The Impact of Elevated Lipoprotein (a) Levels on Postoperative Outcomes in Carotid Endarterectomy: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. The Selection Criteria
2.2. The Search Strategy
2.3. Study Selection and Data Extraction
2.4. Assessment of Study Quality
3. Results
3.1. The Search Results
3.2. Description of the Studies
3.3. Study Quality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACS | Acute coronary syndrome |
| AMSTAR | A Measurement Tool to Assess Systematic Reviews |
| Apo(a) | Apolipoprotein(a) |
| Apo B100 | Apolipoprotein B100 |
| BMT | Best medical therapy |
| CAD | Coronary artery disease |
| CEA | Carotid endarterectomy |
| CI | Confidence interval |
| CVD | Cardiovascular disease |
| CVRF | Cardiovascular risk factor |
| GRADE | Grading of Recommendations, Assessment, Development, and Evaluation |
| LDL | Low-density lipoprotein |
| Lp(a) | Lipoprotein(a) |
| MACE | Major adverse cardiovascular event |
| MMP-9 | Matrix metalloproteinase-9 |
| NHLBI | National Heart, Lung, and Blood Institute |
| ox-LDL | Oxidized low-density lipoprotein |
| PAD | Peripheral arterial disease |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| SMCs | Smooth muscle cells |
References
- Naylor, A.R.; Ricco, J.B.; de Borst, G.J.; Debus, S.; de Haro, J.; Halliday, A.; Hamilton, G.; Kakisis, J.; Kakkos, S.; Lepidi, S.; et al. Editor’s Choice—Management of Atherosclerotic Carotid and Vertebral Artery Disease: 2017 Clinical Practice Guidelines of the European Society for Vascular Surgery (ESVS). Eur. J. Vasc. Endovasc. Surg. 2018, 55, 3–81. [Google Scholar] [CrossRef]
- Naylor, R.; Rantner, B.; Ancetti, S.; de Borst, G.J.; De Carlo, M.; Halliday, A.; Kakkos, S.K.; Markus, H.S.; McCabe, D.J.; Sillesen, H.; et al. Editor’s Choice—European Society for Vascular Surgery (ESVS) 2023 Clinical Practice Guidelines on the Management of Atherosclerotic Carotid and Vertebral Artery Disease. Eur. J. Vasc. Endovasc. Surg. 2023, 65, 7–111. [Google Scholar] [CrossRef]
- Kang, J.; Conrad, M.F.; Patel, V.I.; Mukhopadhyay, S.; Garg, A.; Cambria, M.R.; LaMuraglia, G.M.; Cambria, R.P. Clinical and anatomic outcomes after carotid endarterectomy. J. Vasc. Surg. 2014, 59, 944–949. [Google Scholar] [CrossRef] [PubMed]
- Waissi, F.; Dekker, M.; Timmerman, N.; Hoogeveen, R.M.; van Bennekom, J.; Dzobo, K.E.; Schnitzler, J.G.; Pasterkamp, G.; Grobbee, D.E.; de Borst, G.J.; et al. Elevated Lp(a) (Lipoprotein[a]) Levels Increase Risk of 30-Day Major Adverse Cardiovascular Events in Patients Following Carotid Endarterectomy. Stroke 2020, 51, 2972–2982. [Google Scholar] [CrossRef]
- Nordestgaard, B.G.; Chapman, M.J.; Ray, K.; Borén, J.; Andreotti, F.; Watts, G.F.; Ginsberg, H.; Amarenco, P.; Catapano, A.; Descamps, O.S.; et al. Lipoprotein(a) as a cardiovascular risk factor: Current status. Eur. Heart J. 2010, 31, 2844–2853. [Google Scholar] [CrossRef]
- Emerging Risk Factors Collaboration; Erqou, S.; Kaptoge, S.; Perry, P.L.; Di Angelantonio, E.; Thompson, A.; White, I.R.; Marcovina, S.M.; Collins, R.; Thompson, S.G.; et al. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA 2009, 302, 412–423. [Google Scholar] [CrossRef]
- Tsimikas, S. A Test in Context: Lipoprotein(a): Diagnosis, Prognosis, Controversies, and Emerging Therapies. J. Am. Coll. Cardiol. 2017, 69, 692–711. [Google Scholar] [CrossRef] [PubMed]
- Rigamonti, F.; Carbone, F.A.; Montecucco, F.A.; Bonaventura, A.; Liberale, L.; Burger, F.; Roth, A.; Bertolotto, M.; Spinella, G.; Pane, B.; et al. Serum lipoprotein (a) predicts acute coronary syndromes in patients with severe carotid stenosis. Eur. J. Clin. Investig. 2018, 48, e12888. [Google Scholar] [CrossRef]
- Stinson, J.; D’Arcy, G.; Cooke, T.; Colgan, M.P.; Hall, M.; Tyrrell, J.; Gaffney, K.; Grouden, M.; Moore, D.; Shanik, D.G.; et al. Elevated Lipoprotein (a) in Carotid Artery Atherosclerosis is Unrelated to Restenosis After Endarterectomy. Vasc. Surg. 1995, 29, 23–27. [Google Scholar] [CrossRef]
- Munjal, A.; Khandia, R. Atherosclerosis: Orchestrating cells and biomolecules involved in its activation and inhibition. Adv. Protein Chem. Struct. Biol. 2020, 120, 85–122. [Google Scholar] [CrossRef]
- Dwivedi, A.; Anggård, E.E.; Carrier, M.J. Oxidized LDL-mediated monocyte adhesion to endothelial cells does not involve NFkappaB. Biochem. Biophys. Res. Commun. 2001, 284, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Abilleira, S.; Bevan, S.; Markus, H.S. The role of genetic variants of matrix metalloproteinases in coronary and carotid atherosclerosis. J. Med. Genet. 2006, 43, 897–901. [Google Scholar] [CrossRef] [PubMed]
- Di Minno, A.; Turnu, L.; Porro, B.; Squellerio, I.; Cavalca, V.; Tremoli, E.; Di Minno, M.N. 8-Hydroxy-2-Deoxyguanosine Levels and Cardiovascular Disease: A Systematic Review and Meta-Analysis of the Literature. Antioxid. Redox Signal. 2016, 24, 548–555. [Google Scholar] [CrossRef]
- Gackowski, D.; Kruszewski, M.; Jawien, A.; Ciecierski, M.; Olinski, R. Further evidence that oxidative stress may be a risk factor responsible for the development of atherosclerosis. Free. Radic. Biol. Med. 2001, 31, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, j4008. [Google Scholar]
- National Heart, Lung, and Blood Institute. Study Quality Assessment Tools. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 28 November 2024).
- Atkins, D.; Best, D.; Briss, P.A.; Eccles, M.; Falck-Ytter, Y.; Flottorp, S.; Guyatt, G.H.; Harbour, R.T.; Haugh, M.C.; Henry, D.; et al. Grading quality of evidence and strength of recommendations. BMJ 2004, 328, 1490. [Google Scholar] [CrossRef]
- Woźniak, A.; Satała, J.A.; Gorzelak-Pabiś, P.A.; Pawlos, A.A.; Broncel, M.A.; Kaźmierski, P.A.; Woźniak, E. OxLDL as a prognostic biomarker of plaque instability in patients qualified for carotid endarterectomy. J. Cell. Mol. Med. 2024, 28, e18459. [Google Scholar] [CrossRef]
- Salenius, J.P.; Haapanen, A.; Harju, E.; Jokela, H.; Riekkinen, H. Late carotid restenosis: Aetiologic factors for recurrent carotid artery stenosis during long-term follow-up. Eur. J. Vasc. Surg. 1989, 3, 271–277. [Google Scholar] [CrossRef]
- Gurdasani, D.; Sjouke, B.; Tsimikas, S.; Hovingh, G.K.; Luben, R.N.; Wainwright, N.W.; Pomilla, C.; Wareham, N.J.; Khaw, K.-T.; Boekholdt, S.M.; et al. Lipoprotein(a) and risk of coronary, cerebrovascular, and peripheral artery disease: The EPIC-Norfolk prospective population study. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 3058–3065. [Google Scholar] [CrossRef]
- Banach, M. Lipoprotein (a)—We Know So Much Yet Still Have Much to Learn…. J. Am. Heart Assoc. 2016, 5, e003597. [Google Scholar] [CrossRef] [PubMed]
- Huibers, A.; Calvet, D.; Kennedy, F.; Czuriga-Kovács, K.R.; Featherstone, R.L.; Moll, F.L.; Brown, M.; Richards, T.; de Borst, G. Mechanism of Procedural Stroke Following Carotid Endarterectomy or Carotid Artery Stenting Within the International Carotid Stenting Study (ICSS) Randomised Trial. Eur. J. Vasc. Endovasc. Surg. 2015, 50, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Riles, T.S.; Imparato, A.M.; Jacobowitz, G.R.; Lamparello, P.J.; Giangola, G.; Adelman, M.A.; Landis, R. The cause of perioperative stroke after carotid endarterectomy. J. Vasc. Surg. 1994, 19, 206–214, discussion 215–216. [Google Scholar] [CrossRef] [PubMed]
- Manzato, M.; Wright, R.S.; Jaffe, A.S.; Vasile, V.C. Lipoprotein (a): Underrecognized Risk with a Promising Future. Rev. Cardiovasc. Med. 2024, 25, 393. [Google Scholar] [CrossRef]
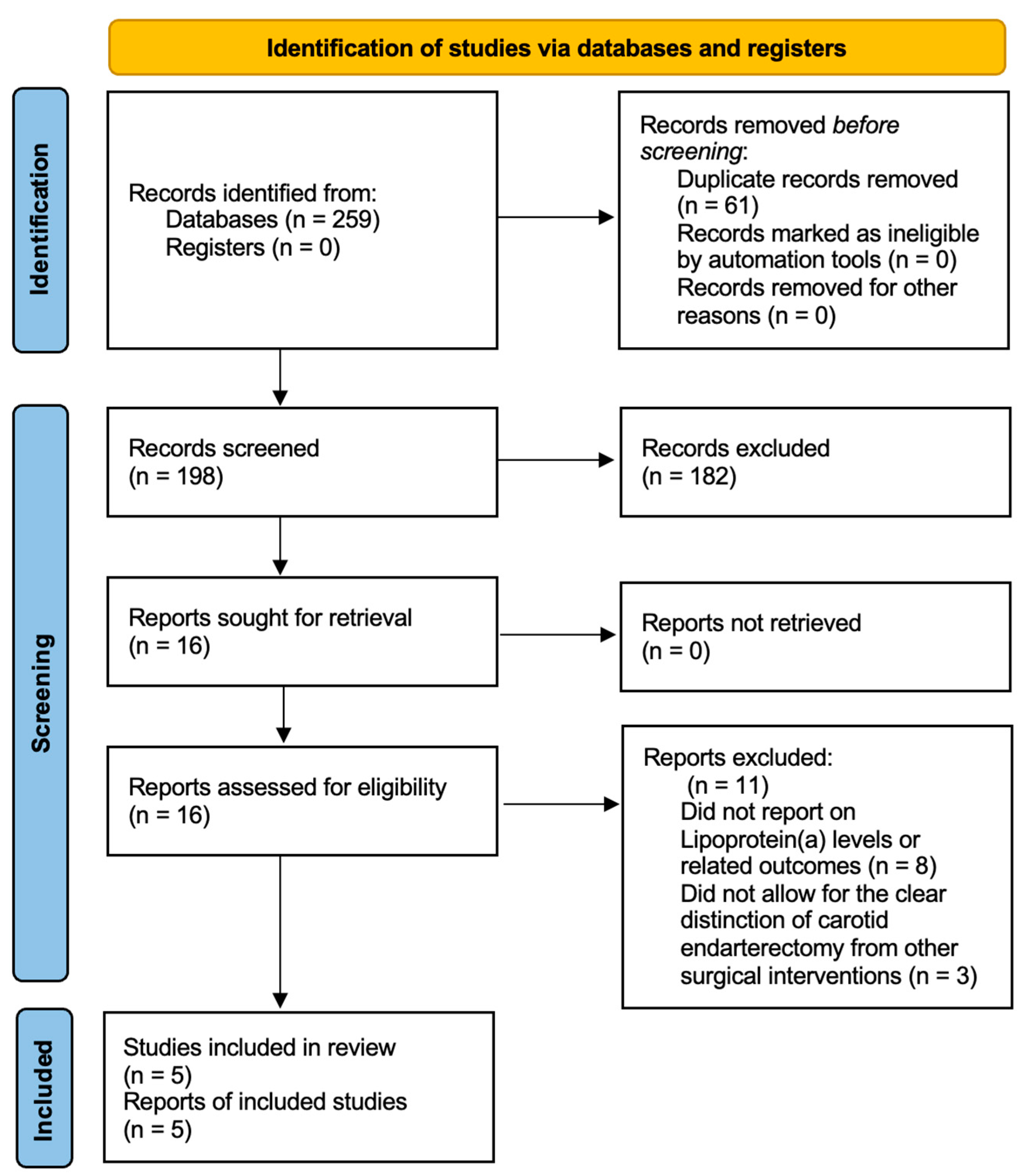
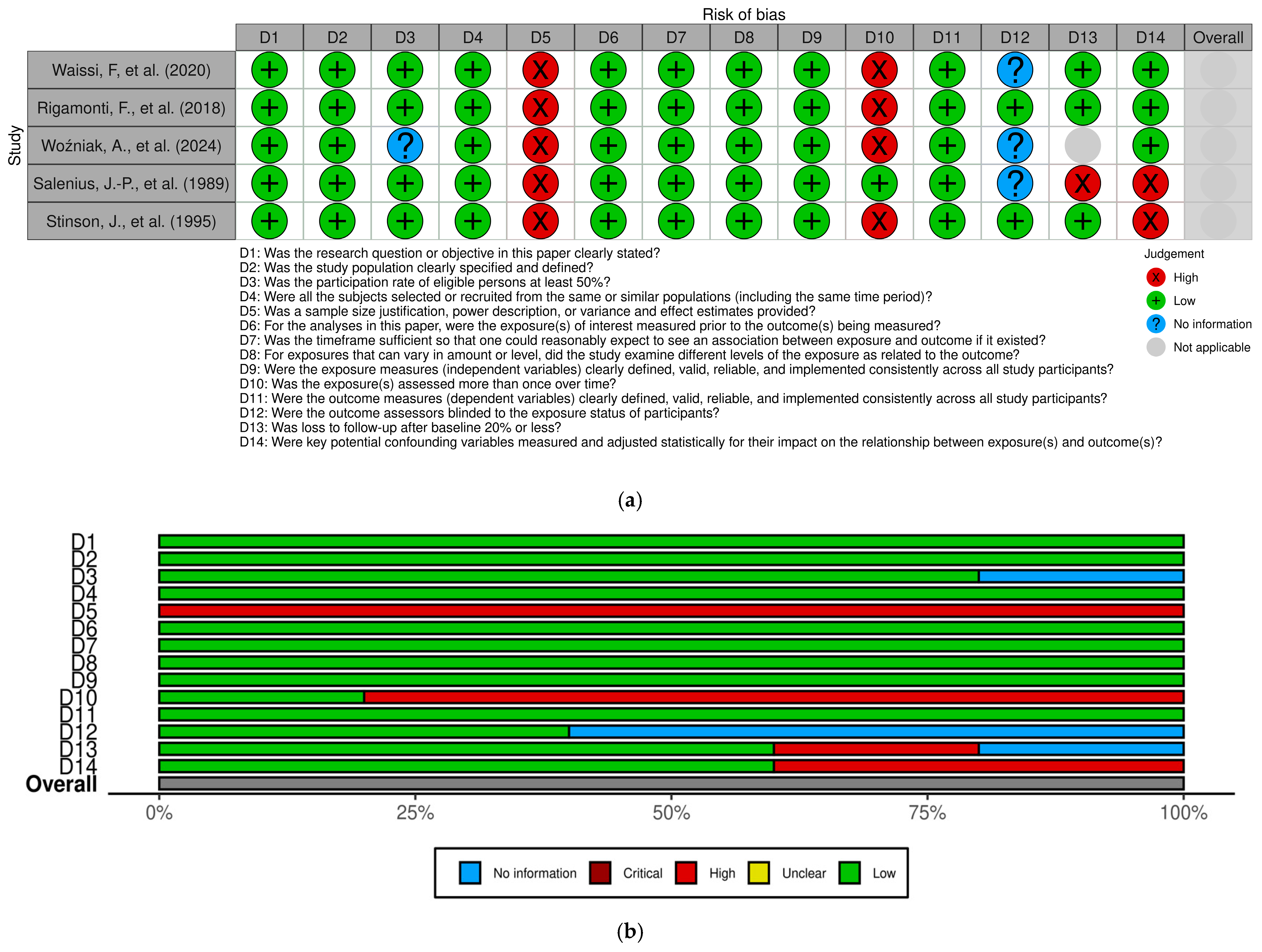
| Author | Journal | Publication Year | Study Design | Study Center | Recruitment Period | Sample Size (Patients) | GRADE Evaluation |
|---|---|---|---|---|---|---|---|
| Waissi F. et al. | Stroke (AHA Journal) | 2020 | Prospective cohort | St. Antonius Hospital Nieuwegein, University Medical Center Utrecht and Amsterdam University Medical Centers, the Netherlands | 2002–2016 | 944 | 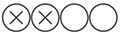 Low |
| Rigamonti F. et al. | European Journal of Clinical Investigation | 2018 | Prospective cohort (sub-study) | Hospital San Martino, Italy | 2008–2012 | 180 | 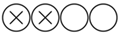 Low |
| Woźniak A. et al. | Journal of Cellular and Molecular Medicine | 2024 | Prospective cohort | Hospital M. Copernicus, Poland | 2018–2019 | 67 | 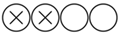 Low |
| Salenius J-P. et al. | European Journal of Vascular Surgery | 1989 | Retrospective cohort | University Central Hospital of Tampere, Finland | 1970–1984 | 116 | 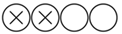 Low |
| Stinson J. et al. | Journal of Vascular Surgery | 2009 | Retrospective, observational study | Trinity Centre for Health Sciences, St. James’s Hospital, Ireland | 1981–1995 | 143 | 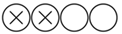 Low |
| Author | Mean Age | Age SD | Male n (%) | Hypertension n (%) | Dyslipidemia n (%) | Obesity n (%) | Diabetes Mellitus n (%) | Chronic Heart Failure (CHF) n (%) | Smoking History n (%) | Coronary Artery Disease n (%) | Peripheral Artery Disease n (%) | Under Antiplatelet Therapy n (%) | Symptomatic n (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Waissi F. et al. | 69.7 | 9 | 651 (68.96) | 668 (70.76) | 753 (79.77) | NA | 209 (22.14) | NA | 321 (34.00) | 268 (28.39) | 182 (19.28) | 833 (88.24) | 825 (87.39) |
| Rigamonti F. et al. | 72 (not elevated Lp(a)); 74 (elevated Lp(a)) | NA | 67 (37.22) | 126 (70.00) | 101 (56.11) | NA | 38 (21.11) | NA | 40 (22.22) | 34 (18.89) | NA | 139 (77.22) | 44 (24.44) |
| Woźniak A. et al. | 71 | NA | 35 (52.24) | 52 (77.61) | NA | 5 (7.46) | 15 (22.39) | NA | 29 (43.28) | NA | NA | NA | NA |
| Salenius J-P. et al. | 57 | NA | 79 (68.10) | 68 (58.62) | 74 (63.79) | 33 (28.45) | 13 (11.21) | NA | 88 (75.86) | 57 (49.14) | 47 (40.52) | NA | 100 (86.21) |
| Stinson J. et al. | 69.5 | NA | 92 (64.33) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Author | Exclusion Criteria | Intervention | Number of Carotids Operated on per Person | Antiplatelet Therapy n (%) | Dual Antiplatelet Therapy n (%) | Anticoagulation n (%) | Contralateral Stenosis n (%) |
|---|---|---|---|---|---|---|---|
| Waissi F. et al. | Carotid endarterectomy for restenosis | All carotid endarterectomy | 1 | NA | NA | NA | 823 (87.18) |
| Rigamonti F. et al. | The absence of angiographic visualization of the symptomatic artery, intracranial stenosis more clinically significant than the cervical lesion, other diseases that limited life expectancy to less than five years, cerebral infarction that resulted in the loss of useful function in the affected arterial territory, non-atherosclerotic carotid disease, cardiac lesions likely to cause cardioembolism, or a history of ipsilateral endarterectomy. | All carotid endarterectomy | 1 | NA | NA | NA | NA |
| Woźniak A. et al. | Causes of non-vascular ischemic stroke, prior hemorrhagic or lacunar strokes, brain disorders, atrial fibrillation treatment, active infection or inflammation, autoimmune conditions, hematological and oncological disorders, severe renal or hepatic failure, venous thrombosis, myocardial infarction or surgical procedures within one year before inclusion, the use of anti-inflammatory or immunosuppressive drugs within 6 months before examination, malnutrition, poisoning, or alcohol and psychoactive substance abuse history. | All carotid endarterectomy | 1 | NA | NA | NA | NA |
| Salenius J-P. et al. | NA | All carotid endarterectomy | 90 persons—1 carotid 22 persons—bilateral 4 persons—two separate endarterectomies on the same side | 32 (27.6) | ASA–dipyridamole combination: 27 (23.3) | 28 (24.1) | NA |
| Stinson J. et al. | NA | All carotid endarterectomy | 1 | NA | NA | NA | NA |
| Author | Stroke—30 Days n (%) | AMI—30 Days n (%) | Cardiovascular-Related Deaths in 30 Days n (%) | 30-Day MACEs n (%) | 30-Day MACEs (Elevated Lp(a) Levels) HR | MACE Definitions |
|---|---|---|---|---|---|---|
| Waissi F. et al. | 28 (2.75) | 6 (0.64) | 1 (0.10) | 35 (3.71) | HR of 2.12 (95% CI: 1.05–4.27) HR of 2.05 (95% CI: 1.01–4.17) (adjusted for age and sex) | Myocardial infarction, stroke, or cardiovascular death |
| Rigamonti F. et al. | NA | NA | NA | NA | NA | NA |
| Woźniak A. et al. | NA | NA | NA | NA | NA | NA |
| Salenius J-P. et al. | NA | NA | NA | NA | NA | NA |
| Stinson J. et al. | NA | NA | NA | NA | NA | NA |
| Author | Follow-Up Time | AMI >30 Days n (%) | AMI Long-Term Follow-Up | Stroke > 30 Days n (%) | Stroke Long-Term Follow-Up | Cardiovascular-Related Death >30 Days n (%) | MACEs in Long-Term Follow-Up | MACEs > 30 Days n (%) | Other Outcomes | Long-Term All-Cause Mortality n (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Waissi F. et al. | 3 years | 21 (2.22) | 27 (2.86) | 35 (3.71) | 63 (6.67) | 11 (1.17) | 102 (10.8%) High Lp(a) levels: HR: 1.54 (95% CI: 1.00–2.39). HR: 1.69 (95% CI: 1.07–2.66) (adjusted for risk factors). | “No significant association between high Lp(a) levels and MACE from time point 30 days to 3 years onward” | NA | NA |
| Rigamonti F. et al. | 24 months | NA | ACS long-term follow-up: High Lp(a)— HR: 6.490 (95% CI: 1.550–27.160, p = 0.010) HR: 8.504 (95% CI: 1.932–37.425], p = 0.005) (adjusted for CV risk factors) | NA | NA | NA | NA | NA | NA | NA |
| Woźniak A. et al. | NA | NA | NA | NA | NA | NA | NA | NA | Regardless of serum Lp(a) levels, in patients with unstable atherosclerotic plaques, other serum components such as ox-LDL, MMP-9, and 8-OHdG are higher than these values in patients without unstable plaques. | NA |
| Salenius J-P. et al. | 28 to 209 months | NA | NA | NA | NA | NA | NA | NA | Low Lp(a) levels indicated a higher frequency of high-grade restenosis: low levels (<80 mg/L): 20.9%; intermediate levels (80–269 mg/L): 7.5%; high levels (>270 mg/L): 12.0%; p = 0.10. | NA |
| Stinson J. et al. | 24 to 168 months | NA | NA | NA | NA | NA | NA | NA | No association was found between Lp(a) concentrations and risk of restenosis. | NA |
| Author | Lp(a) Elevated | Measurement |
|---|---|---|
| Waissi F. et al. | >137 nmol/L | Lp(a) concentrations were measured using a latex-enhanced particle immunoturbidimetric assay with the Cobas c702 analyzer and the LPA2 Tinaquant Lp(a) Gen.2 kit. The method involved the agglutination of Lp(a) in the serum samples using latex particles coated with anti-Lp(a) antibodies. The resulting precipitate was quantified turbidimetrically at 800/660 nm. The assay has a measuring range of 7 to 240 nmol/L. |
| Rigamonti F. et al. | > or = 10 mg/mL | Serum Lp(a) levels were measured using a nephelometric assay. |
| Woźniak A. et al. | > or = 125 nmol/L | Immunoturbidimetric assay. |
| Salenius J-P. et al. | > or = 270 mg/L | NA |
| Stinson J. et al. | Mean Lp(a) levels in patients with carotid stenosis: 390 ± 40.2 mg/L Mean Lp(a) levels in controls: 142 ± 29.7 mg/L | Lipoprotein (a) concentrations were determined using an enzyme-linked immunosorbent assay (ELISA). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marques, J.C.; Marques, M.F.; Ribeiro, H.; Neves, A.P.; Zlatanovic, P.; Neves, J.R. The Impact of Elevated Lipoprotein (a) Levels on Postoperative Outcomes in Carotid Endarterectomy: A Systematic Review. J. Clin. Med. 2025, 14, 2253. https://doi.org/10.3390/jcm14072253
Marques JC, Marques MF, Ribeiro H, Neves AP, Zlatanovic P, Neves JR. The Impact of Elevated Lipoprotein (a) Levels on Postoperative Outcomes in Carotid Endarterectomy: A Systematic Review. Journal of Clinical Medicine. 2025; 14(7):2253. https://doi.org/10.3390/jcm14072253
Chicago/Turabian StyleMarques, João Carvalheiras, Mariana Fragão Marques, Hugo Ribeiro, António Pereira Neves, Peter Zlatanovic, and João Rocha Neves. 2025. "The Impact of Elevated Lipoprotein (a) Levels on Postoperative Outcomes in Carotid Endarterectomy: A Systematic Review" Journal of Clinical Medicine 14, no. 7: 2253. https://doi.org/10.3390/jcm14072253
APA StyleMarques, J. C., Marques, M. F., Ribeiro, H., Neves, A. P., Zlatanovic, P., & Neves, J. R. (2025). The Impact of Elevated Lipoprotein (a) Levels on Postoperative Outcomes in Carotid Endarterectomy: A Systematic Review. Journal of Clinical Medicine, 14(7), 2253. https://doi.org/10.3390/jcm14072253







