Prognostic Value of the C-PLAN Index in Metastatic Renal Cell Carcinoma Treated with Nivolumab
Abstract
1. Introduction
2. Materials and Methods
2.1. Statistical Analysis
2.2. C-PLAN Index Calculation
2.3. Outcome Definitions
3. Results
3.1. Patient Characteristics in the C-PLAN Good and Poor Groups
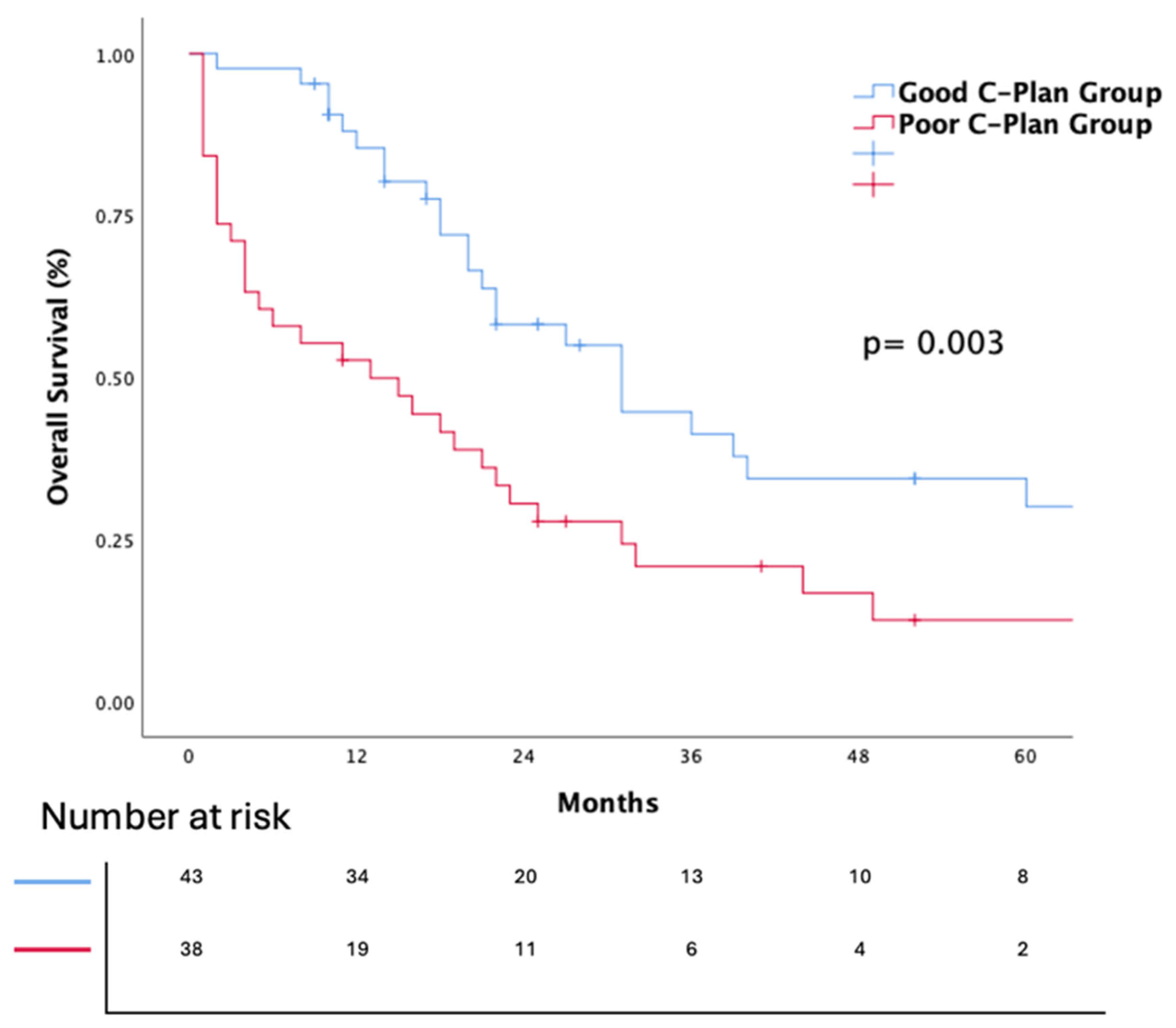
3.2. Survival Analiyses
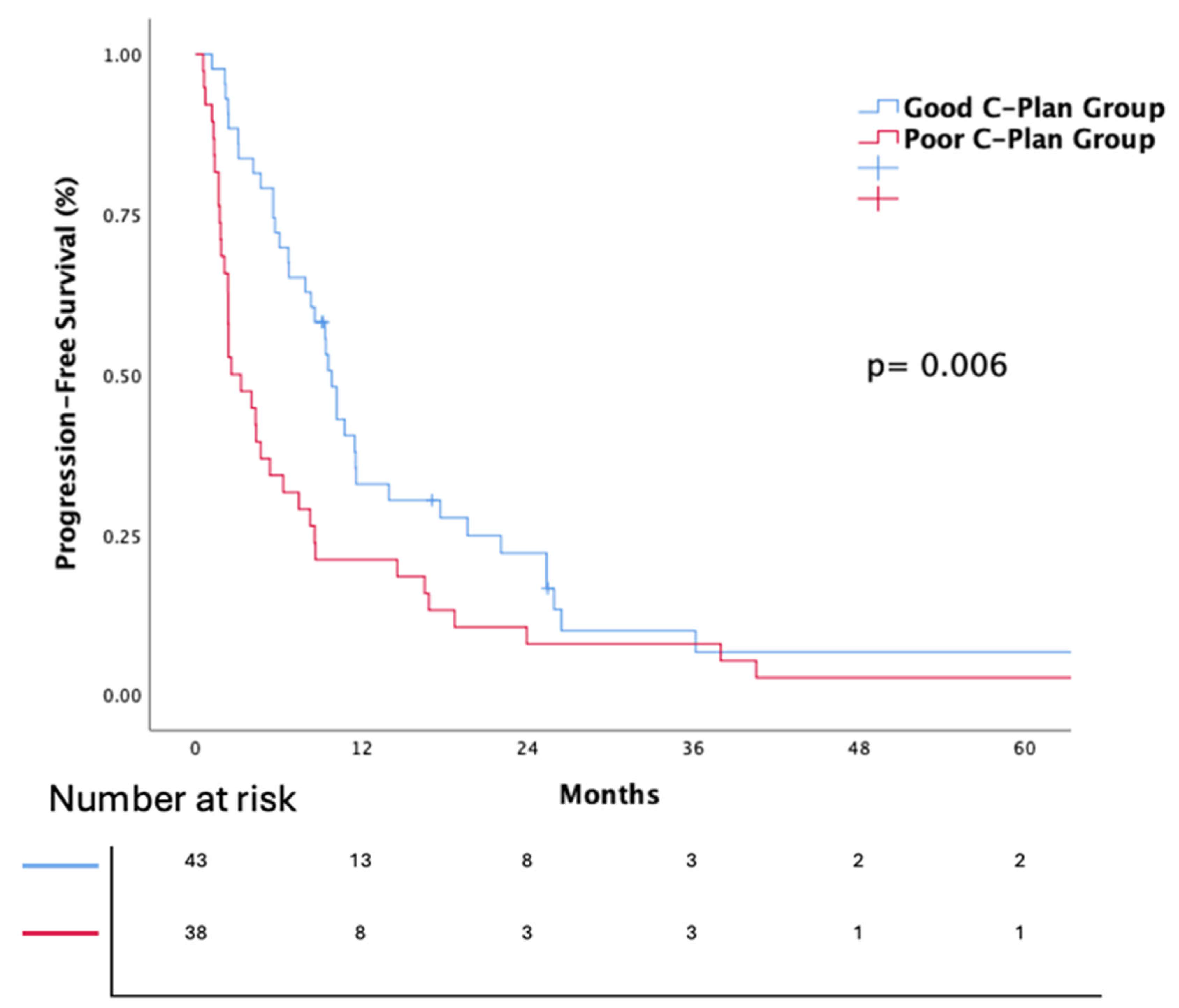
3.3. Efficacy of Nivolumab
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Global Breast Cancer Initiative Implementation Framework: Assessing, Strengthening and Scaling-Up of Services for the Early Detection and Management of Breast Cancer; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Patard, J.-J. Incidental renal tumours. Curr. Opin. Urol. 2009, 19, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.S.; Leppert, J.T.; Belldegrun, A.S.; Figlin, R.A. Novel approaches in the therapy of metastatic renal cell carcinoma. World J. Urol. 2005, 23, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Mlcochova, H.; Machackova, T.; Rabien, A.; Radova, L.; Fabian, P.; Iliev, R.; Slaba, K.; Poprach, A.; Kilic, E.; Stanik, M. Epithelial-mesenchymal transition-associated microRNA/mRNA signature is linked to metastasis and prognosis in clear-cell renal cell carcinoma. Sci. Rep. 2016, 6, 31852. [Google Scholar] [CrossRef]
- Bhat, S. Role of surgery in advanced/metastatic renal cell carcinoma. Indian J. Urol. 2010, 26, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Rini, B.I.; Bukowski, R.M.; Curti, B.D.; George, D.J.; Hudes, G.R.; Redman, B.G.; Margolin, K.A.; Merchan, J.R.; Wilding, G. Sunitinib in patients with metastatic renal cell carcinoma. JAMA 2006, 295, 2516–2524. [Google Scholar] [CrossRef]
- Motzer, R.J.; Hutson, T.E.; Cella, D.; Reeves, J.; Hawkins, R.; Guo, J.; Nathan, P.; Staehler, M.; de Souza, P.; Merchan, J.R. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N. Engl. J. Med. 2013, 369, 722–731. [Google Scholar] [CrossRef]
- Motzer, R.J.; Escudier, B.; McDermott, D.F.; George, S.; Hammers, H.J.; Srinivas, S.; Tykodi, S.S.; Sosman, J.A.; Procopio, G.; Plimack, E.R. Nivolumab versus everolimus in advanced renal-cell carcinoma. N. Engl. J. Med. 2015, 373, 1803–1813. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Powles, T.; Burotto, M.; Escudier, B.; Bourlon, M.T.; Zurawski, B.; Oyervides Juárez, V.M.; Hsieh, J.J.; Basso, U.; Shah, A.Y. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N. Engl. J. Med. 2021, 384, 829–841. [Google Scholar] [CrossRef]
- Rini, B.I.; Plimack, E.R.; Stus, V.; Gafanov, R.; Hawkins, R.; Nosov, D.; Pouliot, F.; Alekseev, B.; Soulières, D.; Melichar, B. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N. Engl. J. Med. 2019, 380, 1116–1127. [Google Scholar] [CrossRef]
- Ko, J.J.; Xie, W.; Kroeger, N.; Lee, J.-L.; Rini, B.I.; Knox, J.J.; Bjarnason, G.A.; Srinivas, S.; Pal, S.K.; Yuasa, T. The International Metastatic Renal Cell Carcinoma Database Consortium model as a prognostic tool in patients with metastatic renal cell carcinoma previously treated with first-line targeted therapy: A population-based study. Lancet Oncol. 2015, 16, 293–300. [Google Scholar] [CrossRef]
- Dudani, S.; Savard, M.-F.; Heng, D.Y. An update on predictive biomarkers in metastatic renal cell carcinoma. Eur. Urol. Focus 2020, 6, 34–36. [Google Scholar] [CrossRef] [PubMed]
- Heng, D.Y.; Xie, W.; Regan, M.M.; Harshman, L.C.; Bjarnason, G.A.; Vaishampayan, U.N.; Mackenzie, M.; Wood, L.; Donskov, F.; Tan, M.-H. External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: A population-based study. Lancet Oncol. 2013, 14, 141–148. [Google Scholar] [CrossRef]
- Rosellini, M.; Marchetti, A.; Mollica, V.; Rizzo, A.; Santoni, M.; Massari, F. Prognostic and predictive biomarkers for immunotherapy in advanced renal cell carcinoma. Nat. Rev. Urol. 2023, 20, 133–157. [Google Scholar] [CrossRef] [PubMed]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef] [PubMed]
- Iinuma, K.; Enomoto, T.; Kawada, K.; Fujimoto, S.; Ishida, T.; Takagi, K.; Nagai, S.; Ito, H.; Kawase, M.; Nakai, C. Utility of neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and systemic immune inflammation index as prognostic, predictive biomarkers in patients with metastatic renal cell carcinoma treated with nivolumab and ipilimumab. J. Clin. Med. 2021, 10, 5325. [Google Scholar] [CrossRef]
- Yildirim, H.C.; Kus, F.; Guven, D.C.; Karaca, E.; Kaygusuz, Y.; Dizdar, O.; Aksoy, S.; Erman, M.; Yalcin, S.; Kilickap, S. Mean Platelet Volume to Lymphocyte Ratio: A New Biomarker Predicting Response in Patients with Solid Tumors Treated with Nivolumab. J. Immunother. Precis. Oncol. 2023, 6, 170–176. [Google Scholar] [CrossRef]
- Roussel, E.; Kinget, L.; Verbiest, A.; Debruyne, P.R.; Baldewijns, M.; Van Poppel, H.; Albersen, M.; Beuselinck, B. C-reactive protein and neutrophil-lymphocyte ratio are prognostic in metastatic clear-cell renal cell carcinoma patients treated with nivolumab. Urol. Oncol. Semin. Orig. Investig. 2021, 39, 239.e217–239.e225. [Google Scholar] [CrossRef]
- Daneshmandi, S.; Wegiel, B.; Seth, P. Blockade of lactate dehydrogenase-A (LDH-A) improves efficacy of anti-programmed cell death-1 (PD-1) therapy in melanoma. Cancers 2019, 11, 450. [Google Scholar] [CrossRef]
- Guven, D.C.; Sahin, T.K.; Erul, E.; Rizzo, A.; Ricci, A.D.; Aksoy, S.; Yalcin, S. The association between albumin levels and survival in patients treated with immune checkpoint inhibitors: A systematic review and meta-analysis. Front. Mol. Biosci. 2022, 9, 1039121. [Google Scholar] [CrossRef]
- Sonehara, K.; Ozawa, R.; Hama, M.; Nozawa, S.; Agatsuma, T.; Nishie, K.; Kato, A.; Matsuo, A.; Araki, T.; Komatsu, M. C-PLAN index as a prognostic factor for patients with previously untreated advanced non-small cell lung cancer who received combination immunotherapy: A multicenter retrospective study. Thorac. Cancer 2023, 14, 636–642. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, R.; Inamura, K.; Yuasa, T.; Numao, N.; Yamamoto, S.; Masuda, H.; Kawauchi, A.; Takeuchi, K.; Yonese, J. Efficacy and safety profile of nivolumab for Japanese patients with metastatic renal cell cancer. Int. J. Clin. Oncol. 2020, 25, 151–157. [Google Scholar] [CrossRef]
- Rebuzzi, S.E.; Signori, A.; Banna, G.L.; Maruzzo, M.; De Giorgi, U.; Pedrazzoli, P.; Sbrana, A.; Zucali, P.A.; Masini, C.; Naglieri, E. Inflammatory indices and clinical factors in metastatic renal cell carcinoma patients treated with nivolumab: The development of a novel prognostic score (Meet-URO 15 study). Ther. Adv. Med. Oncol. 2021, 13, 17588359211019642. [Google Scholar] [CrossRef] [PubMed]
- Mullally, W.J.; Greene, J.; Jordan, E.J.; Horgan, A.M.; O’Connor, M.; Calvert, P.M. The prognostic value of the derived neutrophil-to-lymphocyte ratio (dNLR) in patients treated with immune checkpoint inhibitors. Ir. J. Med. Sci. (1971-) 2023, 192, 83–87. [Google Scholar] [CrossRef]
- Yekedüz, E.; Tural, D.; Ertürk, İ.; Karakaya, S.; Erol, C.; Ercelep, Ö.; Arslan, Ç.; Sever, Ö.N.; Kılıçkap, S.; Şentürk Öztaş, N. The relationship between pan-immune-inflammation value and survival outcomes in patients with metastatic renal cell carcinoma treated with nivolumab in the second line and beyond: A Turkish oncology group kidney cancer consortium (TKCC) study. J. Cancer Res. Clin. Oncol. 2022, 148, 3537–3546. [Google Scholar] [CrossRef] [PubMed]
- Templeton, A.J.; McNamara, M.G.; Šeruga, B.; Vera-Badillo, F.E.; Aneja, P.; Ocaña, A.; Leibowitz-Amit, R.; Sonpavde, G.; Knox, J.J.; Tran, B. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: A systematic review and meta-analysis. J. Natl. Cancer Inst. 2014, 106, dju124. [Google Scholar] [CrossRef]
- Nishiyama, N.; Hirobe, M.; Kikushima, T.; Matsuki, M.; Takahashi, A.; Yanase, M.; Ichimatsu, K.; Egawa, M.; Hayashi, N.; Negishi, T. The neutrophil-lymphocyte ratio has a role in predicting the effectiveness of nivolumab in Japanese patients with metastatic renal cell carcinoma: A multi-institutional retrospective study. BMC Urol. 2020, 20, 1–7. [Google Scholar] [CrossRef]
- Romero-Garcia, S.; Moreno-Altamirano, M.M.B.; Prado-Garcia, H.; Sánchez-García, F.J. Lactate contribution to the tumor microenvironment: Mechanisms, effects on immune cells and therapeutic relevance. Front. Immunol. 2016, 7, 52. [Google Scholar] [CrossRef]
- Tang, Q.; Li, X.; Sun, C.-R. Predictive value of serum albumin levels on cancer survival: A prospective cohort study. Front. Oncol. 2024, 14, 1323192. [Google Scholar] [CrossRef]
- Mezquita, L.; Auclin, E.; Ferrara, R.; Charrier, M.; Remon, J.; Planchard, D.; Ponce, S.; Ares, L.P.; Leroy, L.; Audigier-Valette, C. Association of the lung immune prognostic index with immune checkpoint inhibitor outcomes in patients with advanced non–small cell lung cancer. JAMA Oncol. 2018, 4, 351–357. [Google Scholar] [CrossRef]
- Tsujino, T.; Komura, K.; Matsunaga, T.; Yoshikawa, Y.; Takai, T.; Uchimoto, T.; Saito, K.; Tanda, N.; Oide, R.; Minami, K. Preoperative measurement of the modified glasgow prognostic score predicts patient survival in non-metastatic renal cell carcinoma prior to nephrectomy. Ann. Surg. Oncol. 2017, 24, 2787–2793. [Google Scholar] [CrossRef] [PubMed]
- Carril-Ajuria, L.; Lavaud, P.; Dalban, C.; Negrier, S.; Gravis, G.; Motzer, R.J.; Chevreau, C.; Tannir, N.M.; Oudard, S.; McDermott, D.F. Validation of the Lung Immune Prognostic Index (LIPI) as a prognostic biomarker in metastatic renal cell carcinoma. Eur. J. Cancer 2024, 204, 114048. [Google Scholar] [CrossRef]
- Fujiwara, R.; Takemura, K.; Fujiwara, M.; Yuasa, T.; Yasuoka, S.; Komai, Y.; Numao, N.; Yamamoto, S.; Yonese, J. Modified Glasgow prognostic score as a predictor of prognosis in metastatic renal cell carcinoma treated with nivolumab. Clin. Genitourin. Cancer 2021, 19, e78–e83. [Google Scholar] [CrossRef] [PubMed]
- Hurwitz, M.E.; Considine, B.; Hasson, N.; Savion Gaiger, N.; Nelson, M.; Chiang, V.; Kluger, H.M.; Braun, D.A.; Schoenfeld, D.A.; Sznol, M. Survival of patients with metastatic renal cell carcinoma with or without brain metastases. Am. Soc. Clin. Oncol. 2025, 43, 5. [Google Scholar] [CrossRef]
- Escudier, B.J.; Chabaud, S.; Borchiellini, D.; Gravis, G.; Chevreau, C.; Brachet, P.E.; Geoffrois, L.; Laguerre, B.; Mahammedi, H.; Negrier, S. Efficacy and safety of nivolumab in patients with metastatic renal cell carcinoma (mRCC) and brain metastases: Preliminary results from the GETUG-AFU 26 (Nivoren) study. Am. Soc. Clin. Oncol. 2017, 35, 15. [Google Scholar] [CrossRef]
- Bracarda, S.; Galli, L.; Maruzzo, M.; Lo Re, G.; Buti, S.; Favaretto, A.; Di Costanzo, F.; Sacco, C.; Merlano, M.; Mucciarini, C. Negative prognostic factors and resulting clinical outcome in patients with metastatic renal cell carcinoma included in the Italian nivolumab-expanded access program. Future Oncol. 2018, 14, 1347–1354. [Google Scholar] [CrossRef]
- Dudani, S.; Guillermo de Velasco, J.; Gan, C.L.; Donskov, F.; Porta, C.; Fraccon, A.; Pasini, F.; Hansen, A.; Bjarnason, G.A.; Beuselinck, B. Sites of Metastasis and Survival in Metastatic Renal-Cell Carcinoma (mRCC). Europe 2020, 3474, 34. [Google Scholar]
- Beuselinck, B.; Oudard, S.; Rixe, O.; Wolter, P.; Blesius, A.; Ayllon, J.; Elaidi, R.; Schöffski, P.; Barrascout, E.; Morel, A. Negative impact of bone metastasis on outcome in clear-cell renal cell carcinoma treated with sunitinib. Ann. Oncol. 2011, 22, 794–800. [Google Scholar] [CrossRef]
- Mollica, V.; Rizzo, A.; Tassinari, E.; Giunchi, F.; Schiavina, R.; Fiorentino, M.; Brunocilla, E.; Ardizzoni, A.; Massari, F. Prognostic and predictive factors to nivolumab in patients with metastatic renal cell carcinoma: A single center study. Anti-Cancer Drugs 2021, 32, 74–81. [Google Scholar] [CrossRef]
- Ma, V.T.; Su, C.T.; Hu, M.; Taylor, J.M.; Daignault-Newton, S.; Kellezi, O.; Dahl, M.N.; Shah, M.A.; Erickson, S.; Lora, J. Characterization of outcomes in patients with advanced genitourinary malignancies treated with immune checkpoint inhibitors. Urol. Oncol. Semin. Orig. Investig. 2021, 39, 437.e1–437.e9. [Google Scholar] [CrossRef]
- Gerlinger, M.; Rowan, A.J.; Horswell, S.; Larkin, J.; Endesfelder, D.; Gronroos, E.; Martinez, P.; Matthews, N.; Stewart, A.; Tarpey, P. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N. Engl. J. Med. 2012, 366, 883–892. [Google Scholar] [CrossRef]
- Limmer, A.; Ohl, J.; Kurts, C.; Ljunggren, H.-G.; Reiss, Y.; Groettrup, M.; Momburg, F.; Arnold, B.; Knolle, P.A. Efficient presentation of exogenous antigen by liver endothelial cells to CD8+ T cells results in antigen-specific T-cell tolerance. Nat. Med. 2000, 6, 1348–1354. [Google Scholar] [CrossRef]
| Category | Score 0 | Score 1 |
|---|---|---|
| CRP (mg/dL) | <1.0 | ≥1.0 |
| PS | 0–1 | ≥2 |
| LDH (U/L) | <223 | ≥223 |
| Alb (g/dL) | ≥3.5 | <3.5 |
| Derived NLR | <3.0 | ≥3.0 |
| Characteristics | All Patients (n = 81) (%) | c-Plan Good (n = 43) (%) | C-Plan Poor (n = 38) (%) | p Value |
|---|---|---|---|---|
| Age-years, median (IQR) | 62 (54.5–70.0) | 59 (53–70) | 65(57–70) | 0.302 |
| Age group | 0.169 | |||
| <65 | 47 (58) | 28 (65.1) | 19 ((50) | |
| ≥65 | 34(42) | 15 (34.9) | 19 (50) | |
| Gender | 0.964 | |||
| Male | 62 (76.5) | 33(76.7) | 29 (76.3) | |
| Female | 19 (23.5) | 10(23.3) | 9 (23.7) | |
| Metastase Status | 0.480 | |||
| Denovo | 50 (61.7) | 25 (58.1) | 25 (65.8) | |
| Recurrence | 31 (38.3) | 18 (41.9) | 13 (34.2) | |
| Previous nephrectomy | 0.180 | |||
| Yes | 59 (72.8)) | 34 (79.1) | 25 (65.8) | |
| No | 22 (27.2) | 9 (20.9) | 13 (34.2) | |
| Histological type | 0.859 | |||
| Clear cell | 61(75.3) | 33 (76.7) | 28 (73.7) | |
| Non-clear cell | 11(13.6) | 5 (11.6) | 6 (15.8) | |
| Missing | 9 (11.1) | 5 (11.6) | 4 (10.5) | |
| Sarcomatoid feature | 0.488 | |||
| Yes | 7 (8.6) | 4 (9.3) | 3 (7.9) | |
| No | 63 (77.8) | 35 (81.4) | 28 (73.7) | |
| Missing | 11 (13.6) | 4 (9.3) | 7 (18.4) | |
| First-line TKI | 0.350 | |||
| Cabozantinib | 4 (4.9) | 1 (2.3) | 3 (7.9) | |
| Pazopanib | 42 (51.9) | 21 (48.8) | 21 (55.3) | |
| Sunitinib | 35 (43.2) | 21 (48.8) | 14 (36.8) | |
| Metastatic sites | ||||
| Lung | 52 (64.2) | 27 (62.8) | 25 (65.8) | 0.779 |
| Bone | 42 (51.9) | 25 (58.1) | 17 (44.7) | 0.228 |
| Liver | 20 (24.7) | 8 (18.6) | 12 (31.6) | 0.177 |
| Brain | 4 (4.9) | 0 (0) | 4 (10.5) | 0.044 |
| ECOG | 0.010 | |||
| 0–1 | 58 (71.6) | 38 (88.4) | 20 (52.6) | |
| ≥2 | 21(25.9) | 4 (9.3) | 17 (44.7) | |
| Missing | 2 (2.5) | 1 (2.3) | 1 (2.6) | |
| Nivolumab line | 0.854 | |||
| Second | 52 (64.2) | 28 (65.1) | 24 (63.2) | |
| Third and beyond | 29 (35.8) | 15 (34.9) | 14 (36.8) | |
| IMDC risk | <0.001 | |||
| Favorable | 10 (12.8) | 6 (14) | 4 (10.5) | |
| Intermediate | 45 (57.7) | 32 (74.4) | 13 (34.2) | |
| Poor | 23 (29.5) | 4 (9.3) | 19 (50) | |
| Missing | 3 (14.5) | 1 (2.3) | 2 (5.3) |
| Univariate | p Value a | Multivariate | p Value a | |||
|---|---|---|---|---|---|---|
| HR | %95 CI | HR | %95 CI | |||
| Age group | 0.638 | |||||
| <65 | 1 | |||||
| ≥65 | 1.13 | (0.67–1.93) | ||||
| Gender | 0.730 | |||||
| Male | 1.11 | (0.60–2.07) | ||||
| Female | 1 | |||||
| Metastase Status | 0.697 | |||||
| Denovo | 1.11 | (0.65–1.91) | ||||
| Recurrence | 1 | |||||
| Previous nephrectomy | 0.105 | |||||
| Yes | 1 | |||||
| No | 1.62 | (0.90–2.92) | ||||
| Histological type | 0.448 | |||||
| Clear cell | 1.39 | (0.59–3.3) | ||||
| Non-clear cell | 1 | |||||
| Sarcomatoid feature | 0.469 | |||||
| Yes | 1.37 | (0.583–3.23) | ||||
| No | 1 | |||||
| ECOG | 0.008 | 0.214 | ||||
| 0–1 | 1 | 1 | ||||
| ≥2 | 2.17 | (1.22–3.86) | 1.49 | (0.79–2.83) | ||
| Nivolumab line | 0.659 | |||||
| Second | 1 | |||||
| Third and beyond | 1.13 | (0.65–1.94) | ||||
| IMDC risk | 0.067 | 0.994 | ||||
| Favorable | 1 | 1 | ||||
| Intermediate | 1.07 | (0.46–2.46) | 1.02 | (0.44–2.36) | ||
| Poor | 2.08 | (0.86–5.00) | 1.05 | (0.37–2.97) | ||
| Liver Met | 0.178 | |||||
| Yes | 1.49 | (0.83–2.68) | ||||
| No | 1 | |||||
| Bone Met | 0.726 | |||||
| Yes | 1.1 | (0.64–1.86) | ||||
| No | 1 | |||||
| Lung Met | 0.963 | |||||
| Yes | 1.01 | (0.58–1.75) | ||||
| No | 1 | |||||
| Brain Met | <0.001 | 0.002 | ||||
| Yes | 8.770 | (2.79–27.51) | 6.71 | (2.06–21.89) | ||
| No | 1 | 1 | ||||
| C-Plan | 0.004 | 0.020 | ||||
| Good | 1 | 1 | ||||
| Poor | 2.162 | (1.27–3.67) | 1.19 | (1.11–3.43) | ||
| Univariate | p Value a | Multivariate | p Value a | |||
|---|---|---|---|---|---|---|
| HR | %95 CI | HR | %95 CI | |||
| Age group | 0.472 | |||||
| <65 | 1.18 | (0.74–1.89) | ||||
| ≥65 | 1 | |||||
| Gender | 0.675 | |||||
| Male | 1 | |||||
| Female | 1.12 | (0.65–1.91) | ||||
| Metastase Status | 0.883 | |||||
| Denovo | 1.04 | (0.65–1.65) | ||||
| Recurrence | 1 | |||||
| Previous nephrectomy | 0.425 | |||||
| Yes | 1 | |||||
| No | 1.24 | (0.73–2.12) | ||||
| Histological type | 0.837 | |||||
| Clear cell | 1 | |||||
| Non-clear cell | 1.07 | (0.54–2.11) | ||||
| Sarcomatoid feature | 0.289 | |||||
| Yes | 1 | |||||
| No | 1.58 | (0.67–3.71) | ||||
| ECOG | 0.047 | 0.271 | ||||
| 0–1 | 1 | 1 | ||||
| ≥2 | 1.68 | (1.01–2.80) | 1.38 | (0.77–2.47) | ||
| Nivolumab line | 0.761 | |||||
| Second | 1 | |||||
| Third and beyond | 1.01 | (0.66–1.73) | ||||
| IMDC risk | 0.116 | |||||
| Favorable | 1 | |||||
| Intermediate | 1.73 | (0.74–3.50) | ||||
| Poor | 2.29 | (1.02–5.17) | ||||
| Liver Met | 0.021 | 0.012 | ||||
| Yes | 1.84 | (1.09–3.11) | 2.03 | (1.17–3.52) | ||
| No | 1 | 1 | ||||
| Bone Met | 0.731 | |||||
| Yes | 1.08 | (0.68–1.72) | ||||
| No | 1 | |||||
| Lung Met | 0.873 | |||||
| Yes | 1 | |||||
| No | 1.04 | (0.64–1.67) | ||||
| Brain Met | 0.007 | |||||
| Yes | 5.89 | (1.96–17.72) | 0.002 | 4.83 | (1.55–15.03) | |
| No | 1 | 1 | ||||
| C-Plan | 0.008 | 0.032 | ||||
| Good | 1 | 1 | ||||
| Poor | 1.87 | (1.18–2.96) | 1.71 | (1.04–2.78) | ||
| Efficacy | All Patients, N (%) | Good C-PLAN Index Group, N (%) | Poor C-PLAN Index Group, N (%) | p-Value |
|---|---|---|---|---|
| CR | 1 (1.2) | 1 (2.3) | 0 (0.0) | |
| PR | 17 (21) | 12(27.9) | 5 (13.2) | |
| SD | 22 (27.2) | 15 (34.9) | 7 (18.4) | |
| PD | 39 (48.1) | 14 (32.6) | 25 (65.8) | |
| NE | 2 (2.5) | 1 (2.3) | 1 (2.6) | |
| ORR, % (95%, CI) | 22.8 (11.1–35.0) | 31 (11.1–49.6) | 13.5% (1.1–24.7) | 0.065 |
| DCR, % (95%, CI) | 50.6 (40.0–61.2) | 66.7(44.6–81.7) | 32.4% (18.7–40.0) | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Şahin, G.; Acar, C.; Yüksel, H.Ç.; Tünbekici, S.; Açar, F.P.; Gökmen, E.; Karaca, B. Prognostic Value of the C-PLAN Index in Metastatic Renal Cell Carcinoma Treated with Nivolumab. J. Clin. Med. 2025, 14, 2217. https://doi.org/10.3390/jcm14072217
Şahin G, Acar C, Yüksel HÇ, Tünbekici S, Açar FP, Gökmen E, Karaca B. Prognostic Value of the C-PLAN Index in Metastatic Renal Cell Carcinoma Treated with Nivolumab. Journal of Clinical Medicine. 2025; 14(7):2217. https://doi.org/10.3390/jcm14072217
Chicago/Turabian StyleŞahin, Gökhan, Caner Acar, Haydar Çağatay Yüksel, Salih Tünbekici, Fatma Pınar Açar, Erhan Gökmen, and Burçak Karaca. 2025. "Prognostic Value of the C-PLAN Index in Metastatic Renal Cell Carcinoma Treated with Nivolumab" Journal of Clinical Medicine 14, no. 7: 2217. https://doi.org/10.3390/jcm14072217
APA StyleŞahin, G., Acar, C., Yüksel, H. Ç., Tünbekici, S., Açar, F. P., Gökmen, E., & Karaca, B. (2025). Prognostic Value of the C-PLAN Index in Metastatic Renal Cell Carcinoma Treated with Nivolumab. Journal of Clinical Medicine, 14(7), 2217. https://doi.org/10.3390/jcm14072217






