Four Faces of Infective Endocarditis: Where Thinking Outside the Box Was Crucial
Abstract
1. Introduction
1.1. Case Series Presentation
1.1.1. Case 1
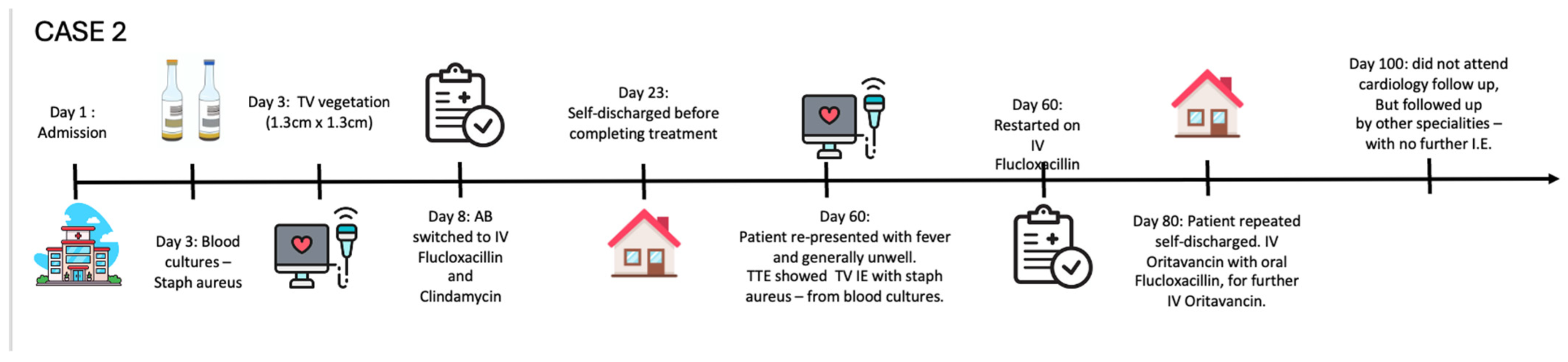
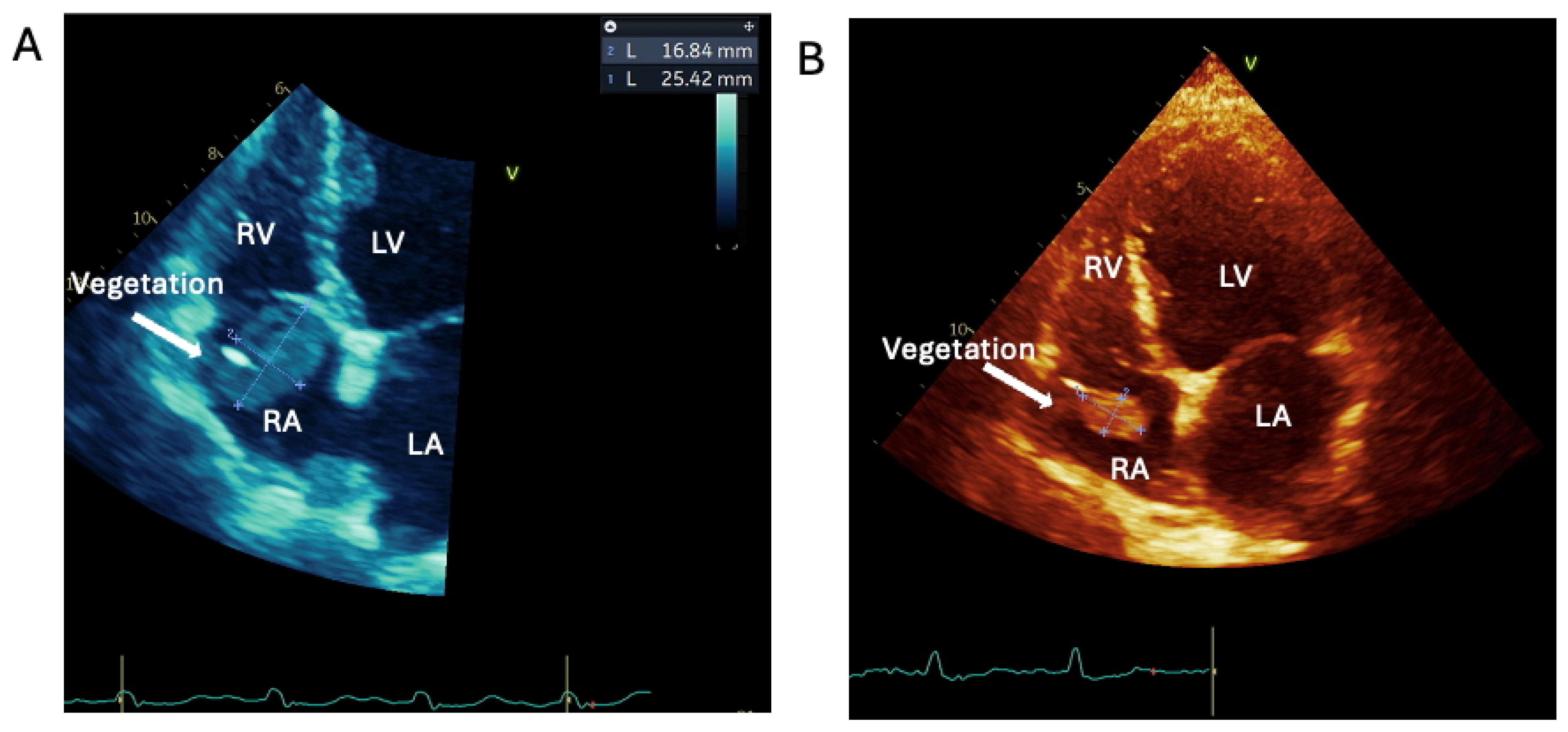
1.1.2. Case 2
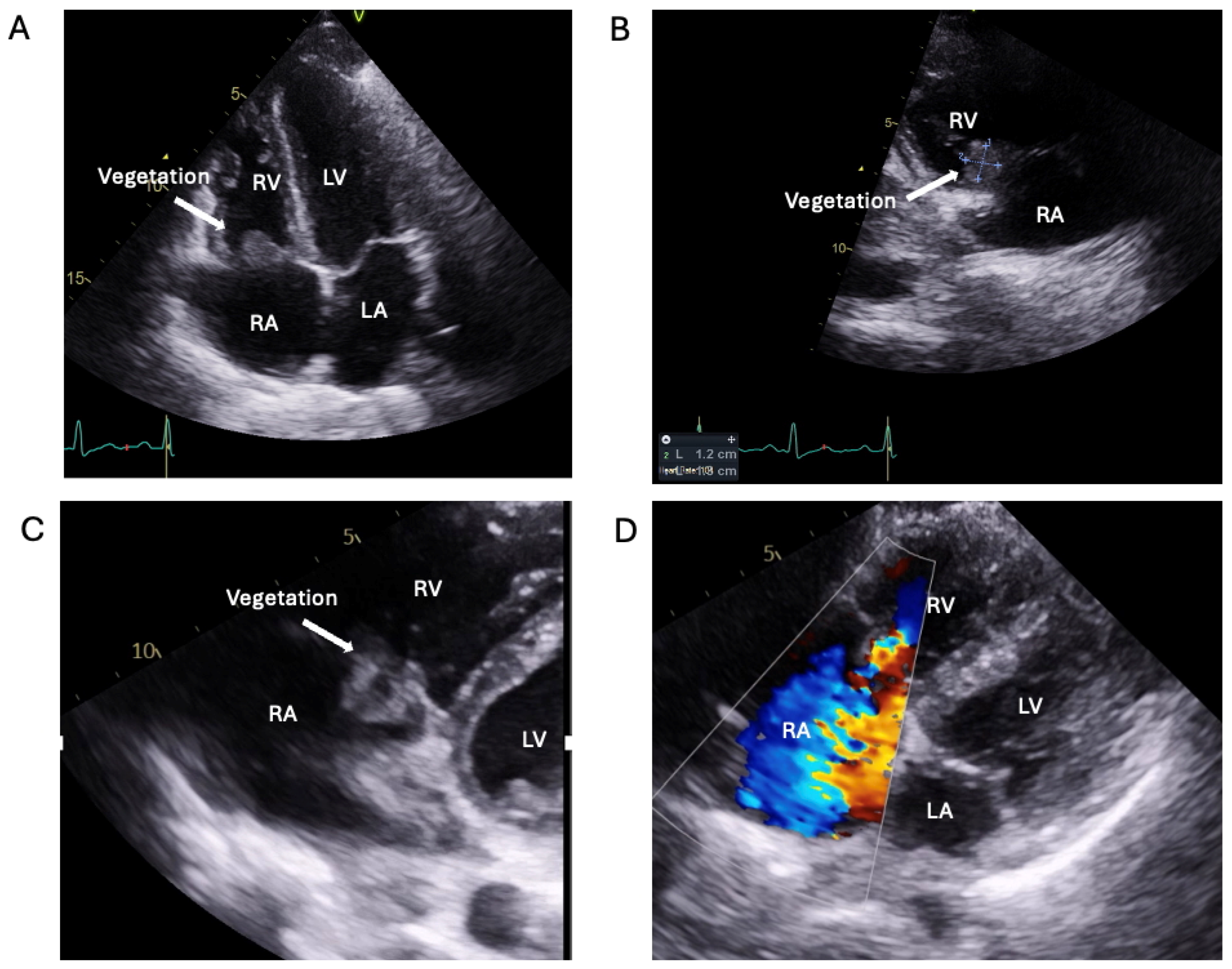
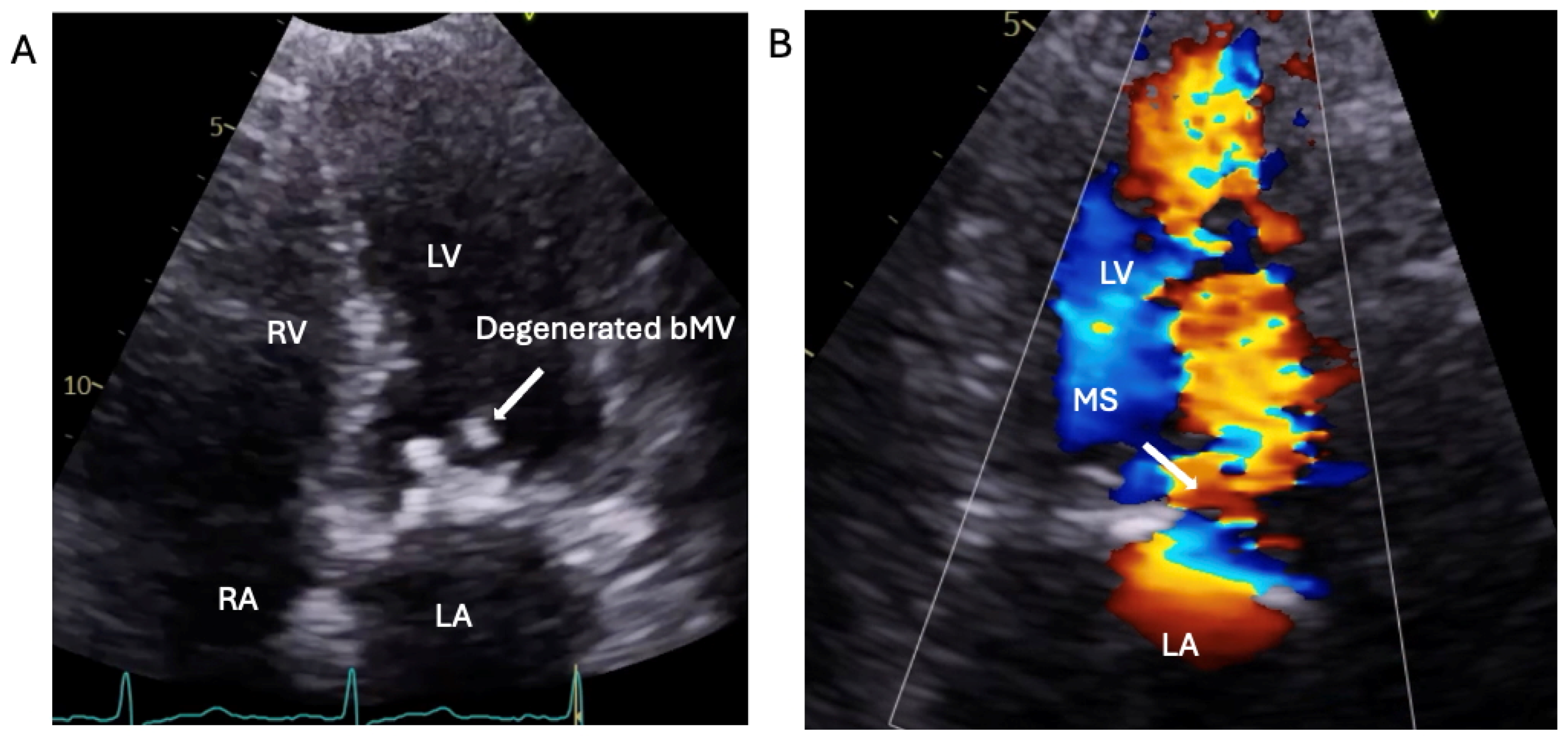
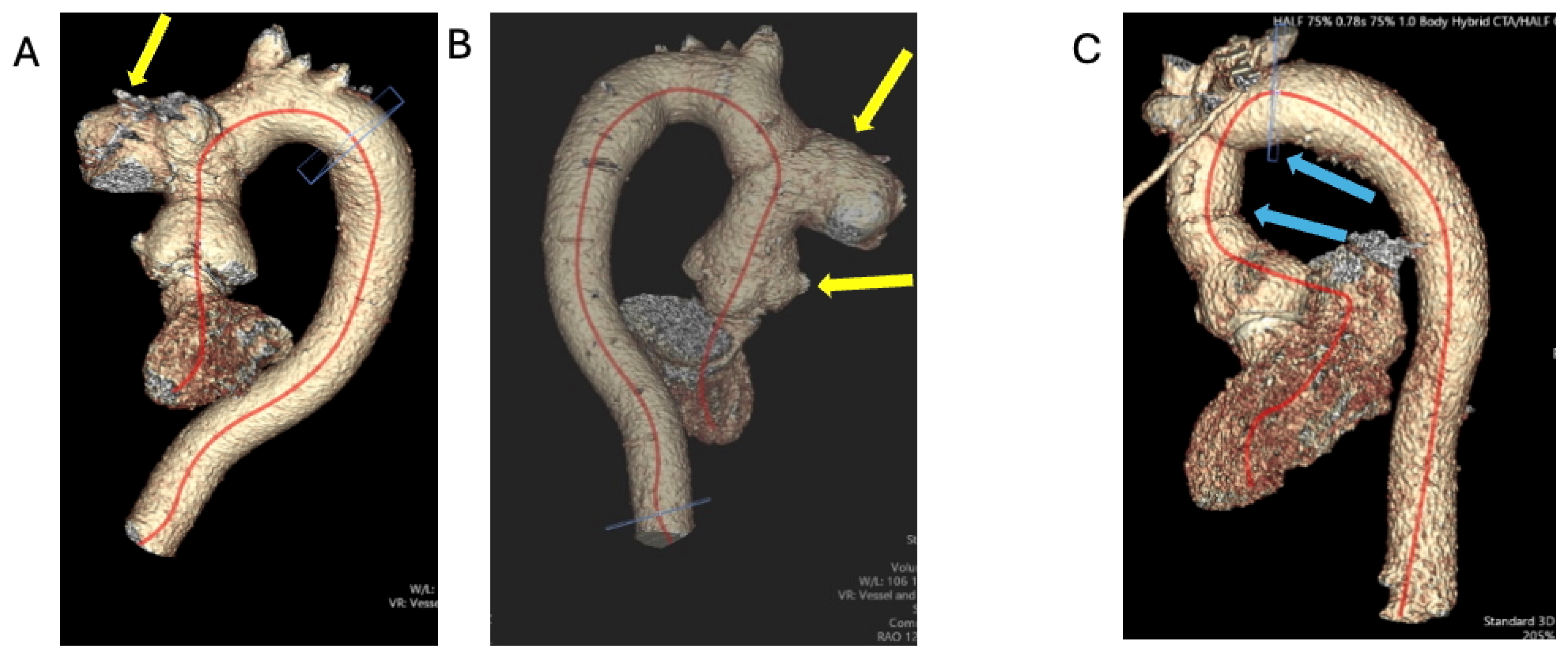
1.1.3. Case 3
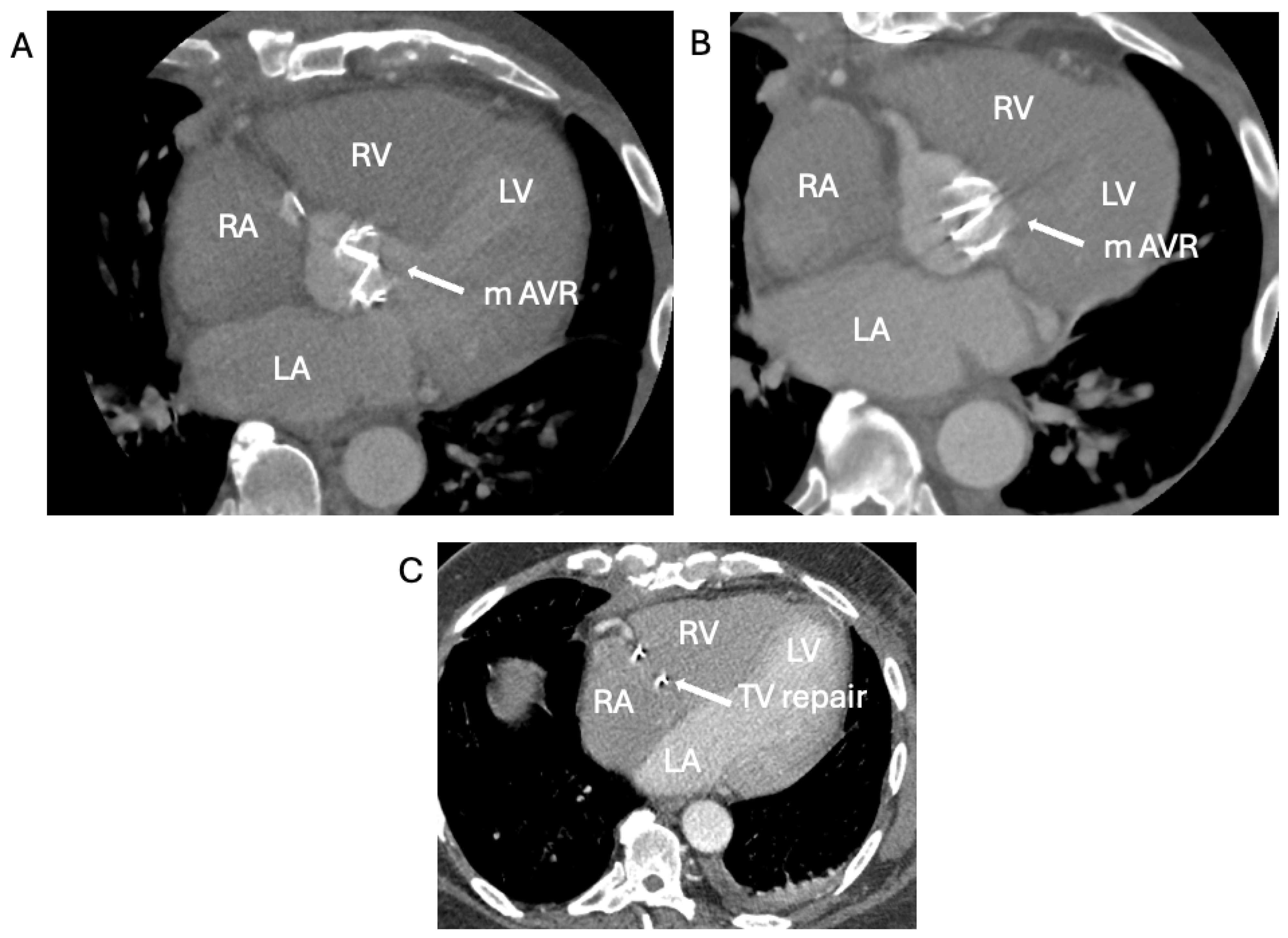
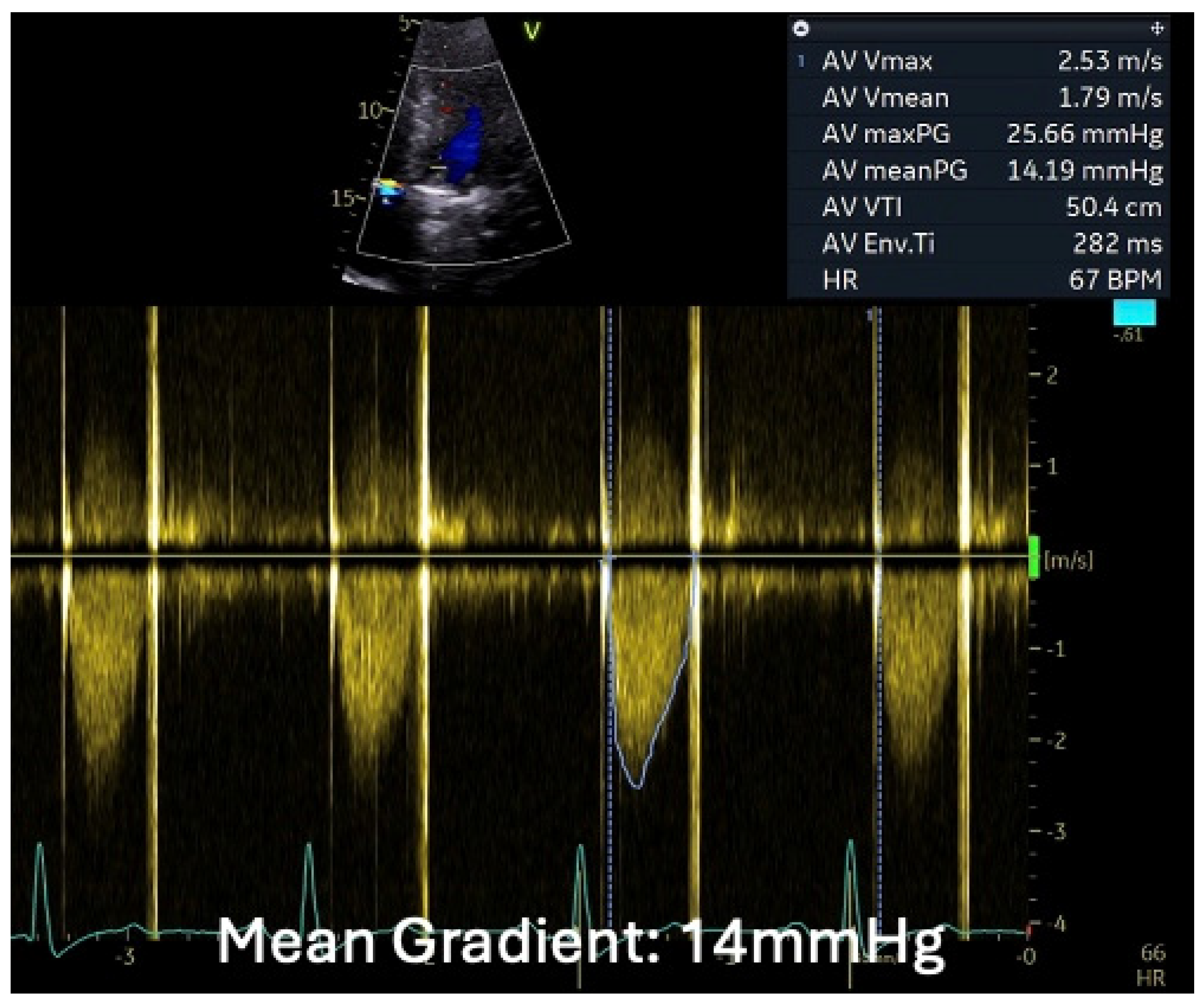
1.1.4. Case 4
2. Discussion
2.1. The Conversion of High Risk Open-Heart-Surgery to Lower-Risk Lead-Extraction in a CIED-IE Case
2.2. Logistical Management Challenges in a Non-Compliant High-Risk Young PWID with Relapsing Right-Sided IE
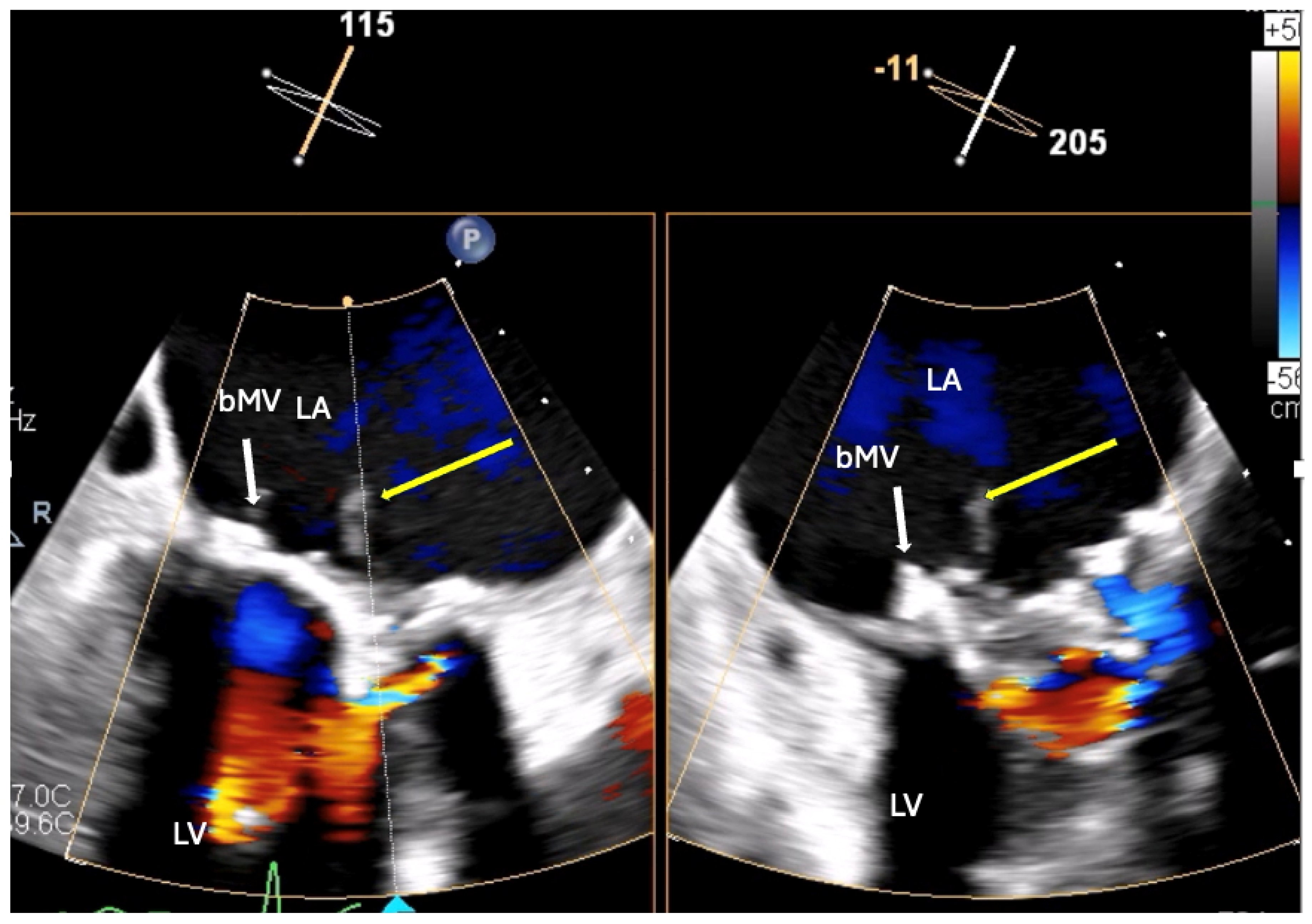
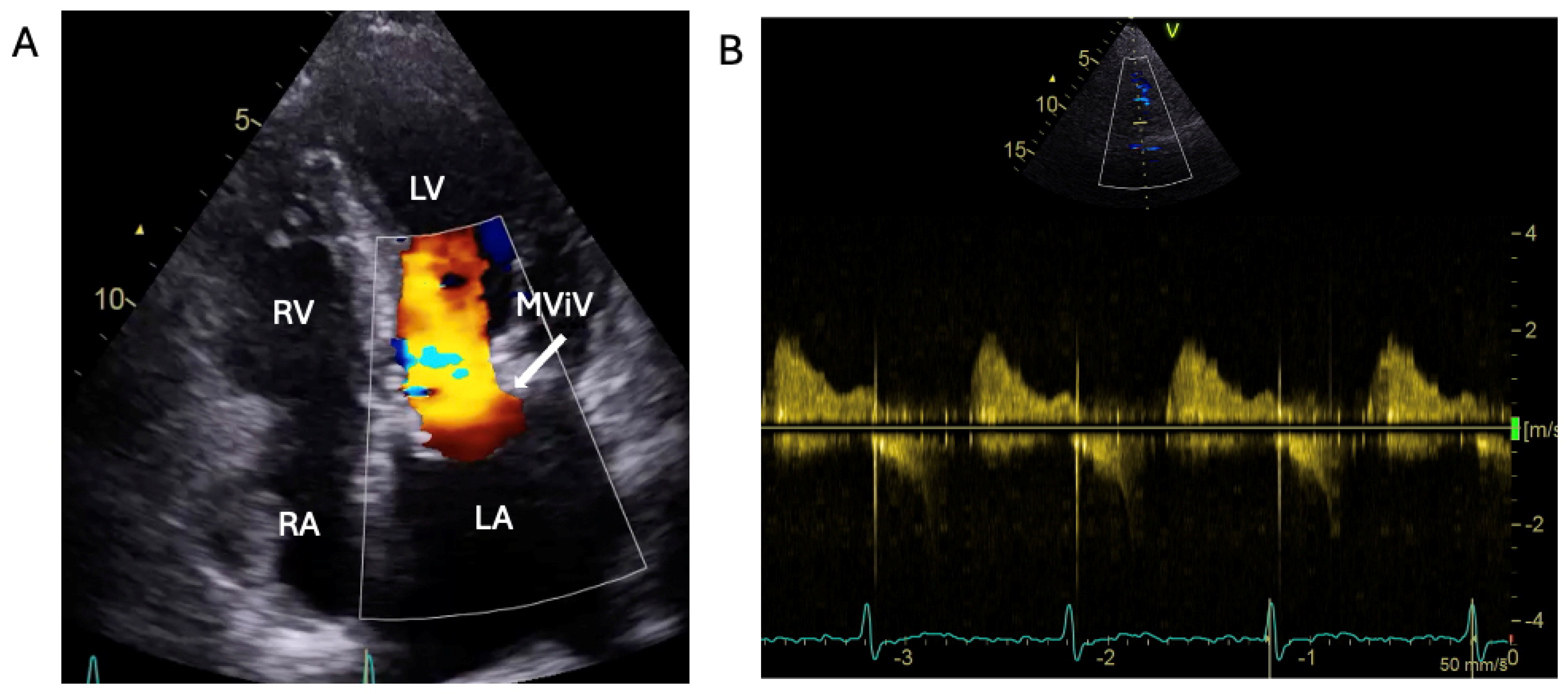
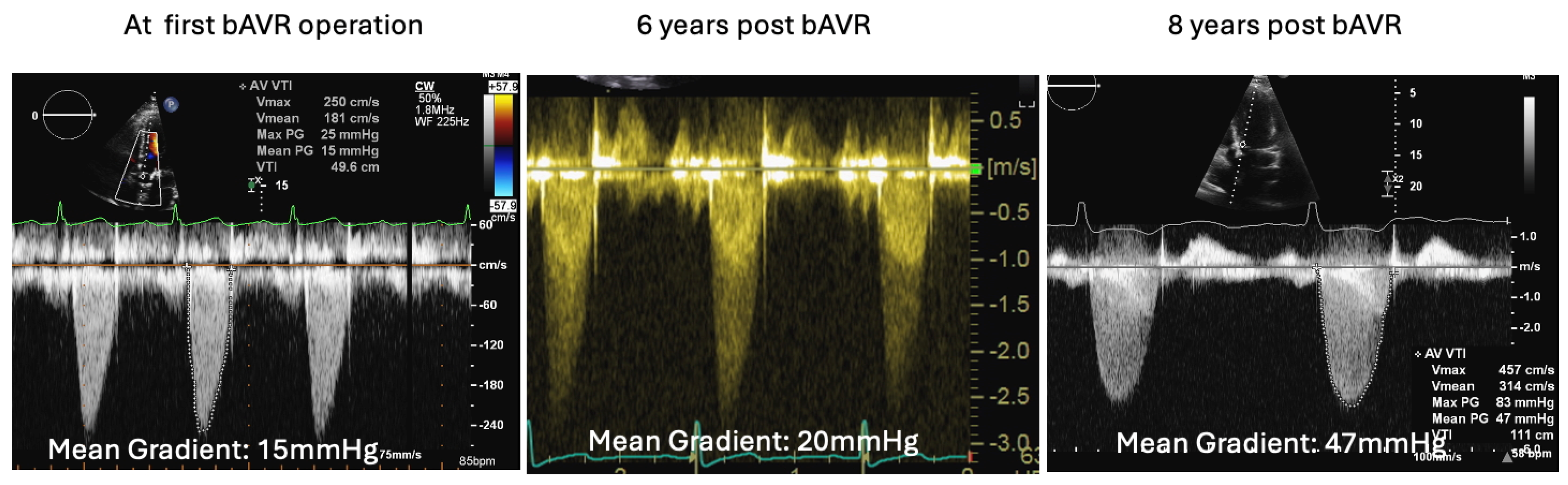
2.3. The Saga of Relapsing IE of the Prosthetic Mitral Valve Causing Structural Valve Degeneration and Transcutaneous MViV Prosthesis
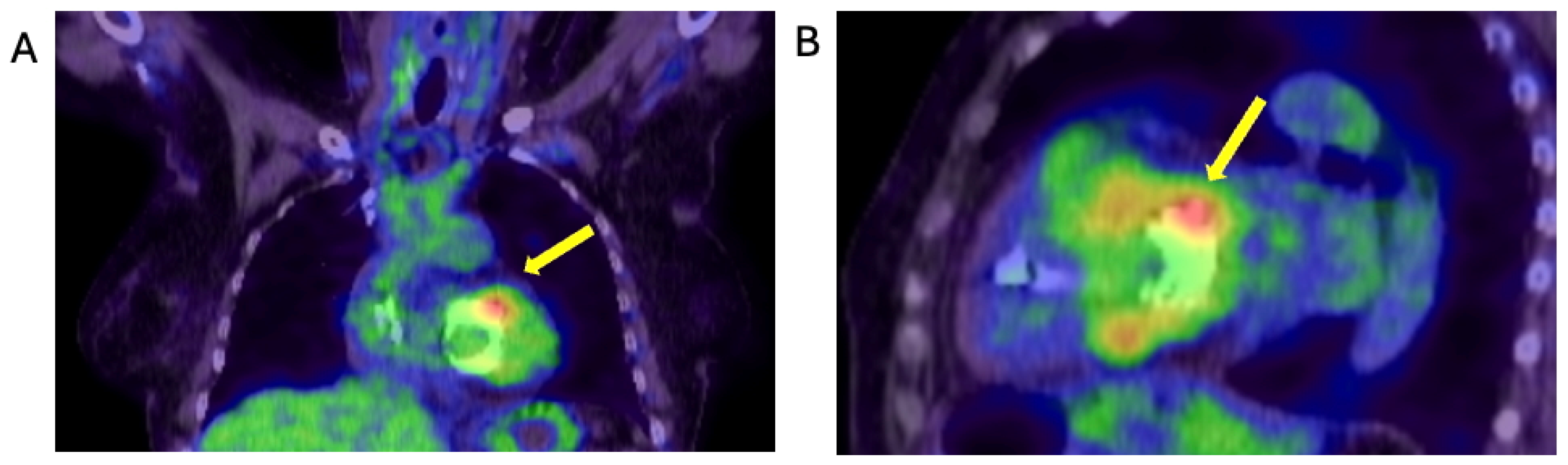
2.4. When the Diagnosis of IE Is Only Confirmed by the Tissue Culture of the Removed Degenerated tAVR
3. Conclusions
Learning Objectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
List of Abbreviations and Acronyms
| EEMT | Extended Endocarditis Multidisciplinary Team |
| CIED | Cardiac Implantable Electronic Device |
| PWID | Person Who Injected Drugs |
| ViV | Valve in Valve |
| IE | Infective Endocarditis |
| WCC | Wight Cell Count |
| CRP | C-Reactive Protein |
| PICC | Peripherally Inserted Central Catheter |
| OPAT | Outpatient Parenteral Antibiotic Therapy |
| TTE | Trans Thoracic Echocardiogram |
| ASA score | American Anaesthetists Society score |
| TV | Tricuspid Valve |
| ESC | European Society of Cardiology |
| tAVR | tissue Aortic Valve Replacement |
| RSISE | Right-Sided Infective Endocarditis |
References
- Rajani, R.; Klein, J.L. Infective endocarditis: A contemporary update. CME Cardiovasc. Med. 2020, 20, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Delgado, V.; Marsan, N.A.; de Waha, S.; Bonaros, N.; Brida, M.; Burri, H.; Caselli, S.; Doenst, T.; Ederhy, S.; Erba, P.A.; et al. 2023 ESC Guidelines for the management of endocarditis. Eur. Heart J. 2023, 44, 3948–4042. [Google Scholar] [CrossRef] [PubMed]
- Corey, G.R.; Loutit, J.; Moeck, G.; Wikler, M.; Dudley, M.N.; O’Riordan, W., on behalf of the SOLO I and SOLO II Investigators. Single Intravenous Dose of Oritavancin for Treatment of Acute Skin and Skin Structure Infections Caused by Gram-Positive Bacteria: Summary of Safety Analysis from the Phase 3 SOLO Studies. Antimicrob. Agents Chemother. 2018, 62, e01919-17. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, A.; Baddour, L.M.; Sohail, M.R. Microbiology and Pathogenesis of Cardiovascular Implantable Electronic Device Infections. Circ. Arrhythmia Electrophysiol. 2012, 5, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Udo, E.O.; Zuithoff, N.P.; van Hemel, N.M.; de Cock, C.C.; Hendriks, T.; Doevendans, P.A.; Moons, K.G. Incidence and predictors of short- and long-term complications in pacemaker therapy: The FOLLOWPACE study. Heart Rhythm. 2012, 9, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Poole, J.E.; Gleva, M.J.; Mela, T.; Chung, M.K.; Uslan, D.Z.; Borge, R.; Gottipaty, V.; Shinn, T.; Dan, D.; Feldman, L.A.; et al. Complication rates associated with pacemaker or implantable cardioverter-defibrillator generator replacements and upgrade procedures: Results from the REPLACE registry. Circulation 2010, 122, 1553–1561. [Google Scholar] [CrossRef] [PubMed]
- DeSimone, D.C.; Sohail, M.R. Infection Management. Card. Electrophysiol. Clin. 2018, 10, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Lassen, H.; Nielsen, S.L.; Gill, S.U.A.; Johansen, I.S. The epidemiology of infective endocarditis with focus on non-device related right-sided infective endocarditis: A retrospective register-based study in the region of Southern Denmark. Int. J. Infect. Dis. 2020, 95, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Saravolatz, L.D.; Stein, G.E. Oritavancin: A Long-Half-Life Lipoglycopeptide. Clin. Infect. Dis. 2015, 61, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Riasat, M.; Hanumanthu, B.K.J.; Khan, A.; Riaz, A.H.; Anjum, Z.; Ehtesham, M.; Rehman, S.U.; Javed, A.; Muhammad, A.; Misra, D. Outcomes and survival of patients undergoing percutaneous vegetectomy for right heart endocarditis. IJC Heart Vasc. 2023, 47, 101–231. [Google Scholar] [CrossRef] [PubMed]
- Barnes, A.M.T.; Frank, K.L.; Dunny, G.M. Enterococcal Endocarditis: Hiding in Plain Sight. Front. Cell. Infect. Microbiol. 2021, 11, 722482. [Google Scholar] [CrossRef]
- Llopis, J.; Muñoz, P.; Gálvez-Acebal, J.; Kestler, M.; Valerio, M.; Hernández-Meneses, M.; Cobo-Belaustegui, M.; Montejo, M.; Ojeda-Burgos, G.; Sousa-Regueiro, M.D.; et al. A Contemporary Picture of Enterococcal Endocarditis. J. Am. Coll. Cardiol. 2020, 75, 482–494. [Google Scholar] [CrossRef]
- Asgar, A.W.; Ducharme, A.; Pellerin, M.; Garceau, P.; Basmadjian, A.; Bouchard, D.; Bonan, R. The Evolution of Transcatheter Therapies for Mitral Valve Disease: From Mitral Valvuloplasty to Transcatheter Mitral Valve Replacement. Can. J. Cardiol. 2022, 38, S54–S65. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kelly, R.; Mbai, M.; Chandrashekhar, Y.; Bertog, S. Transcatheter Mitral Valve Lithotripsy as a Pretreatment to Percutaneous Balloon Mitral Valvuloplasty for Heavily Calcified Rheumatic Mitral Stenosis. Circ. Cardiovasc. Interv. 2020, 13, e009357. [Google Scholar] [CrossRef] [PubMed]
- García-Granja, P.E.; López, J.; Vilacosta, I.; Ortiz-Bautista, C.; Sevilla, T.; Olmos, C.; Sarriá, C.; Ferrera, C.; Gómez, I.; San Román, J.A. Polymicrobial Infective Endocarditis: Clinical Features and Prognosis. Medicine 2015, 94, e2000. [Google Scholar] [CrossRef] [PubMed]
- Heinen, F.J.; Arregle, F.; Brink, F.S.V.D.; Marsan, N.A.; Bernts, L.H.P.; Kamp, O.; Roescher, N.; Verkaik, N.J.; Roos-Hesselink, J.W.; Post, M.C.; et al. Cutibacterium acnes endocarditis: A multicenter case series. Eur. Heart J. 2022, 43 (Suppl. S2), ehac544.1676. [Google Scholar] [CrossRef]
- Bando, G.; Okada, T.; Tsubota, H.; Furukawa, Y. Prosthetic valve infective endocarditis with severe mitral stenosis caused by Cutibacterium acnes: A case report. Eur. Heart J.-Case Rep. 2024, 8, ytae205. [Google Scholar] [CrossRef] [PubMed]
- La Canna, G.; Torracca, L.; Barbone, A.; Scarfò, I. Unexpected Infective Endocarditis: Towards a New Alert for Clinicians. J. Clin. Med. 2024, 13, 5058. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Shamy, A.; Man, P.; Vijapurkar, G.; Hanna, B.; Kardos, A. Four Faces of Infective Endocarditis: Where Thinking Outside the Box Was Crucial. J. Clin. Med. 2025, 14, 2162. https://doi.org/10.3390/jcm14072162
El-Shamy A, Man P, Vijapurkar G, Hanna B, Kardos A. Four Faces of Infective Endocarditis: Where Thinking Outside the Box Was Crucial. Journal of Clinical Medicine. 2025; 14(7):2162. https://doi.org/10.3390/jcm14072162
Chicago/Turabian StyleEl-Shamy, Ali, Patrick Man, Gayatri Vijapurkar, Baher Hanna, and Attila Kardos. 2025. "Four Faces of Infective Endocarditis: Where Thinking Outside the Box Was Crucial" Journal of Clinical Medicine 14, no. 7: 2162. https://doi.org/10.3390/jcm14072162
APA StyleEl-Shamy, A., Man, P., Vijapurkar, G., Hanna, B., & Kardos, A. (2025). Four Faces of Infective Endocarditis: Where Thinking Outside the Box Was Crucial. Journal of Clinical Medicine, 14(7), 2162. https://doi.org/10.3390/jcm14072162