Hemoadsorption in the Management of Septic Shock: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Material and Methods
2.1. Selection Criteria
2.2. Primary and Secondary Outcomes
2.3. Search Methods
2.4. Study Selection and Data Extraction Process
2.5. Assessment of Bias Risk and Evidence of Certainty in Included Studies
2.6. Data Synthesis and Statistical Analysis
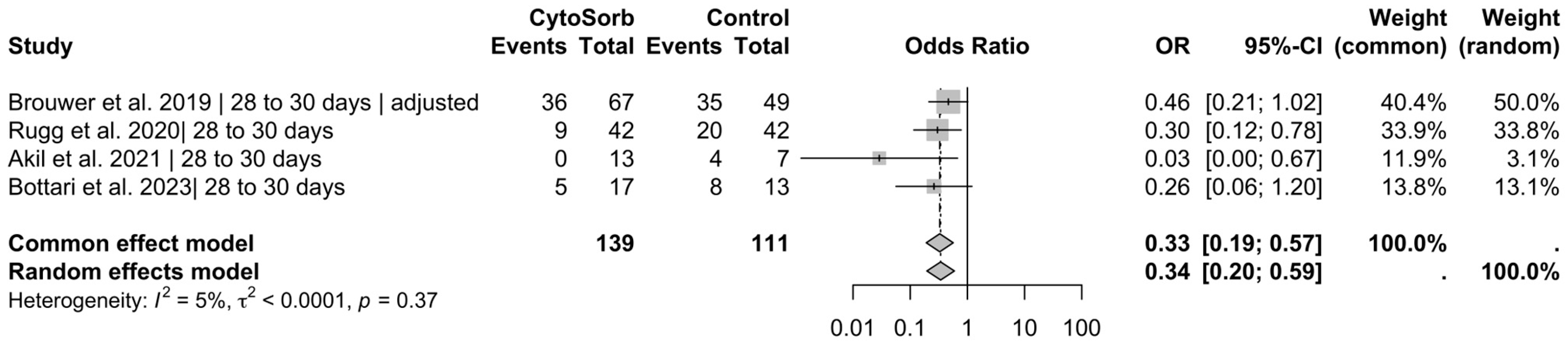
3. Results
3.1. Risk of Bias Assessment and Quality Assessment
3.2. Primary Outcomes
3.3. Secondary Outcomes
3.4. Outcomes with Insufficient Reporting
4. Discussion
4.1. Previous Findings
4.2. Current Analysis
4.3. Safety
4.4. Strengths and Limitations
4.5. Implications of This Meta-Analysis and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Bauer, M.; Gerlach, H.; Vogelmann, T.; Preissing, F.; Stiefel, J.; Adam, D. Mortality in sepsis and septic shock in Europe, North America and Australia between 2009 and 2019—Results from a systematic review and meta-analysis. Crit. Care 2020, 24, 239. [Google Scholar] [CrossRef] [PubMed]
- La Via, L.; Sangiorgio, G.; Stefani, S.; Marino, A.; Nunnari, G.; Cocuzza, S.; La Mantia, I.; Cacopardo, B.; Stracquadanio, S.; Spampinato, S.; et al. The Global Burden of Sepsis and Septic Shock. Epidemiologia 2024, 5, 456–478. [Google Scholar] [CrossRef] [PubMed]
- Gerlach, H. Agents to reduce cytokine storm. F1000Research 2016, 5, 2909. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; He, F.; Li, P.; Hardwidge, P.R.; Li, N.; Peng, Y. The Role of Innate Immunity in Pulmonary Infections. BioMed Res. Int. 2021, 2021, 6646071. [Google Scholar] [CrossRef]
- Mantovani, A.; Cassatella, M.A.; Costantini, C.; Jaillon, S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 2011, 11, 519–531. [Google Scholar] [CrossRef]
- Bode, C.; Weis, S.; Sauer, A.; Wendel-Garcia, P.; David, S. Targeting the host response in sepsis: Current approaches and future evidence. Crit. Care 2023, 27, 478. [Google Scholar] [CrossRef]
- Ronco, C.; Chawla, L.; Husain-Syed, F.; Kellum, J.A. Rationale for sequential extracorporeal therapy (SET) in sepsis. Crit. Care 2023, 27, 50. [Google Scholar] [CrossRef]
- Mitzner, S.; Kogelmann, K.; Ince, C.; Molnár, Z.; Ferrer, R.; Nierhaus, A. Adjunctive Hemoadsorption Therapy with CytoSorb in Patients with Septic/Vasoplegic Shock: A Best Practice Consensus Statement. J. Clin. Med. 2023, 12, 7199. [Google Scholar] [CrossRef]
- Winchester, J.F.; Kellum, J.A.; Ronco, C.; Brady, J.A.; Quartararo, P.J.; Salsberg, J.A.; Levin, N.W. Sorbents in acute renal failure and the systemic inflammatory response syndrome. Blood Purif. 2003, 21, 79–84. [Google Scholar] [CrossRef]
- Song, M.; Winchester, J.; Albright, R.L.; Capponi, V.J.; Choquette, M.D.; Kellum, J.A. Cytokine removal with a novel adsorbent polymer. Blood Purif. 2004, 22, 428–434. [Google Scholar] [CrossRef]
- Kellum, J.A.; Song, M.; Venkataraman, R. Hemoadsorption removes tumor necrosis factor, interleukin-6, and interleukin-10, reduces nuclear factor-kappaB DNA binding, and improves short-term survival in lethal endotoxemia. Crit. Care Med. 2004, 32, 801–805. [Google Scholar] [CrossRef] [PubMed]
- Jansen, A.; Waalders, N.J.B.; van Lier, D.P.T.; Kox, M.; Pickkers, P. CytoSorb hemoperfusion markedly attenuates circulating cytokine concentrations during systemic inflammation in humans in vivo. Crit. Care 2023, 27, 117. [Google Scholar] [CrossRef] [PubMed]
- Cochrane Handbook for Systematic Reviews and Interventions. Available online: http://training.cochrane.org/handbook (accessed on 3 June 2020).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- JBI Global Wiki. Systematic Reviews of Etiology and RISK—JBI Manual for Evidence Synthesis. Available online: https://jbi-global-wiki.refined.site/space/MANUAL/355598596/7.+Systematic+reviews+of+etiology+and+risk (accessed on 15 September 2024).
- Mantel, N.; Haenszel, W. Statistical aspects of the analysis of data from retrospective studies of disease. J. Natl. Cancer Inst. 1959, 22, 719–748. [Google Scholar]
- Brouwer, W.P.; Duran, S.; Kuijper, M.; Ince, C. Hemoadsorption with CytoSorb shows a decreased observed versus expected 28-day all-cause mortality in ICU patients with septic shock: A propensity-score-weighted retrospective study. Crit. Care 2019, 23, 317. [Google Scholar] [CrossRef]
- Brouwer, W.P.; Duran, S.; Ince, C. Improved Survival beyond 28 Days up to 1 Year after CytoSorb Treatment for Refractory Septic Shock: A Propensity-Weighted Retrospective Survival Analysis. Blood Purif. 2020, 50, 539–545. [Google Scholar] [CrossRef]
- Hawchar, F.; László, I.; Öveges, N.; Trásy, D.; Ondrik, Z.; Molnar, Z. Extracorporeal cytokine adsorption in septic shock: A proof of concept randomized, controlled pilot study. J. Crit. Care 2019, 49, 172–178. [Google Scholar] [CrossRef]
- Rugg, C.; Klose, R.; Hornung, R.; Innerhofer, N.; Bachler, M.; Schmid, S.; Fries, D.; Ströhle, M. Hemoadsorption with CytoSorb in Septic Shock Reduces Catecholamine Requirements and In-Hospital Mortality: A Single-Center Retrospective “Genetic” Matched Analysis. Biomedicines 2020, 8, 539. [Google Scholar] [CrossRef]
- Schittek, G.A.; Zoidl, P.; Eichinger, M.; Orlob, S.; Simonis, H.; Rief, M.; Metnitz, P.; Fellinger, T.; Soukup, J. Adsorption therapy in critically ill with septic shock and acute kidney injury: A retrospective and prospective cohort study. Ann. Intensiv. Care 2020, 10, 154. [Google Scholar] [CrossRef]
- Akil, A.; Ziegeler, S.; Reichelt, J.; Rehers, S.; Abdalla, O.; Semik, M.; Fischer, S. Combined Use of CytoSorb and ECMO in Patients with Severe Pneumogenic Sepsis. Thorac. Cardiovasc. Surg. 2021, 69, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Kogelmann, K.; Hübner, T.; Schwameis, F.; Drüner, M.; Scheller, M.; Jarczak, D. First Evaluation of a New Dynamic Scoring System Intended to Support Prescription of Adjuvant CytoSorb Hemoadsorption Therapy in Patients with Septic Shock. J. Clin. Med. 2021, 10, 2939. [Google Scholar] [CrossRef] [PubMed]
- Garcia, P.D.W.; Hilty, M.P.; Held, U.; Kleinert, E.-M.; Maggiorini, M. Cytokine adsorption in severe, refractory septic shock. Intensiv. Care Med. 2021, 47, 1334–1336. [Google Scholar] [CrossRef]
- Bottari, G.; Guzzo, I.; Cappoli, A.; Labbadia, R.; Perdichizzi, S.; Serpe, C.; Creteur, J.; Cecchetti, C.; Taccone, F.S. Impact of CytoSorb and CKRT on hemodynamics in pediatric patients with septic shock: The PedCyto study. Front. Pediatr. 2023, 11, 1259384. [Google Scholar] [CrossRef]
- Mariano, F.; Greco’, D.; Depetris, N.; Mella, A.; Sciarrillo, A.; Stella, M.; Berardino, M.; Risso, D.; Gambino, R.; Biancone, L. CytoSorb® in burn patients with septic shock and Acute Kidney Injury on Continuous Kidney Replacement Therapy is associated with improved clinical outcome and survival. Burns 2024, 50, 1213–1222. [Google Scholar] [CrossRef]
- Schädler, D.; Pausch, C.; Heise, D.; Meier-Hellmann, A.; Brederlau, J.; Weiler, N.; Marx, G.; Putensen, C.; Spies, C.; Jörres, A.; et al. The effect of a novel extracorporeal cytokine hemoadsorption device on IL-6 elimination in septic patients: A randomized controlled trial. PLoS ONE 2017, 12, e0187015. [Google Scholar] [CrossRef]
- Becker, S.; Lang, H.; Barbosa, C.V.; Tian, Z.; Melk, A.; Schmidt, B.M.W. Efficacy of CytoSorb®: A systematic review and meta-analysis. Crit. Care 2023, 27, 215. [Google Scholar] [CrossRef]
- Kasper, R.; Rodriguez-Alfonso, A.; Ständker, L.; Wiese, S.; Schneider, E.M. Major endothelial damage markers identified from hemadsorption filters derived from treated patients with septic shock—Endoplasmic reticulum stress and bikunin may play a role. Front. Immunol. 2024, 15, 1359097. [Google Scholar] [CrossRef]
- Piskovatska, V.; Santos, A.N.; Kalies, K.; Korca, E.; Stiller, M.; Szabó, G.; Simm, A.; Wächter, K. Proteins Adsorbed during Intraoperative Hemoadsorption and Their In Vitro Effects on Endothelium. Healthcare 2023, 11, 310. [Google Scholar] [CrossRef]
- Bottari, G.; Confalone, V.; Creteur, J.; Cecchetti, C.; Taccone, F.S. The Sublingual Microcirculation in Critically Ill Children with Septic Shock Undergoing Hemoadsorption: A Pilot Study. Biomedicines 2024, 12, 1435. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.M.W.; Lang, H.; Tian, Z.J.; Becker, S.; Melk, A. Cytokine removal: Do not ban it, but learn in whom and when to use it. Crit. Care 2023, 27, 444. [Google Scholar] [CrossRef] [PubMed]
- Pappalardo, F.; Cardinale, A.; D’ettore, N.; Maj, G. Blood purification in critically ill patients: Not enough, but still helpful. Crit. Care 2023, 27, 357. [Google Scholar] [CrossRef] [PubMed]
- Raasveld, S.J.; Oord, C.v.D.; Schenk, J.; Bergh, W.M.v.D.; Hartgring, A.O.L.-; van der Velde, F.; Maas, J.J.; van de Berg, P.; Lorusso, R.; Delnoij, T.S.R.; et al. The interaction of thrombocytopenia, hemorrhage, and platelet transfusion in venoarterial extracorporeal membrane oxygenation: A multicenter observational study. Crit. Care 2023, 27, 321. [Google Scholar] [CrossRef]
- Heymann, M.; Schorer, R.; Putzu, A. Mortality and adverse events of hemoadsorption with CytoSorb® in critically ill patients: A systematic review and meta-analysis of randomized controlled trials. Acta Anaesthesiol. Scand. 2022, 66, 1037–1050. [Google Scholar] [CrossRef]
- Liebchen, U.; Scharf, C.; Zoller, M.; Weinelt, F.; Kloft, C.; Michelet, R.; Schroeder, I.; Paal, M.; Vogeser, M.; Irlbeck, M.; et al. No clinically relevant removal of meropenem by cytokine adsorber CytoSorb® in critically ill patients with sepsis or septic shock. Intensiv. Care Med. 2021, 47, 1332–1333. [Google Scholar] [CrossRef]
- Bottari, G.; Goffredo, B.M.; Marano, M.; Maccarrone, C.; Simeoli, R.; Bianco, G.; Vallesi, L.; Beetham, J.C.C.; Mazzeo, A.T.; Cappoli, A.; et al. Impact of Continuous Kidney Replacement Therapy and Hemoadsorption with CytoSorb on Antimicrobial Drug Removal in Critically Ill Children with Septic Shock: A Single-Center Prospective Study on a Pediatric Cohort. Antibiotics 2023, 12, 1395. [Google Scholar] [CrossRef]
- Schneider, A.G.; André, P.; Scheier, J.; Schmidt, M.; Ziervogel, H.; Buclin, T.; Kindgen-Milles, D. Pharmacokinetics of anti-infective agents during CytoSorb hemoadsorption. Sci. Rep. 2021, 11, 10493. [Google Scholar] [CrossRef]
- Asgarpur, G.; Weber, F.; Kiessling, P.; Akbari, N.; Stroben, F.; Kleikamp, B.; Kloft, C.; Treskatsch, S.; Angermair, S. Impact of hemoadsorption with CytoSorb® on meropenem and piperacillin exposure in critically ill patients in a post-CKRT setup: A single-center, retrospective data analysis. Intensiv. Care Med. Exp. 2025, 13, 7. [Google Scholar] [CrossRef]



| Author | Year | Study Design | N (CS) | N (CG) | Median Age (CS/CG) | Sex; Male (%) CS/CG | |
|---|---|---|---|---|---|---|---|
| 1 | Brouwer et al. [19,20] | 2019/2021 | Retrospective IPTW-weighted study; single-center study | 67 | 49 | 61/69 | 55/61 |
| 2 | Hawchar et al. [21] | 2019 | Randomized controlled trial (RCT), open-label, single-center study | 10 | 10 | 60/71 | 70/60 |
| 3 | Rugg et al. [22] | 2020 | Retrospective propensity score-matched study; single-center study | 42 | 42 | 64/68 | 64/60 |
| 4 | Schittek et al. [23] | 2020 | Retrospective control group with a prospective intervention group; single-center study | 43 | 33 | 63/62 | 88/72 |
| 5 | Akil et al. [24] | 2021 | Single-center study with a retrospective control group and a prospective intervention group | 13 | 7 | 61/61 | 38/29 |
| 6 | Kogelmann et al. [25] | 2021 | Retrospective study; multicenter study | 198 | 69 | 62/66 | 61/NA |
| 7 | Wendel-Garcia et al. [26] | 2021 | Single-center propensity score-matched study with a retrospective control group and a prospective intervention group | 48 | 48 | 57/58 | 65/65 |
| 8 | Bottari et al. [27] | 2023 | Retrospective control group with a prospective intervention group; single-center study | 17 | 13 | 9/6 | 8/11 |
| 9 | Mariano et al. [28] | 2024 | Retrospective study; single-center study | 11 | 24 | 63/72 | 8/20 |
| Total: | 449 | 295 |
| Study | Change in Vasopressor Dosage or VIS in CytoSorb® Group | Time Window of Measurement | Statistical Significance Within CytoSorb® Group | Statistical Significance Between Groups | Mortality Benefit CS |
|---|---|---|---|---|---|
| Akil et al. (2021) [24] | Significant reduction at 12, 24, and 48 h; none after 72 h | 12, 24, 48, 72 h | p < 0.001 | <0.03 # | Yes, 30 days |
| Rugg et al. (2020) [22] | Halving of median NE equivalents to 0.26 μg/kg/min | 24 h | NR | <0.01 # | Yes, hospital and 28 days |
| Hawchar et al. (2019) [21] | Reduction from 0.54 to 0.16 μg/kg/min | 48 h | p = 0.016 | <0.35 # | Yes, 48 h |
| Wendel-Garcia et al. (2021) [26] | NR, reduction in requirement | 72 h | NR | p = 0.555 | No |
| Bottari et al. (2023) [27] | Significant reduction in VIS | 72 h | NR | p = 0.001 | Yes, 28 days |
| Mariano et al. (2024) [28] | Significant reduction at various time points | Days 1–4 | NR | p = 0.02 | Yes, hospital |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steindl, D.; Schroeder, T.; Krannich, A.; Nee, J. Hemoadsorption in the Management of Septic Shock: A Systematic Review and Meta-Analysis. J. Clin. Med. 2025, 14, 2285. https://doi.org/10.3390/jcm14072285
Steindl D, Schroeder T, Krannich A, Nee J. Hemoadsorption in the Management of Septic Shock: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2025; 14(7):2285. https://doi.org/10.3390/jcm14072285
Chicago/Turabian StyleSteindl, David, Tim Schroeder, Alexander Krannich, and Jens Nee. 2025. "Hemoadsorption in the Management of Septic Shock: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 14, no. 7: 2285. https://doi.org/10.3390/jcm14072285
APA StyleSteindl, D., Schroeder, T., Krannich, A., & Nee, J. (2025). Hemoadsorption in the Management of Septic Shock: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 14(7), 2285. https://doi.org/10.3390/jcm14072285





