The Effectiveness of an Electronic Decision Support Algorithm to Optimize Recommendations of SGLT2i and GLP-1RA in Patients with Type 2 Diabetes upon Discharge from Internal Medicine Wards †
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Setting, and Population
2.2. The Algorithm
2.3. Data Collection
2.4. Study Outcomes
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Overall Rate of Recommendation
3.3. Analysis by Age and Sex
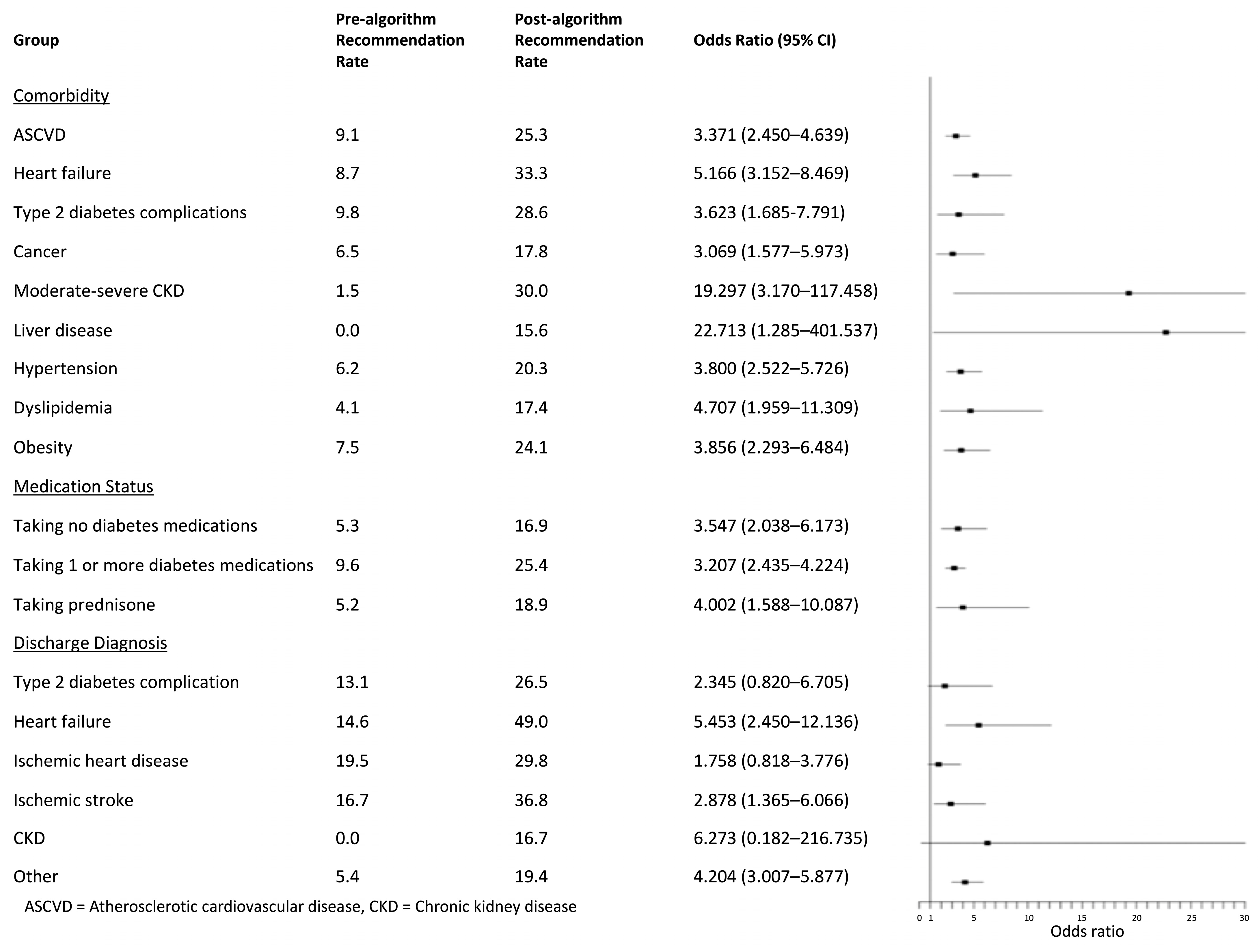
3.4. Analysis by Comorbidity
3.5. Analysis by Medication Status
3.6. Analysis by Discharge Diagnosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Ong, K.L.; Stafford, L.K.; McLaughlin, S.A.; Boyko, E.J.; Vollset, S.E.; Smith, A.E.; Dalton, B.E.; Duprey, J.; Cruz, J.A.; Hagins, H.; et al. Global, Regional, and National Burden of Diabetes from 1990 to 2021, with Projections of Prevalence to 2050: A Systematic Analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402, 203–234. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.B.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Al Kaabi, J. Epidemiology of Type 2 Diabetes—Global Burden of Disease and Forecasted Trends. J. Epidemiol. Glob. Health 2019, 10, 107–111. [Google Scholar] [CrossRef]
- The Emerging Risk Factors Collaboration. Diabetes Mellitus, Fasting Blood Glucose Concentration, and Risk of Vascular Disease: A Collaborative Meta-Analysis of 102 Prospective Studies. Lancet 2010, 375, 2215–2222. [Google Scholar] [CrossRef] [PubMed]
- Mariani, M.V.; Lavalle, C.; Palombi, M.; Pierucci, N.; Trivigno, S.; D’Amato, A.; Filomena, D.; Cipollone, P.; Laviola, D.; Vizza, C.D.; et al. SGLT2i reduce arrhythmic events in heart failure patients with cardiac implantable electronic devices. ESC Heart Fail. 2025. [Google Scholar] [CrossRef]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef]
- Zelniker, T.A.; Wiviott, S.D.; Raz, I.; Im, K.; Goodrich, E.L.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Furtado, R.H.M.; et al. SGLT2 Inhibitors for Primary and Secondary Prevention of Cardiovascular and Renal Outcomes in Type 2 Diabetes: A Systematic Review and Meta-Analysis of Cardiovascular Outcome Trials. Lancet 2019, 393, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Palmer, S.C.; Tendal, B.; Mustafa, R.A.; Vandvik, P.O.; Li, S.; Hao, Q.; Tunnicliffe, D.; Ruospo, M.; Natale, P.; Saglimbene, V.; et al. Sodium-Glucose Cotransporter Protein-2 (SGLT-2) Inhibitors and Glucagon-like Peptide-1 (GLP-1) Receptor Agonists for Type 2 Diabetes: Systematic Review and Network Meta-Analysis of Randomised Controlled Trials. BMJ 2021, 372, m4573. [Google Scholar] [CrossRef]
- Perkovic, V.; Tuttle, K.R.; Rossing, P.; Mahaffey, K.W.; Mann, J.F.E.; Bakris, G.; Baeres, F.M.M.; Idorn, T.; Bosch-Traberg, H.; Lausvig, N.L.; et al. Effects of Semaglutide on Chronic Kidney Disease in Patients with Type 2 Diabetes. N. Engl. J. Med. 2024, 391, 109–121. [Google Scholar] [CrossRef]
- Das, S.R.; Everett, B.M.; Birtcher, K.K.; Brown, J.M.; Januzzi, J.L.; Kalyani, R.R.; Kosiborod, M.; Magwire, M.; Morris, P.B.; Neumiller, J.J.; et al. 2020 Expert Consensus Decision Pathway on Novel Therapies for Cardiovascular Risk Reduction in Patients with Type 2 Diabetes. J. Am. Coll. Cardiol. 2020, 76, 1117–1145. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee; ElSayed, N.A.; Aleppo, G.; Bannuru, R.R.; Beverly, E.A.; Bruemmer, D.; Collins, B.S.; Cusi, K.; Darville, A.; Das, S.R.; et al. Introduction and Methodology: Standards of Care in Diabetes—2024. Diabetes Care 2024, 47, S1–S4. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee; ElSayed, N.A.; Aleppo, G.; Bannuru, R.R.; Bruemmer, D.; Collins, B.S.; Ekhlaspour, L.; Gaglia, J.L.; Hilliard, M.E.; Johnson, E.L.; et al. Pharmacologic Approaches to Glycemic Treatment: Standards of Care in Diabetes—2024. Diabetes Care 2024, 47, S158–S178. [Google Scholar] [CrossRef]
- Cosentino, F.; Grant, P.J.; Aboyans, V.; Bailey, C.J.; Ceriello, A.; Delgado, V.; Federici, M.; Filippatos, G.; Grobbee, D.E.; Hansen, T.B.; et al. 2019 ESC Guidelines on Diabetes, Pre-Diabetes, and Cardiovascular Diseases Developed in Collaboration with the EASD. Eur. Heart J. 2020, 41, 255–323. [Google Scholar] [CrossRef]
- The Israeli Ministry of Health. The Israeli Drug Registry. Available online: https://israeldrugs.health.gov.il/#!/byDrug (accessed on 2 March 2025).
- Mahtta, D.; Ramsey, D.J.; Lee, M.T.; Chen, L.; Al Rifai, M.; Akeroyd, J.M.; Vaughan, E.M.; Matheny, M.E.; Santo, K.R.D.E.; Navaneethan, S.D.; et al. Utilization Rates of SGLT2 Inhibitors and GLP-1 Receptor Agonists and Their Facility-Level Variation Among Patients with Atherosclerotic Cardiovascular Disease and Type 2 Diabetes: Insights From the Department of Veterans Affairs. Diabetes Care 2022, 45, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.J.; Ardissino, M.; Haynes, K.; Shambhu, S.; Eapen, Z.J.; McGuire, D.K.; Carnicelli, A.; Lopes, R.D.; Green, J.B.; O’Brien, E.C.; et al. Gaps in Evidence-Based Therapy Use in Insured Patients in the United States with Type 2 Diabetes Mellitus and Atherosclerotic Cardiovascular Disease. J. Am. Heart Assoc. 2021, 10, e016835. [Google Scholar] [CrossRef] [PubMed]
- Ganz, M.; Ustyugova, A.; Sawalhi-Leckenby, N.; De Souza, S.; Zhang, L.; Gunnarsson, E.; Gao, R.; Desai, N.R. Utilization of Glucose-Lowering Drugs in Patients with T2DM and Established CVD in US: A Descriptive Study Using Optum Clinformatics Data. J. Am. Coll. Cardiol. 2020, 75, 2017. [Google Scholar] [CrossRef]
- Rattelman, C.R.; Ciemins, E.L.; Cuddeback, J.K. 1247-P: Anticipating the Impact of 2019 Guidelines: Use of SGLT2i and GLP-1RA in Patients with Diabetes and Cardiovascular Disease. Diabetes 2019, 68, 1247-P. [Google Scholar] [CrossRef]
- Hamid, A.; Vaduganathan, M.; Oshunbade, A.A.; Ayyalasomayajula, K.K.; Kalogeropoulos, A.P.; Lien, L.F.; Shafi, T.; Hall, M.E.; Butler, J. Antihyperglycemic Therapies with Expansions of US Food and Drug Administration Indications to Reduce Cardiovascular Events: Prescribing Patterns Within an Academic Medical Center. J. Cardiovasc. Pharmacol. 2020, 76, 313–320. [Google Scholar] [CrossRef]
- Almigbal, T.H.; Alzarah, S.A.; Aljanoubi, F.A.; Alhafez, N.A.; Aldawsari, M.R.; Alghadeer, Z.Y.; Alrasheed, A.A. Clinical Inertia in the Management of Type 2 Diabetes Mellitus: A Systematic Review. Medicina 2023, 59, 182. [Google Scholar] [CrossRef]
- Griffith, M.L.; Boord, J.B.; Eden, S.K.; Matheny, M.E. Clinical Inertia of Discharge Planning among Patients with Poorly Controlled Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2012, 97, 2019–2026. [Google Scholar] [CrossRef]
- Lee, P.H.; Franks, A.S.; Barlow, P.B.; Farland, M.Z. Hospital Readmission and Emergency Department Use Based on Prescribing Patterns in Patients with Severely Uncontrolled Type 2 Diabetes Mellitus. Diabetes Technol. Ther. 2014, 16, 150–155. [Google Scholar] [CrossRef]
- Pagidipati, N.J.; Nelson, A.J.; Kaltenbach, L.A.; Leyva, M.; McGuire, D.K.; Pop-Busui, R.; Cavender, M.A.; Aroda, V.R.; Magwire, M.L.; Richardson, C.R.; et al. Coordinated Care to Optimize Cardiovascular Preventive Therapies in Type 2 Diabetes: A Randomized Clinical Trial. JAMA 2023, 329, 1261–1270. [Google Scholar] [CrossRef] [PubMed]
- Sutton, R.T.; Pincock, D.; Baumgart, D.C.; Sadowski, D.C.; Fedorak, R.N.; Kroeker, K.I. An Overview of Clinical Decision Support Systems: Benefits, Risks, and Strategies for Success. NPJ Digit. Med. 2020, 3, 17. [Google Scholar] [CrossRef]
- Nirantharakumar, K.; Chen, Y.F.; Marshall, T.; Webber, J.; Coleman, J.J. Clinical Decision Support Systems in the Care of Inpatients with Diabetes in Non-critical Care Setting: Systematic Review. Diabet. Med. 2012, 29, 698–708. [Google Scholar] [CrossRef] [PubMed]
- Gill, J.; Kucharski, K.; Turk, B.; Pan, C.; Wei, W. Using Electronic Clinical Decision Support in Patient-Centered Medical Homes to Improve Management of Diabetes in Primary Care: The DECIDE Study. J. Ambul. Care Manag. 2019, 42, 105–115. [Google Scholar] [CrossRef]
- O’Connor, P.J.; Sperl-Hillen, J.M.; Rush, W.A.; Johnson, P.E.; Amundson, G.H.; Asche, S.E.; Ekstrom, H.L.; Gilmer, T.P. Impact of Electronic Health Record Clinical Decision Support on Diabetes Care: A Randomized Trial. Ann. Fam. Med. 2011, 9, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A New Method of Classifying Prognostic Comorbidity in Longitudinal Studies: Development and Validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Lobach, D.F.; Hammond, W.E. Computerized Decision Support Based on a Clinical Practice Guideline Improves Compliance with Care Standards. Am. J. Med. 1997, 102, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Liang, Y.; Li, J.; Li, X. Applications of Clinical Decision Support Systems in Diabetes Care: Scoping Review. J. Med. Int. Res. 2023, 25, e51024. [Google Scholar] [CrossRef]
- Zhang, X.; Svec, M.; Tracy, R.; Ozanich, G. Clinical Decision Support Systems with Team-Based Care on Type 2 Diabetes Improvement for Medicaid Patients: A Quality Improvement Project. Int. J. Med. Inf. 2022, 158, 104626. [Google Scholar] [CrossRef]
- Ozaki, A.F.; Ko, D.T.; Chong, A.; Fang, J.; Atzema, C.L.; Austin, P.C.; Stukel, T.A.; Tu, K.; Udell, J.A.; Naimark, D.; et al. Prescribing Patterns and Factors Associated with Sodium–Glucose Cotransporter-2 Inhibitor Prescribing in Patients with Diabetes Mellitus and Atherosclerotic Cardiovascular Disease. CMAJ Open 2023, 11, E494–E503. [Google Scholar] [CrossRef]
- Malik, M.E.; Butt, J.H.; Strange, J.E.; Falkentoft, A.C.; Jensen, J.; Andersson, C.; Zahir, D.; Fosbøl, E.; Petrie, M.C.; Sattar, N.; et al. Initiation of SGLT2 Inhibitors and GLP-1 Receptor Agonists According to Level of Frailty in People with Type 2 Diabetes and Cardiovascular Disease in Denmark: A Cross-Sectional, Nationwide Study. Lancet Healthy Longev. 2023, 4, e552–e560. [Google Scholar] [CrossRef]
- Diallo, A.; Carlos-Bolumbu, M.; Galtier, F. Age, Sex, Race, BMI, and Duration of Diabetes Differences in Cardiovascular Outcomes with Glucose Lowering Drugs in Type 2 Diabetes: A Systematic Review and Meta-Analysis. eClinicalMedicine 2022, 54, 101697. [Google Scholar] [CrossRef]
- Strain, W.D.; Griffiths, J. A Systematic Review and Meta-Analysis of the Impact of GLP-1 Receptor Agonists and SGLT-2 Inhibitors on Cardiovascular Outcomes in Biologically Healthy Older Adults. Br. J. Diabetes 2021, 21, 30–35. [Google Scholar] [CrossRef]
- Bae, J.; Liu, D.; Chinthammit, C.; Kadziola, Z.; Boye, K.; Mather, K. Type 2 Diabetes Pharmacotherapy Trends in high-risk Subgroups. Diabetes Obes. Metab. 2022, 24, 1166–1171. [Google Scholar] [CrossRef] [PubMed]
- Garnica, P. Transition of Care for Patients with Diabetes. Curr. Diabetes Rev. 2017, 13, 263–279. [Google Scholar] [CrossRef]
- Donihi, A.C. Practical Recommendations for Transitioning Patients with Type 2 Diabetes from Hospital to Home. Curr. Diab. Rep. 2017, 17, 52. [Google Scholar] [CrossRef] [PubMed]
- Black, R.L.; Duval, C. Diabetes Discharge Planning and Transitions of Care: A Focused Review. Curr. Diabetes Rev. 2019, 15, 111–117. [Google Scholar] [CrossRef]
- Umpierrez, G.E.; Reyes, D.; Smiley, D.; Hermayer, K.; Khan, A.; Olson, D.E.; Pasquel, F.; Jacobs, S.; Newton, C.; Peng, L.; et al. Hospital Discharge Algorithm Based on Admission HbA1c for the Management of Patients with Type 2 Diabetes. Diabetes Care 2014, 37, 2934–2939. [Google Scholar] [CrossRef]
- Hofer, F.; Kazem, N.; Schweitzer, R.; Hammer, A.; Jakse, F.; Koller, L.; Hengstenberg, C.; Sulzgruber, P.; Niessner, A. Prescription Patterns of Sodium-Glucose Cotransporter 2 Inhibitors and Glucagon-Like Peptide-1 Receptor Agonists in Patients with Coronary Artery Disease. Cardiovasc. Drugs Ther. 2021, 35, 1161–1170. [Google Scholar] [CrossRef]
- Arnold, S.V.; Inzucchi, S.E.; Tang, F.; McGuire, D.K.; Mehta, S.N.; Maddox, T.M.; Goyal, A.; Sperling, L.S.; Einhorn, D.; Wong, N.D.; et al. Real-World Use and Modeled Impact of Glucose-Lowering Therapies Evaluated in Recent Cardiovascular Outcomes Trials: An NCDR® Research to Practice Project. Eur. J. Prev. Cardiol. 2017, 24, 1637–1645. [Google Scholar] [CrossRef]

| Pre-Algorithm (N = 1318) | Post-Algorithm (N = 970) | p Value | ||
|---|---|---|---|---|
| Age (years) | Mean ± SD | 74.2 ± 11.7 | 71.3 ± 12.5 | <0.0001 |
| Sex | Male | 61.5% | 60.4% | 0.603 |
| Female | 38.5% | 39.6% | ||
| LOS (days) | Median (IQR) | 3 (2–6) | 4 (3–7) | <0.0001 |
| Comorbidity | ASCVD | 60.8% | 47.2% | <0.001 |
| Heart failure | 25.2% | 17.6% | <0.001 | |
| Type 2 diabetes complications | 10.1% | 7.2% | 0.017 | |
| Cancer | 18.7% | 13.9% | 0.003 | |
| Obesity | 20.3% | 32.5% | <0.001 | |
| Moderate–severe CKD | 5.0% | 3.1% | 0.026 | |
| Liver disease | 4.5% | 7.9% | <0.001 | |
| Hypertension | 47.3% | 38.0% | <0.001 | |
| Dyslipidemia | 13.0% | 11.9% | 0.443 | |
| Type 2 Diabetes Medications | Taking one or more | 74.4% | 67.7% | <0.001 |
| Taking none | 25.6% | 32.3% | ||
| Prednisone | 8.7% | 11.4% | 0.033 | |
| Discharge Diagnosis | Type 2 diabetes complication | 4.6% | 3.5% | 0.204 |
| Heart failure | 6.8% | 5.3% | 0.158 | |
| Ischemic heart disease | 9.3% | 4.8% | <0.001 | |
| Ischemic stroke | 7.7% | 5.9% | 0.096 | |
| CKD | 0.8% | 0.6% | 0.629 | |
| Other | 70.7% | 79.9% | <0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayalon-Dangur, I.; Jaffe, E.; Grossman, A.; Hendel, H.; Oved, Y.; Shaked, A.; Shimon, I.; Basharim, B.; Abo Molhem, M.; McNeil, R.; et al. The Effectiveness of an Electronic Decision Support Algorithm to Optimize Recommendations of SGLT2i and GLP-1RA in Patients with Type 2 Diabetes upon Discharge from Internal Medicine Wards. J. Clin. Med. 2025, 14, 2170. https://doi.org/10.3390/jcm14072170
Ayalon-Dangur I, Jaffe E, Grossman A, Hendel H, Oved Y, Shaked A, Shimon I, Basharim B, Abo Molhem M, McNeil R, et al. The Effectiveness of an Electronic Decision Support Algorithm to Optimize Recommendations of SGLT2i and GLP-1RA in Patients with Type 2 Diabetes upon Discharge from Internal Medicine Wards. Journal of Clinical Medicine. 2025; 14(7):2170. https://doi.org/10.3390/jcm14072170
Chicago/Turabian StyleAyalon-Dangur, Irit, Emily Jaffe, Alon Grossman, Hagit Hendel, Yossi Oved, Amir Shaked, Ilan Shimon, Bar Basharim, Mohamad Abo Molhem, Rotem McNeil, and et al. 2025. "The Effectiveness of an Electronic Decision Support Algorithm to Optimize Recommendations of SGLT2i and GLP-1RA in Patients with Type 2 Diabetes upon Discharge from Internal Medicine Wards" Journal of Clinical Medicine 14, no. 7: 2170. https://doi.org/10.3390/jcm14072170
APA StyleAyalon-Dangur, I., Jaffe, E., Grossman, A., Hendel, H., Oved, Y., Shaked, A., Shimon, I., Basharim, B., Abo Molhem, M., McNeil, R., Abuhasira, R., Shitrit, T., Azulay Gitter, L., El Saleh, R., Shochat, T., & Eliakim-Raz, N. (2025). The Effectiveness of an Electronic Decision Support Algorithm to Optimize Recommendations of SGLT2i and GLP-1RA in Patients with Type 2 Diabetes upon Discharge from Internal Medicine Wards. Journal of Clinical Medicine, 14(7), 2170. https://doi.org/10.3390/jcm14072170






