evidence, Limitations and Future Directions" Journal of Clinical Medicine 14, no. 7: 2300. https://doi.org/10.3390/jcm14072300
APA StyleImperatore, G., Lochy, S., Ben Yedder, M., Galea, R., & Aminian, A. (2025). Percutaneous Left Atrial Appendage Closure: Supporting Evidence, Limitations and Future Directions. Journal of Clinical Medicine, 14(7), 2300. https://doi.org/10.3390/jcm14072300







