Chronic Kidney Disease After Lung Transplantation in Spain: A Retrospective Single-Center Analysis
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AKI | acute kidney injury |
| CF | cystic fibrosis |
| COPD | chronic obstructive pulmonary disease |
| ILD | interstitial lung disease |
| LTx | lung transplant |
| MARE | major adverse renal events |
| SOT | solid organ transplants |
References
- Organización Nacional de Trasplantes. Memoria de Actividad de Donación y Trasplante Pulmonar España 2022. Available online: https://www.ont.es/wp-content/uploads/2023/06/DONACION-Y-TRASPLANTE-GENERAL-2022.pdf (accessed on 21 March 2025).
- Solé, A.; Zurbano, F.; Borro, J.M.; Monforte, V.; Ussetti, P.; Santos, F. Prevalence and Diagnosis of Chronic Kidney Disease in Maintenance Lung Transplant Patients: ICEBERG Study. Transpl. Proc. 2015, 47, 1966–1971. [Google Scholar] [CrossRef]
- De La Morena, M.P.; Bravos, M.D.L.T.; Prado, R.F.; Delgado Roel, M.; García Salcedo, J.A.; Fieira Costa, E.; Rivas, D.G.; Maté, J.B. Chronic kidney disease after lung transplantation: Incidence, risk factors, and treatment. Transpl. Proc. 2010, 42, 3217–3219. [Google Scholar] [CrossRef]
- Ojo, A.O.; Held, P.J.; Port, F.K.; Wolfe, R.A.; Leichtman, A.B.; Young, E.W.; Arndorfer, J.; Christensen, L.; Merion, R.M. Chronic Renal Failure after Transplantation of a Nonrenal Organ. N. Engl. J. Med. 2003, 349, 931–940. [Google Scholar] [PubMed]
- Bloom, R.D.; Reese, P.P. Chronic kidney disease after nonrenal solid-organ transplantation. J. Am. Soc. Nephrol. 2007, 18, 3031. [Google Scholar] [CrossRef]
- Lamas, S. Cellular mechanisms of vascular injury mediated by calcineurin inhibitors. Kidney Int. 2005, 68, 898. [Google Scholar] [CrossRef]
- Remuzzi, G.; Bertani, T. Renal vascular and thrombotic effects of cyclosporine. Am. J. Kidney Dis. 1989, 13, 261–272. [Google Scholar] [CrossRef]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024, 105, S117–S314. [Google Scholar]
- Nelson, J.; Alvey, N.; Bowman, L.; Schulte, J.; Segovia, M.C.; McDermott, J.; Te, H.S.; Kapila, N.; Levine, D.J.; Gottlieb, R.L.; et al. Consensus recommendations for use of maintenance immunosuppression in solid organ transplantation: Endorsed by the American College of Clinical Pharmacy, American Society of Transplantation, and the International Society for Heart and Lung Transplantation. Pharmacotherapy 2022, 42, 599–633. [Google Scholar] [CrossRef]
- Prischl, F.C.; Rossing, P.; Bakris, G.; Mayer, G.; Wanner, C. Major adverse renal events (MARE): A proposal to unify renal endpoints. Nephrol. Dial. Transpl. 2021, 36, 491–497. [Google Scholar] [CrossRef]
- Pham, P.T.T.; Slavov, C.; Pham, P.C.T. Acute Kidney Injury After Liver, Heart, and Lung Transplants: Dialysis Modality, Predictors of Renal Function Recovery, and Impact on Survival. Adv. Chronic Kidney Dis. 2009, 16, 256–267. [Google Scholar] [CrossRef]
- Canales, M.; Youssef, P.; Spong, R.; Ishani, A.; Savik, K.; Hertz, M.; Ibrahim, H.N. Predictors of chronic kidney disease in long-term survivors of lung and heart-lung transplantation. Am. J. Transpl. 2006, 6, 2157–2163. [Google Scholar] [CrossRef]
- Osho, A.A.; Castleberry, A.W.; Snyder, L.D.; Palmer, S.M.; Stafford-Smith, M.; Lin, S.S.; Davis, R.D.; Hartwig, M.G. The Chronic Kidney Disease Epidemiology Collaboration (CKDEPI) equation best characterizes kidney function in patients being considered for lung transplantation. J. Heart Lung Transpl. 2014, 33, 1248–1254. [Google Scholar] [CrossRef]
- Carillo, C.; Pecoraro, Y.; Anile, M.; Mantovani, S.; Oliva, A.; D’Abramo, A.; Amore, D.; Pagini, A.; De Giacomo, T.; Pugliese, F.; et al. Evaluation of Renal Function in Patients Undergoing Lung Transplantation. Transpl. Proc. 2017, 49, 699–701. [Google Scholar] [CrossRef] [PubMed]
- Husain-Syed, F.; Ferrari, F.; Birk, H.W.; Weimer, R.; Ronco, C.; Poll, K.; Hecker, M.; Walmrath, H.-D.; Seeger, W.; Kuhnert, S.; et al. Pre-transplant renal functional reserve and renal function after lung transplantation. J. Heart Lung Transpl. 2020, 39, 970–974. [Google Scholar] [CrossRef]
- Weber, N.T.; Bonani, M.; Benden, C.; Schleich, A.; Fehr, T.; Mueller, T.F.; Schuurmans, M.M. Evolution of lung and kidney allograft function in patients receiving kidney after lung transplantation. Clin. Transpl. 2018, 32, e13169. [Google Scholar] [CrossRef]
- Wehbe, E.; Brock, R.; Budev, M.; Xu, M.; Demirjian, S.; Schreiber, M.J., Jr.; Stephany, B. Short-term and long-term outcomes of acute kidney injury after lung transplantation. J. Heart Lung Transpl. 2012, 31, 244–251. [Google Scholar] [CrossRef]
- Sikma, M.A.; Hunault, C.C.; van de Graaf, E.A.; Verhaar, M.C.; Kesecioglu, J.; de Lange, D.W.; Meulenbelt, J. High tacrolimus blood concentrations early after lung transplantation and the risk of kidney injury. Eur. J. Clin. Pharmacol. 2017, 73, 573–580. [Google Scholar] [CrossRef]
- Ishikawa, S.; Griesdale, D.E.G.; Lohser, J. Acute kidney injury within 72 hours after lung transplantation: Incidence and perioperative risk factors. J. Cardiothorac. Vasc. Anesth. 2014, 28, 931–935. [Google Scholar] [CrossRef]
- Hennessy, S.A.; Gillen, J.R.; Hranjec, T.; Kozower, B.D.; Jones, D.R.; Kron, I.L.; Lau, C.L. Influence of hemodialysis on clinical outcomes after lung transplantation. J. Surg. Res. 2013, 183, 916–921. [Google Scholar] [CrossRef]
- Banga, A.; Mohanka, M.; Mullins, J.; Bollineni, S.; Kaza, V.; Tanriover, B.; Torres, F. Characteristics and outcomes among patients with need for early dialysis after lung transplantation surgery. Clin. Transpl. 2017, 31. [Google Scholar] [CrossRef]
- Sang, L.; Chen, S.; Nong, L.; Xu, Y.; Liang, W.; Zheng, H.; Zhou, L.; Sun, H.; He, J.; Liu, X.; et al. The Prevalence, Risk Factors, and Prognosis of Acute Kidney Injury After Lung Transplantation: A Single-Center Cohort Study in China. Transpl. Proc. 2021, 53, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Atchade, E.; Barour, S.; Tran-Dinh, A.; Jean-Baptiste, S.; Tanaka, S.; Tashk, P.; Snauwaert, A.; Lortat-Jacob, B.; Mourin, G.; Mordant, P.; et al. Acute Kidney Injury After Lung Transplantation: Perioperative Risk Factors and Outcome. Transpl. Proc. 2020, 52, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; Chen, W.; Zhao, L.; Guo, L.; Liang, C.; Chen, J.; Wang, C. Acute kidney injury following adult lung transplantation. Chin. Med. J. 2022, 135, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Du, W.W.; Wang, X.X.; Zhang, D.; Chen, W.Q.; Zhang, X.L.; Li, P.M. Retrospective analysis on incidence and risk factors of early onset acute kidney injury after lung transplantation and its association with mortality. Ren. Fail. 2021, 43, 535–542. [Google Scholar] [CrossRef]
- Shashaty, M.G.S.; Forker, C.M.; Miano, T.A.; Wu, Q.; Yang, W.; Oyster, M.L.; Porteous, M.K.; Cantu, E.E.; Diamond, J.M.; Christie, J.D. The association of post–lung transplant acute kidney injury with mortality is independent of primary graft dysfunction: A cohort study. Clin. Transpl. 2019, 33, e13678. [Google Scholar] [CrossRef]
- Stephany, B.R.; Boumitri, M.; Budev, M.; Alao, B.; Poggio, E.D. Absence of Proteinuria Predicts Improvement in Renal Function After Conversion to Sirolimus-based Immunosuppressive Regimens in Lung Transplant Survivors with Chronic Kidney Disease. J. Heart Lung Transpl. 2009, 28, 564–571. [Google Scholar] [CrossRef]
- Cassuto, J.R.; Levine, M.H.; Reese, P.P.; Bloom, R.D.; Goral, S.; Naji, A.; Abt, P.L. The Influence of Induction Therapy for Kidney Transplantation after a Non-Renal Transplant. Clin. J. Am. Soc. Nephrol. 2012, 7, 158–166. [Google Scholar] [CrossRef]
- Högerle, B.A.; Kohli, N.; Habibi-Parker, K.; Lyster, H.; Reed, A.; Carby, M.; Zeriouh, M.; Weymann, A.; Simon, A.R.; Sabashnikov, A.; et al. Challenging immunosuppression treatment in lung transplant recipients with kidney failure. Transpl. Immunol. 2016, 35, 18–22. [Google Scholar] [CrossRef]
- Miano, T.A.; Flesch, J.D.; Feng, R.; Forker, C.M.; Brown, M.; Oyster, M.; Kalman, L.; Rushefski, M.; Cantu, E., III; Porteus, M.; et al. Early Tacrolimus Concentrations After Lung Transplant Are Predicted by Combined Clinical and Genetic Factors and Associated with Acute Kidney Injury. Clin. Pharmacol. Ther. 2020, 107, 462–470. [Google Scholar] [CrossRef]
- López-Ibor, J.V.; Citores, M.J.; Portoles, J.; Gomez-Bueno, M.; Sanchez Sobrino, B.; Muñoz, A.; Cuervas-Mons, V.; Segovia-Cubero, J. Role of TGF-β1 +869T>C polymorphism in renal dysfunction one year after heart transplantation. J. Heart Lung Transpl. 2022, 41, 1672–1678. [Google Scholar] [CrossRef]
- Bloom, R.D.; Doyle, A.M. Kidney disease after heart and lung transplantation. Am. J. Transpl. 2006, 6, 671–679. [Google Scholar] [CrossRef]
- Portoles, J.; Huerta, A.; Arjona, E.; Gavela, E.; Agüera, M.; Jiménez, C.; Cavero, T.; Marrero, D.; de Córdoba, S.R.; Diekmann, F.; et al. Characteristics, management and outcomes of atypical haemolytic uraemic syndrome in kidney transplant patients: A retrospective national study. Clin. Kidney J. 2021, 14, 1173–1180. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Salazar, M.; Medina-Zahonero, L.; Janeiro-Marín, D.; Contreras-Lorenzo, C.; Aguilar-Perez, M.; Sanchez-Sobrino, B.; López-Sánchez, P.; Ussetti-Gil, P.; Portoles-Perez, J. Kidney Transplantation in Patients With Chronic Kidney Disease After a Previous Lung Transplantation. Transpl. Proc. 2019, 51, 324–327. [Google Scholar] [CrossRef]
- Lonze, B.E.; Warren, D.S.; Stewart, Z.A.; Dagher, N.N.; Singer, A.L.; Shah, A.S.; Montgomery, R.A.; Segev, D.L. Kidney transplantation in previous heart or lung recipients. Am. J. Transpl. 2009, 9, 578–585. [Google Scholar] [CrossRef]
- Srinivas, T.R.; Stephany, B.R.; Budev, M.; Mason, D.P.; Starling, R.C.; Miller, C.; Goldfarb, D.A.; Flechner, S.M.; Poggio, E.D.; Schold, J.D. An Emerging Population Kidney Transplant Candidates Who Are Placed on the Waiting List after Liver, Heart, and Lung Transplantation. Clin. J. Am. Soc. Nephrol. 2010, 5, 1881–1886. [Google Scholar]
- Cassuto, J.R.; Reese, P.P.; Sonnad, S.; Bloom, R.D.; Levine, M.H.; Olthoff, K.M.; Shaked, A.; Naji, A.; Abt, P. Wait list death and survival benefit of kidney transplantation among nonrenal transplant recipients. Am. J. Transpl. 2010, 10, 2502–2511. [Google Scholar] [CrossRef]
- Otani, S.; Levvey, B.J.; Westall, G.P.; Paraskeva, M.; Whitford, H.; Williams, T.; McGiffin, D.C.; Walker, R.; Menahem, S.; Snell, G.I. Long-term successful outcomes from kidney transplantation after lung and heart-lung transplantation. Ann. Thorac. Surg. 2015, 99, 1032–1038. [Google Scholar] [CrossRef]
- Buffet, A.; Guillouët, S.; Lobbedez, T.; Ficheux, M.; Lanot, A.; Béchade, C. Safety of peritoneal dialysis after nonrenal solid-organ transplantation. Perit. Dial. Int. 2018, 38, 37–43. [Google Scholar] [CrossRef]
- El-Husseini, A.; Aghil, A.; Ramirez, J.; Sawaya, B.; Rajagopalan, N.; Baz, M.; Mei, X.; Davenport, D.L.; Gedaly, R. Outcome of kidney transplant in primary, repeat, and kidney-after-nonrenal solid-organ transplantation: 15-year analysis of recent UNOS database. Clin. Transpl. 2017, 31, e13108. [Google Scholar] [CrossRef]
- Osho, A.A.; Hirji, S.A.; Castleberry, A.W.; Mulvihill, M.S.; Ganapathi, A.M.; Speicher, P.J.; Yerokun, B.; Snyder, L.D.; Davis, R.D.; Hartwig, M.G. Long-term survival following kidney transplantation in previous lung transplant recipients—An analysis of the UNOS registry. Clin. Transpl. 2017, 31, e12953. [Google Scholar] [CrossRef]


| Total | COPD | CF | ILD | Others | p Value | |
|---|---|---|---|---|---|---|
| N | 80 | 27 | 23 | 26 | 4 | |
| Previous Lung Tx | ||||||
| Age (years) | 49.7 (15.9) | 59.9 (5.1) | 28.0 (10.9) | 56.9 (7.8) | 54.5 (9.3) | <0.001 b |
| Male (%) | 56.3 | 66.7 | 47.8 | 57.7 | 25 | 0.3 c |
| Former smokers | 60.8 | 100 | 4.4 | 0.68 | 75 | <0.001 c |
| Lung transplant | ||||||
| Bipulmonary (%) | 81 | 77.8 | 95.7 | 65.4 | 100 | 0.02 c |
| Lung disease (%) | - | 33.8 | 28.8 | 28.8 | 5 | - |
| Retransplant (n) | 5 | 0 | 4 | 1 | 0 | - |
| eGFR (mL/min/1.72 m2) | 101.6 (20.1) | 94.7 (17.1) | 122.4 (17.5) | 93.3 (13.8) | 94.1 (6.1) | <0.001 b |
| Referral to Nephrology | ||||||
| eGFR (mL/min/1.72 m2) | 31.7 (15.5) | 32.6 (16) | 33.2 (16.5) | 30.5 (15.6) | 23.9 (4.7) | 0.7 b |
| Time since LTx | 4.7 [2.7–8.2] | 3.1 [1.9–4.8] | 9.0 [5.7–13.7] | 4.7 [1.8–6.5] | 12.7 [3.2–19.5] | <0.001 a |
| Comorbidities (%) | ||||||
| High blood pressure | 80 | 85.2 | 78.3 | 73.1 | 100 | 0.5 c |
| Diabetes mellitus | 53.8 | 40.7 | 82.6 | 38.5 | 75 | 0.005 c |
| Dyslipidemia | 46.3 | 55.6 | 34.8 | 42.3 | 75 | 0.3 c |
| Previous cardiovascular events | 27.5 | 22.2 | 17.4 | 42.3 | 25 | 0.2 c |
| UACR at consultation (mg/g) | 41.7 [8.1–249.7] | 88 [11.4–243] | 44.8 [6.5–559] | 40.6 [2.7–114] | 14.9 [0–376] | 0.7 a |
| UACR stage (%) | 0.2 c | |||||
| A1 | 32.0 | 30.8 | 19.1 | 37.5 | 75.0 | |
| A2 | 36.0 | 46.2 | 28.6 | 37.5 | 0 | |
| A3 | 32.0 | 23.1 | 52.4 | 25.0 | 25.0 | |
| Leukocyturia (%) | 14.7 | 7.7 | 23.8 | 12.5 | 25.0 | 0.4 c |
| Microhematuria (%) | 26.7 | 19.2 | 42.9 | 25.0 | 0 | 0.2 c |
| KF loss 1st year (%) | −50.1 [36.3–59.2] | −52.6 [37.0–59.08] | −56.5 [37.2–64.4] | −48.9 [36.3–55.8] | −49.0 [0.1–65.1] | 0.57 a |
| KF loss 2nd year (%) | −9.9 [12.2 to−25.5] | −9.6 [12.2 to −25.4] | −0.7 [16.7 to −21.2] | −14.4 [8.8 to −27.1] | −25.1 [−57.1 to −4.6] | 0.4 a |
| Time since LTx until RRT (years) | 12.7 [8.1–17.0] | 7.8 | 12 | 13 | 21.3 | 0.2 d |
| Clinical Outcomes | ||||||
| Dialysis (%) | 43.8 | 44.4 | 56.5 | 34.6 | 25 | 0.44 c |
| Death (%) | 32.5 | 33.3 | 21.7 | 38.5 | 50 | 0.53 c |
| Dialysis or death (%) | 53.8 | 55.6 | 56.5 | 50 | 50 | 0.96 c |
| Total | Non-Biopsy | Biopsy | p Value | |
|---|---|---|---|---|
| N | 80 | 63 | 17 | |
| Previous LTx | ||||
| High blood pressure (%) | 80 | 82.5 | 70.6 | 0.3 |
| Diabetes mellitus (%) | 53.8 | 50.8 | 64.7 | 0.3 |
| eGFR (mL/min/1.72 m2) | 101.6 (20.1) | 101.8 (30.0) | 99.1 (27.3) | 0.8 |
| Referral to Nephrology | ||||
| eGFR (mL/min/1.72 m2) | 31.7 (15.5) | 32.2 (14.5) | 29.8 (19.5) | 0.6 |
| Time since LTx | 4.7 [2.7–8.2] | |||
| UACR at consultation (mg/g) | 41.7 [8.1–249.7] | 25 [6.5–199] | 207.5 [41.25–541.5] | 0.07 |
| UACR stage (%) | 0.006 | |||
| A1 | 32.0 | 39.0 | 6.3 | |
| A2 | 36.0 | 37.3 | 31.3 | |
| A3 | 32.0 | 23.7 | 62.5 | |
| Time since LTx until RRT (years) | 12.7 [8.1–17.0] | 15.2 [8.1–21.3] | 10.1 [5.4–12.7] | 0.03 |
| UNIVARIATE | MULTIVARIATE | |||||||
|---|---|---|---|---|---|---|---|---|
| sHR | p Value | CI 95% | sHR | p Value | CI 95% | |||
| Demographics | ||||||||
| CV | 1.24 | 0.57 | 0.60 | 2.58 | ||||
| DM | 1.22 | 0.58 | 0.61 | 2.44 | ||||
| Female | 1.12 | 0.74 | 0.59 | 2.12 | ||||
| Kidney disease | ||||||||
| Other | 1.00 | |||||||
| CNI toxicity | 0.74 | 0.371 | 0.38 | 1.44 | ||||
| Lung disease | ||||||||
| COPD | 1.00 | 1.00 | ||||||
| Cystic fibrosis | 1.51 | 0.31 | 0.69 | 3.32 | 1.56 | 0.27 | 0.70 | 3.46 |
| ILD | 1.68 | 0.25 | 0.70 | 4.06 | 1.53 | 0.35 | 0.63 | 3.74 |
| Others | 4.26 | 0.047 | 1.02 | 17.77 | 7.73 | <0.001 | 3.27 | 18.25 |
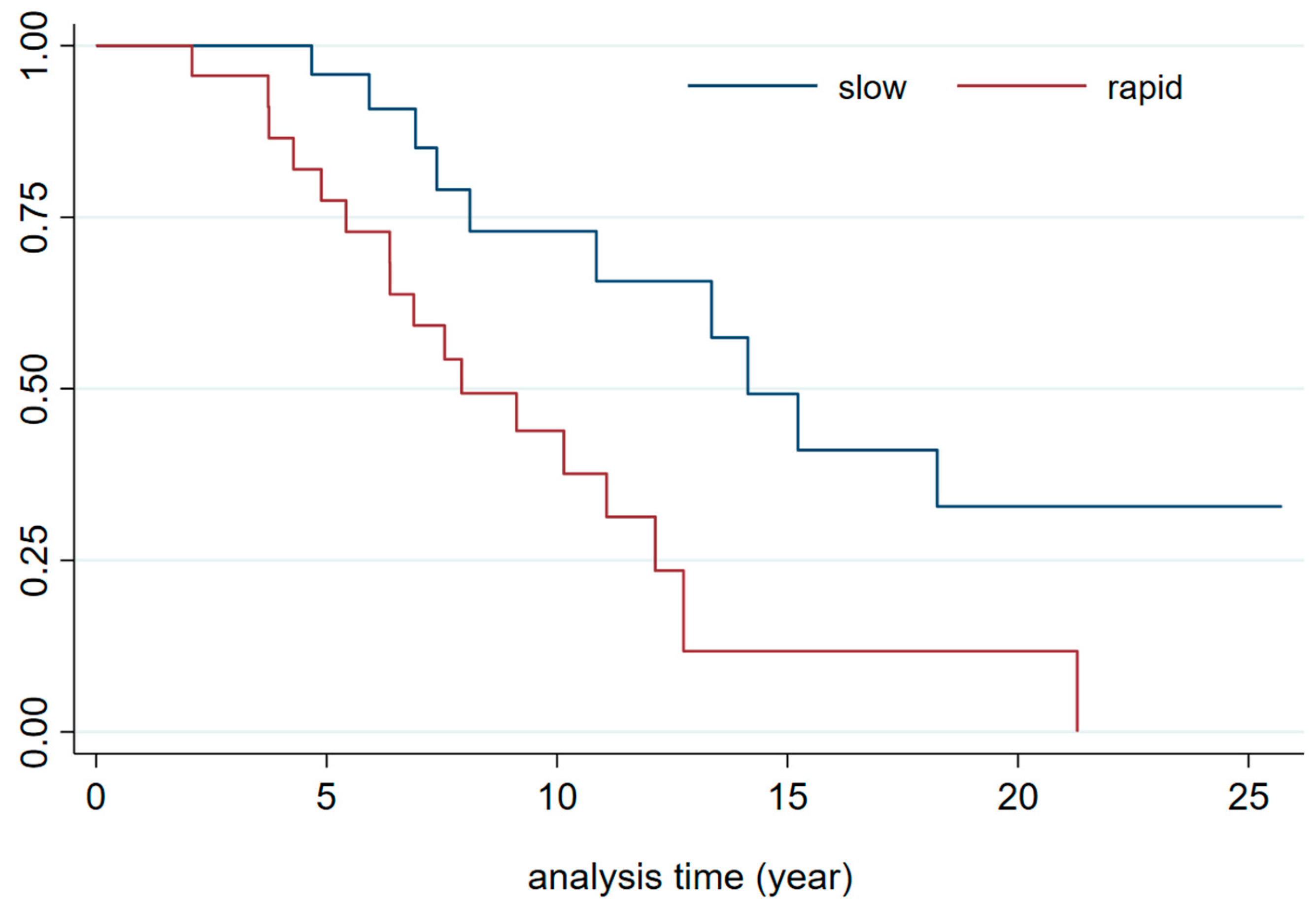
| CKD progression | ||||||||
| Slow progressors | 1.00 | 1.0 | ||||||
| medium | 1.22 | 0.66 | 0.49 | 3.05 | 1.21 | 0.70 | 0.46 | 3.20 |
| Rapid progressors | 3.40 | 0.002 | 1.57 | 7.39 | 4.29 | 0.001 | 1.89 | 9.75 |
| Albuminuria | ||||||||
| A1 (<30 mg/g) | 1.00 | |||||||
| A2 (30–300 mg/g) | 1.97 | 0.6 | 6.45 | |||||
| A3 (>300 mg/g) | 2.29 | 0.737 | 7.084 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serrano Salazar, M.L.; Almonacid, C.; Marques Vidas, M.; López-Sánchez, P.; Sánchez Sobrino, B.; Aguilar, M.; Rubio Arboli, L.; Martínez Morales, E.; Huerta, A.; Valdenebro Recio, M.; et al. Chronic Kidney Disease After Lung Transplantation in Spain: A Retrospective Single-Center Analysis. J. Clin. Med. 2025, 14, 2241. https://doi.org/10.3390/jcm14072241
Serrano Salazar ML, Almonacid C, Marques Vidas M, López-Sánchez P, Sánchez Sobrino B, Aguilar M, Rubio Arboli L, Martínez Morales E, Huerta A, Valdenebro Recio M, et al. Chronic Kidney Disease After Lung Transplantation in Spain: A Retrospective Single-Center Analysis. Journal of Clinical Medicine. 2025; 14(7):2241. https://doi.org/10.3390/jcm14072241
Chicago/Turabian StyleSerrano Salazar, Maria Luisa, Carlos Almonacid, Maria Marques Vidas, Paula López-Sánchez, Beatriz Sánchez Sobrino, Myriam Aguilar, Lucia Rubio Arboli, Eduardo Martínez Morales, Ana Huerta, Maria Valdenebro Recio, and et al. 2025. "Chronic Kidney Disease After Lung Transplantation in Spain: A Retrospective Single-Center Analysis" Journal of Clinical Medicine 14, no. 7: 2241. https://doi.org/10.3390/jcm14072241
APA StyleSerrano Salazar, M. L., Almonacid, C., Marques Vidas, M., López-Sánchez, P., Sánchez Sobrino, B., Aguilar, M., Rubio Arboli, L., Martínez Morales, E., Huerta, A., Valdenebro Recio, M., Ussetti, P., & Portoles, J. (2025). Chronic Kidney Disease After Lung Transplantation in Spain: A Retrospective Single-Center Analysis. Journal of Clinical Medicine, 14(7), 2241. https://doi.org/10.3390/jcm14072241






