The Relation of Angiotensin-Converting Enzyme 2, Renin-Angiotensin-Aldosterone System Inhibitors, and Arterial Stiffness in Acute COVID-19 Emergency Department Patients—A Prospective Observational Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Objectives
- What effect does COVID-19 have on sACE2 levels?
- What effect does RAASi therapy have on sACE2 levels in COVID-19 patients compared to non-COVID-19 patients?
- What effect does upregulated sACE2 have on arterial stiffness, measured via PWV, in acutely ill COVID-19 patients?
2.2. Study Population and Data Acquisition
2.3. Statistical Analysis
3. Results
4. Discussion
4.1. sACE2 Levels in COVID-19
4.2. RAASi and sACE2 in COVID-19
4.3. Arterial Stiffness
4.4. Research Outlook
4.5. Relevance for Medical Practice
4.6. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organisation. WHO COVID-19 Dashboard. COVID-19 Cases|WHO COVID-19 Dashboard. Available online: https://data.who.int/dashboards/covid19/cases (accessed on 24 August 2024).
- Li, W.; Moore, M.J.; Vasilieva, N.; Sui, J.; Wong, S.K.; Berne, M.A.; Somasundaran, M.; Sullivan, J.L.; Luzuriaga, K.; Greenough, T.C.; et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003, 426, 450–454. [Google Scholar] [PubMed]
- Lopes, R.D.; Macedo, A.V.S.; de Barros ESilva, P.G.M.; Moll-Bernardes, R.J.; Feldman, A.; D’Andréa Saba Arruda, G.; de Souza, A.S.; de Albuquerque, D.C.; Mazza, L. Continuing versus suspending angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: Impact on adverse outcomes in hospitalized patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)--The BRACE CORONA Trial. Am. Heart J. 2020, 226, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Letko, M.; Marzi, A.; Munster, V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat. Microbiol. 2020, 5, 562–569. [Google Scholar] [CrossRef]
- Tai, W.; He, L.; Zhang, X.; Pu, J.; Voronin, D.; Jiang, S.; Zhou, Y.; Du, L. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: Implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell Mol Immunol. 2020, 17, 613–620. [Google Scholar]
- Wrapp, D.; Wang, N.; Corbett, K.S.; Goldsmith, J.A.; Hsieh, C.L.; Abiona, O.; Graham, B.S.; McLellan, J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020, 367, 1260–1263. [Google Scholar]
- Beyerstedt, S.; Casaro, E.B.; Rangel, É.B. COVID-19: Angiotensin-converting enzyme 2 (ACE2) expression and tissue susceptibility to SARS-CoV-2 infection. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 905–919. [Google Scholar] [PubMed]
- Gustafson, D.; Raju, S.; Wu, R.; Ching, C.; Veitch, S.; Rathnakumar, K.; Boudreau, E.; Howe, K.L.; Fish, J.E. Overcoming Barriers: The Endothelium As a Linchpin of Coronavirus Disease 2019 Pathogenesis? Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1818–1829. [Google Scholar]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar]
- Renu, K.; Prasanna, P.L.; Valsala Gopalakrishnan, A. Coronaviruses pathogenesis, comorbidities and multi-organ damage—A review. Life Sci. 2020, 255, 117839. [Google Scholar] [CrossRef]
- Long, B.; Brady, W.J.; Koyfman, A.; Gottlieb, M. Cardiovascular complications in COVID-19. Am. J. Emerg. Med. 2020, 38, 1504–1507. [Google Scholar]
- Goshua, G.; Pine, A.B.; Meizlish, M.L.; Chang, C.-H.; Zhang, H.; Bahel, P.; Baluha, A.; Bar, N.; Bona, R.D.; Burns, A.J.; et al. Endotheliopathy in COVID-19-associated coagulopathy: Evidence from a single-centre, cross-sectional study. Lancet Haematol. 2020, 7, e575–e582. [Google Scholar] [CrossRef]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef] [PubMed]
- Vlachopoulos, C.; Dima, I.; Aznaouridis, K.; Vasiliadou, C.; Ioakeimidis, N.; Aggeli, C.; Toutouza, M.; Stefanadis, C. Acute systemic inflammation increases arterial stiffness and decreases wave reflections in healthy individuals. Circulation 2005, 112, 2193–2200. [Google Scholar] [CrossRef]
- Saeed, S.; Mancia, G. Arterial stiffness and COVID-19: A bidirectional cause-effect relationship. J. Clin. Hypertens. 2021, 23, 1099–1103. [Google Scholar] [CrossRef]
- Iwai, M.; Horiuchi, M. Devil and angel in the renin-angiotensin system: ACE-angiotensin II-AT1 receptor axis vs. ACE2-angiotensin-(1-7)-Mas receptor axis. Hypertens. Res. 2009, 32, 533–536. [Google Scholar] [CrossRef]
- Silhol, F.; Sarlon, G.; Deharo, J.C.; Vaïsse, B. Downregulation of ACE2 induces overstimulation of the renin-angiotensin system in COVID-19: Should we block the renin-angiotensin system? Hypertens Res. 2020, 43, 854–856. [Google Scholar] [CrossRef] [PubMed]
- Murray, E.; Tomaszewski, M.; Guzik, T.J. Binding of SARS-CoV-2 and angiotensin-converting enzyme 2: Clinical implications. Cardiovasc. Res. 2020, 116, e87–e89. [Google Scholar] [CrossRef] [PubMed]
- Tipnis, S.R.; Hooper, N.M.; Hyde, R.; Karran, E.; Christie, G.; Turner, A.J. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J. Biol. Chem. 2000, 275, 33238–33243. [Google Scholar] [CrossRef]
- Douglas, G.C.; O’bryan, M.K.; Hedger, M.P.; Lee, D.K.L.; Yarski, M.A.; Smith, A.I.; Lew, R.A. The novel angiotensin-converting enzyme (ACE) homolog, ACE2, is selectively expressed by adult Leydig cells of the testis. Endocrinology 2004, 145, 4703–4711. [Google Scholar] [CrossRef]
- Tikellis, C.; Bernardi, S.; Burns, W.C. Angiotensin-converting enzyme 2 is a key modulator of the renin-angiotensin system in cardiovascular and renal disease. Curr. Opin. Nephrol. Hypertens 2011, 20, 62–68. [Google Scholar] [CrossRef]
- Kuba, K.; Imai, Y.; Rao, S.; Gao, H.; Guo, F.; Guan, B.; Huan, Y.; Yang, P.; Zhang, Y.; Deng, W.; et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus–induced lung injury. Nat. Med. 2005, 11, 875–879. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Yan, Y.; Shu, Y.; Gao, R.; Sun, Y.; Li, X.; Ju, X.; Liang, Z.; Liu, Q.; Zhao, Y.; et al. Angiotensin-converting enzyme 2 protects from lethal avian influenza A H5N1 infections. Nat. Commun. 2014, 5, 3594. [Google Scholar]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [PubMed]
- Danser, A.H.J.; Epstein, M.; Batlle, D. Renin-Angiotensin System Blockers and the COVID-19 Pandemic: At Present There Is No Evidence to Abandon Renin-Angiotensin System Blockers. Hypertension 2020, 75, 1382–1385. [Google Scholar] [PubMed]
- Fang, L.; Karakiulakis, G.; Roth, M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir. Med. 2020, 8, e21. [Google Scholar] [CrossRef]
- Wenzel, U.O.; Kintscher, U. ACE2 and SARS-CoV-2: Tissue or Plasma, Good or Bad? Am. J. Hypertens. 2021, 34, 274–277. [Google Scholar]
- Lopes, R.D.; Macedo, A.V.S.; de Barros ESilva, P.G.M.; Moll-Bernardes, R.J.; Dos Santos, T.M.; Mazza, L.; Feldman, A. Effect of Discontinuing vs Continuing Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers on Days Alive and Out of the Hospital in Patients Admitted With COVID-19: A Randomized Clinical Trial. JAMA 2021, 325, 254–264. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Chen, J.; Zhang, H.; Deng, A. Association of Renin-Angiotensin System Inhibitors With Severity or Risk of Death in Patients With Hypertension Hospitalized for Coronavirus Disease 2019 (COVID-19) Infection in Wuhan, China. JAMA Cardiol. 2020, 5, 825–830. [Google Scholar]
- Zhang, P.; Zhu, L.; Cai, J.; Lei, F.; Qin, J.-J.; Xie, J.; Liu, Y.-M.; Zhao, Y.-C.; Huang, X.; Lin, L.; et al. Association of Inpatient Use of Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers With Mortality Among Patients With Hypertension Hospitalized With COVID-19. Circ. Res. 2020, 126, 1671–1681. [Google Scholar] [CrossRef]
- Karthika, T.; Joseph, J.; Das, V.R.A.; Nair, N.; Charulekha, P.; Roji, M.D.; Raj, V.S. SARS-CoV-2 Cellular Entry Is Independent of the ACE2 Cytoplasmic Domain Signaling. Cells 2021, 10, 1814. [Google Scholar] [CrossRef]
- Yeung, M.L.; Teng, J.L.L.; Jia, L.; Zhang, C.; Huang, C.; Cai, J.-P.; Zhou, R.; Chan, K.-H.; Zhao, H.; Zhu, L.; et al. Soluble ACE2-mediated cell entry of SARS-CoV-2 via interaction with proteins related to the renin-angiotensin system. Cell 2023, 186, 5428–5432. [Google Scholar]
- Kintscher, U.; Slagman, A.; Domenig, O.; Röhle, R.; Konietschke, F.; Poglitsch, M.; Möckel, M. Plasma Angiotensin Peptide Profiling and ACE (Angiotensin-Converting Enzyme)-2 Activity in COVID-19 Patients Treated With Pharmacological Blockers of the Renin-Angiotensin System. Hypertension 2020, 76, e34–e36. [Google Scholar]
- Vaduganathan, M.; Vardeny, O.; Michel, T.; McMurray, J.J.V.; Pfeffer, M.A.; Solomon, S.D. Renin-Angiotensin-Aldosterone System Inhibitors in Patients with Covid-19. N. Engl. J. Med. 2020, 382, 1653–1659. [Google Scholar] [CrossRef] [PubMed]
- Schmieder, R.E.; Hilgers, K.F.; Schlaich, M.P.; Schmidt, B.M.W. Renin-angiotensin system and cardiovascular risk. Lancet 2007, 369, 1208–1219. [Google Scholar] [CrossRef]
- Guy, J.L.; Lambert, D.W.; Warner, F.J.; Hooper, N.M.; Turner, A.J. Membrane-associated zinc peptidase families: Comparing ACE and ACE2. Biochim. Biophys. Acta 2005, 1751, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, H.; An, Y. ACE2 Shedding and the Role in COVID-19. Front. Cell Infect. Microbiol. 2021, 11, 789180. [Google Scholar]
- Lambert, D.W.; Yarski, M.; Warner, F.J.; Thornhill, P.; Parkin, E.T.; Smith, A.I.; Hooper, N.M.; Turner, A.J. Tumor necrosis factor-alpha convertase (ADAM17) mediates regulated ectodomain shedding of the severe-acute respiratory syndrome-coronavirus (SARS-CoV) receptor, angiotensin-converting enzyme-2 (ACE2). J. Biol. Chem. 2005, 280, 30113–30119. [Google Scholar] [PubMed]
- Chan, K.K.; Dorosky, D.; Sharma, P.; Abbasi, S.A.; Dye, J.M.; Kranz, D.M.; Herbert, A.S.; Procko, E. Engineering human ACE2 to optimize binding to the spike protein of SARS coronavirus 2. Science 2020, 369, 1261–1265. [Google Scholar]
- Chan, K.K.; Tan, T.J.C.; Narayanan, K.K.; Procko, E. An engineered decoy receptor for SARS-CoV-2 broadly binds protein S sequence variants. Sci. Adv. 2021, 7, eabf1738. [Google Scholar] [CrossRef]
- Monteil, V.; Kwon, H.; Prado, P.; Hagelkrüys, A.; Wimmer, R.A.; Stahl, M.; Leopoldi, A.; Garreta, E.; Del Pozo, C.H.; Prosper, F.; et al. Inhibition of SARS-CoV-2 Infections in Engineered Human Tissues Using Clinical-Grade Soluble Human ACE2. Cell. 2020, 181, 905–913.e7. [Google Scholar]
- Batlle, D.; Wysocki, J.; Satchell, K. Soluble angiotensin-converting enzyme 2: A potential approach for coronavirus infection therapy? Clin. Sci. 2020, 134, 543–545. [Google Scholar]
- Kornilov, S.A.; Lucas, I.; Jade, K.; Dai, C.L.; Lovejoy, J.C.; Magis, A.T. Plasma levels of soluble ACE2are associated with sex, Metabolic Syndrome, and its biomarkers in a large cohort, pointing to a possible mechanism for increased severity in COVID-19. Crit. Care 2020, 24, 452. [Google Scholar] [PubMed]
- Schnaubelt, S.; Oppenauer, J.; Tihanyi, D.; Mueller, M.; Maldonado-Gonzalez, E.; Zejnilovic, S.; Haslacher, H.; Perkmann, T.; Strassl, R.; Anders, S.; et al. Arterial stiffness in acute COVID-19 and potential associations with clinical outcome. J. Intern. Med. 2021.
- den Ruijter, H.M.; Haitjema, S.; Asselbergs, F.W.; Pasterkamp, G. Sex matters to the heart: A special issue dedicated to the impact of sex related differences of cardiovascular diseases. Atherosclerosis 2015, 241, 205–207. [Google Scholar]
- Brown, N.J.; Vaughan, D.E. Angiotensin-converting enzyme inhibitors. Circulation 1998, 97, 1411–1420. [Google Scholar] [CrossRef]
| COVID-19 (N = 103) | Non-COVID-19 (N = 49) | p-Value | |
|---|---|---|---|
| ABI [IQR] | 1.08 [0.99–1.14] | 1.0 [0.96–1.10] | 0.03 |
| baPWV, m/s; [IQR] | 14.9 [13.2–18.6] | 15.1 [12.4–19.5] | 0.83 |
| baPWV ≥ 16, m/s; x of N (%) | 31 of 87 (36%) | 22 of 48 (46%) | 0.33 |
| cfPWV, m/s; [IQR] | 10.1 [8.7–12.6] | 9.6 [7.8–13.1] | 0.77 |
| cfPWV ≥ 10, m/s; x of N (%) | 44 of 83 (53%) | 21 of 43 (49%) | 0.80 |
| sACE2, ng/mL; [IQR] | 0.485 [0.364–1.329] | 0.458 [0.356–1.138] | 0.70 |
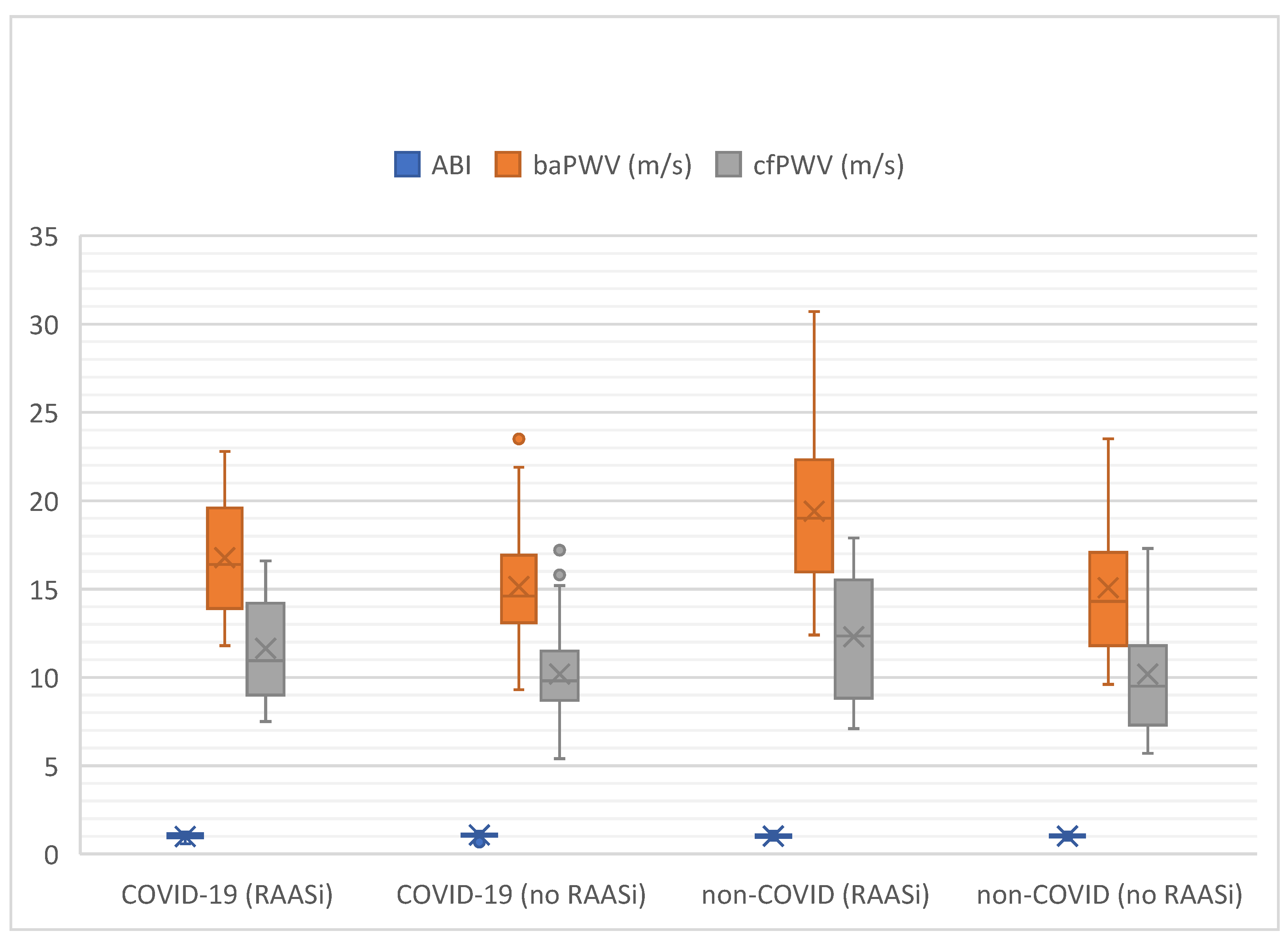
| COVID-19 with RAASi (N = 32) | COVID-19 Without RAASi (N = 71) | p-Value | Non-COVID-19 with RAASi (N = 12) | Non-COVID-19 Without RAASi (N = 37) | p-Value | |
|---|---|---|---|---|---|---|
| ABI [IQR] | 1.06 [0.91–1.15] | 1.09 [1.01–1.13] | 0.13 | 1.01 [0.94–1.10] | 1.00 [0.96–1.10] | 0.95 |
| baPWV, m/s [IQR] | 16.4 [13.9–19.6] | 14.6 [13.1–16.9] | 0.06 | 19.0 [16.0–22.3] | 14.3 [11.8–17.1] | 0.01 |
| baPWV ≥ 16, m/s x of N (%) | 12 von 23 (52%) | 19 of 64 (30%) | 0.09 | 75% (9 of 12) | 36% (13 of 36) | 0.044 |
| cfPWV, m/s [IQR] | 11.0 [9.0–14.2] | 10.2 [8.7–11.5] | 0.06 | 12.4 [8.8–15.5] | 9.5 [7.3–11.8] | 0.15 |
| cfPWV ≥ 10, m/s x of N (%) | 15 von 22 (68%) | 29 of 61 (48%) | 0.16 | 60% (6 of 10) | 45% (15 of 33) | 0.66 |
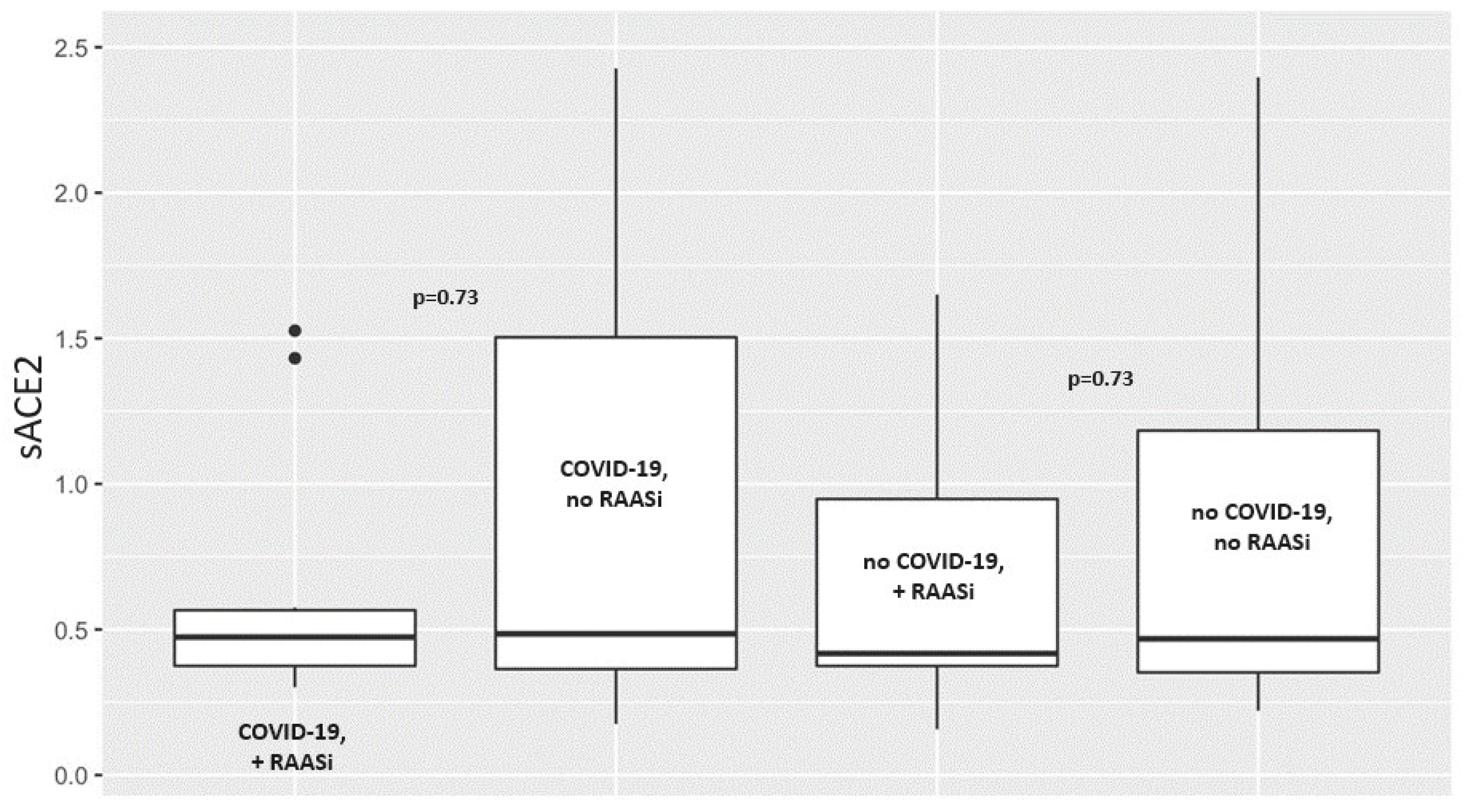
| sACE2, ng/mL [IQR] | 0.474 [0.375–0.566] | 0.485 [0.364–1.504] | 0.73 | 0.417 [0.375–0.948] | 0.468 [0.353–1.183] | 0.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schnaubelt, S.; Jakobljevich, A.; Brock, R.; Oppenauer, J.; Kornfehl, A.; Eibensteiner, F.; Veigl, C.; Perkmann, T.; Haslacher, H.; Strassl, R.; et al. The Relation of Angiotensin-Converting Enzyme 2, Renin-Angiotensin-Aldosterone System Inhibitors, and Arterial Stiffness in Acute COVID-19 Emergency Department Patients—A Prospective Observational Study. J. Clin. Med. 2025, 14, 2233. https://doi.org/10.3390/jcm14072233
Schnaubelt S, Jakobljevich A, Brock R, Oppenauer J, Kornfehl A, Eibensteiner F, Veigl C, Perkmann T, Haslacher H, Strassl R, et al. The Relation of Angiotensin-Converting Enzyme 2, Renin-Angiotensin-Aldosterone System Inhibitors, and Arterial Stiffness in Acute COVID-19 Emergency Department Patients—A Prospective Observational Study. Journal of Clinical Medicine. 2025; 14(7):2233. https://doi.org/10.3390/jcm14072233
Chicago/Turabian StyleSchnaubelt, Sebastian, Anna Jakobljevich, Roman Brock, Julia Oppenauer, Andrea Kornfehl, Felix Eibensteiner, Christoph Veigl, Thomas Perkmann, Helmuth Haslacher, Robert Strassl, and et al. 2025. "The Relation of Angiotensin-Converting Enzyme 2, Renin-Angiotensin-Aldosterone System Inhibitors, and Arterial Stiffness in Acute COVID-19 Emergency Department Patients—A Prospective Observational Study" Journal of Clinical Medicine 14, no. 7: 2233. https://doi.org/10.3390/jcm14072233
APA StyleSchnaubelt, S., Jakobljevich, A., Brock, R., Oppenauer, J., Kornfehl, A., Eibensteiner, F., Veigl, C., Perkmann, T., Haslacher, H., Strassl, R., Reindl-Schwaighofer, R., Schlager, O., & Sulzgruber, P. (2025). The Relation of Angiotensin-Converting Enzyme 2, Renin-Angiotensin-Aldosterone System Inhibitors, and Arterial Stiffness in Acute COVID-19 Emergency Department Patients—A Prospective Observational Study. Journal of Clinical Medicine, 14(7), 2233. https://doi.org/10.3390/jcm14072233





