2. Methods
The presented method focuses on the usage of wearable sensors and a subsequent coarse-graining of large amounts of microscopic equidistant self-similar time sequences with (multi-)fractal structures. The complexity/fractal mesoscale/medical projection using the Hurst exponent [
10,
11,
12,
13] allows for analytic indexing replacing, e.g., Young Mania Rating Scale indices. It allows the prediction of mental disorders using a stochastic approach as well as probability characterization of BD episodes.
The Hurst exponent can be interpreted as a probability measure of a decay of time-series autocorrelation. The decay indicates that an observed signal is losing its repetitive pattern, i.e., a delayed copy of itself. Thus, decay of autocorrelation can be considered to be an increasing absence of “memory”, characterized by an absence of predictable behavioral patterns. Moreover, such decay also exhibits itself as a lack of a measured response to both external and/or internal stimuli. We hypothesize that the presence of a mental disorder can be detected and quantified by the time evolution of complexity surrogate signs of partial behavioral patterns autocorrelation in high-dimensional spaces modeling physiological, behavioral, topological, and environmental influences. Accepting this hypothesis, we conjecture that various mental disorders differ by their complexity/fractal dimension.
The presented approach, applicable, in general, to various objective diagnoses of mental disorders, combines longer time acquisition of surrogate data with subsequent mathematical coarse-graining of the raw input using complexity projections as well as stochastic optimization to achieve short-term predictability.
The first focal point is the introduction of
Digital Mental Biomarkers (
DMB). Specifically, the complexity projections of bio-sensor-generated time sequences open possibilities for quantitative re-definition of the Young Mania Rating Scale (YMRS), c.f.,
Section 2.2.
The second focal point concerns the predictability of mental states, particularly BD manic episodes. This possibility has not been explored so-far to our best knowledge. Our approach is based on finding the best fit of mono-fractal Gaussian white-noise approximation of a given complexity projection of, possibly, multifractal microscale vital sensory data, c.f.,
Section 2.3.
Thirdly, we are proposing a way to evaluate the probability of stable, meta-stable, and observable BD episodes.
The mathematical approaches to the above two areas are described in some detail in
Section 3.
2.1. Limitations
Several issues are not addressed in the presented communications.
The paper does not provide a broad longitudinal study of the applicability of accelerometer-based measurements related to BD, as this is well established in the literature. Nevertheless, the patient data used in this communication are based on 20 weeks of continuous measurements representing about 20,000 time data points.
The paper is solely focused on the application of complexity projections of sensory data and the possibility of providing objective evaluations of BD episodes, their probability of appearing, as well as their predictability. Consequently, this study focuses on novel techniques rather than comparative analysis with existing methods. We chose a patient, code name C207, as an example to demonstrate the presented phenomenology and methodology.
The efficiency of the presented approach is mentioned only briefly in
Section 2.2 as it is not the main focus of the presented paper.
2.2. Digital Mental Biomarkers
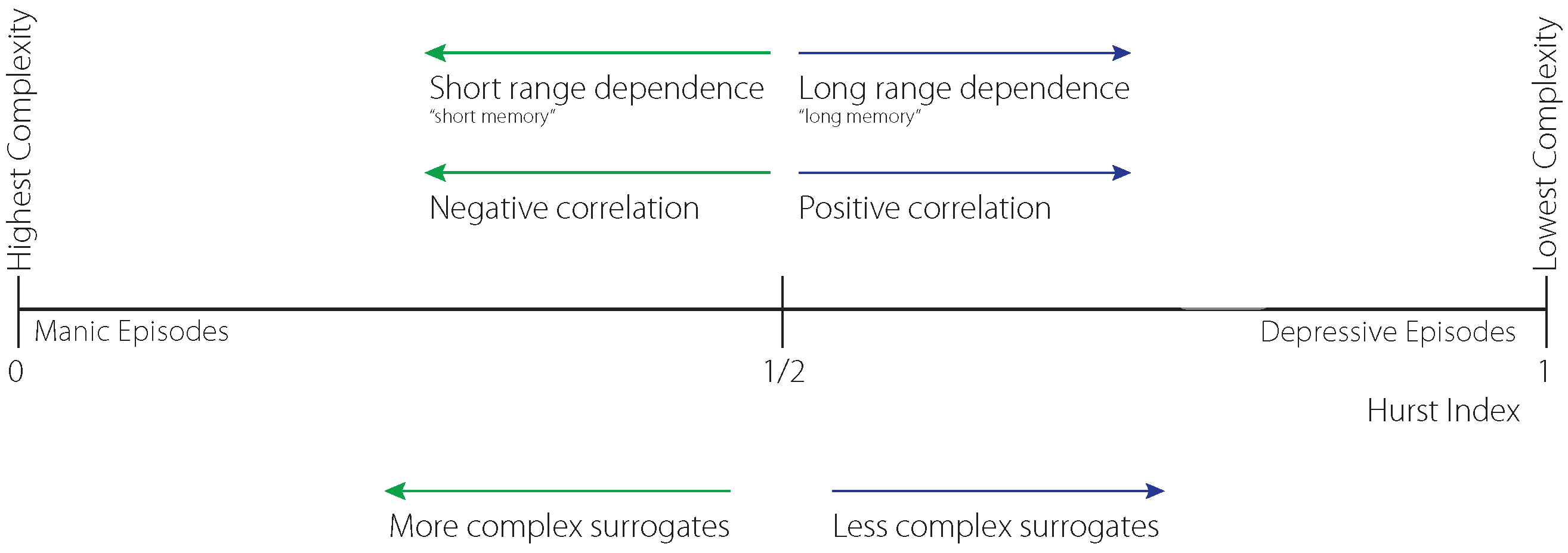
The origin of the presented Digital Mental Biomarkers is rooted in the autocorrelation of repetition of various (past) time-sequence patterns. This autocorrelation is expressed by the Hurst exponent, i.e., the Hasdorff–Besicovitch dimension of fractal structures of self-similar sequences. The underlying complexity (low probability of repetition) and its interpretation as “memory” is shown in
Figure 2.
The fractal scale is represented by the Hurst exponent (H), with a range . The exponent is important because it characterizes persistent, positively correlated, i.e., “longer memory” , sequences if and negatively correlated, anti-persistent sequences otherwise. A Wiener process is characterized by yielding neither persistent nor anti-persistent behavior represented by surrogate data.
It is important to mention that H is related to fractal dimensions by the formula , d being the dimension of the ambient space in the case of self-similar time series.
The complexity representation of vital time sequences removes the physical units that are present in vital surrogate sensor data. This feature is fundamental when analyzing different time sequences.
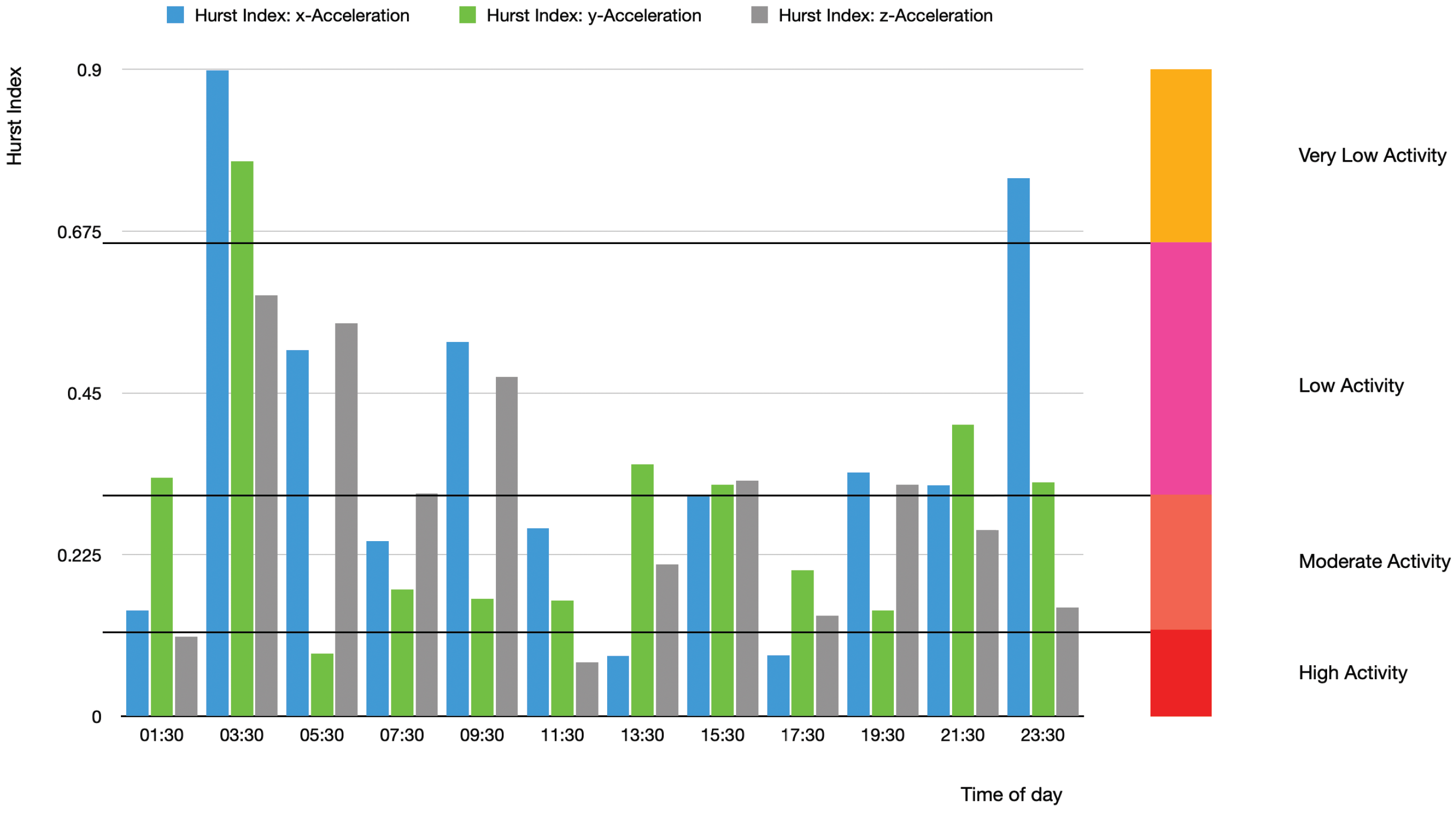
An application of the presented complexity projection approach is shown in
Figure 3 and
Table 1 using the acceleration of the left hand of a BD patient in three dimensions. The
H values below
indicate rather erratic hand movements corresponding to an anti-persistent state that can be possibly interpreted as “manic state ” which is negatively correlated.
While the Hurst exponent provides valuable insights into the predictability of rhythm, it is essential to consider its limitations. The calculation of the Hurst exponent assumes stationarity and fractal properties, which may not hold true for all types of time-series data. Additionally, the interpretation of the Hurst exponent should be context-dependent and complemented by other analysis techniques. Understanding the specific characteristics and context of the rhythm being analyzed is crucial to applying the Hurst exponent effectively, c.f.,
Table 2.
A clinical application of the proposed methodology is mentioned at
Appendix A.
Global View of the Complexity Projection of Acceleration Data
The Hurst exponent is particularly useful in understanding the underlying structure and predictability of complex rhythmic patterns. Rhythmic patterns often display fractal properties, meaning that they exhibit similar structures and behaviors across multiple scales of observation. By calculating the Hurst exponent, we assess the probability of a rhythm exhibiting persistent or anti-persistent behavior.
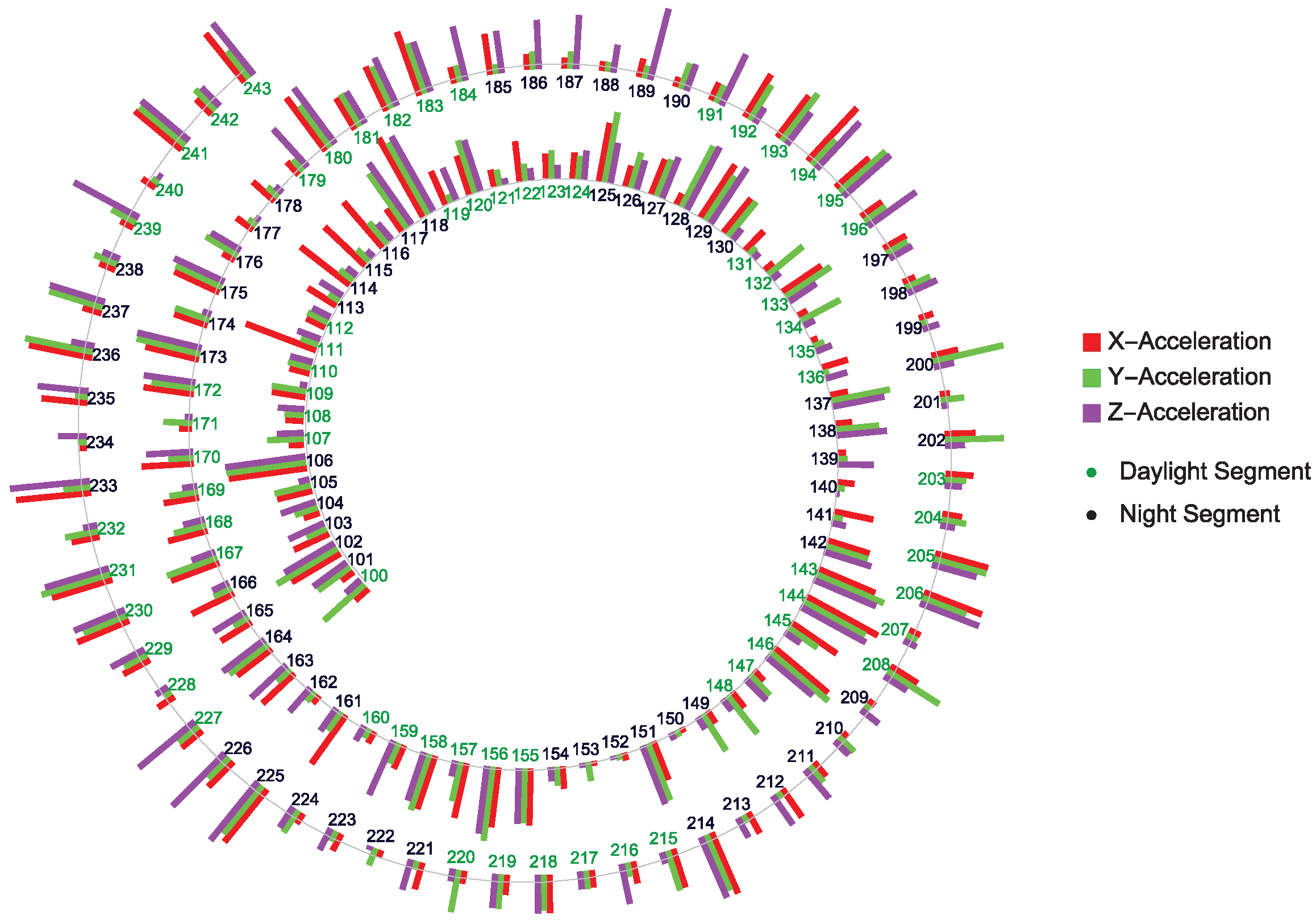
A global view of BD evolution spanning about 20 days is shown in
Figure 4. The acquired data are segmented into 120 min with a one-minute frequency of data collection. The plot displays quasi-periodic, non-chaotic patterns of, e.g., depression periods represented by the longer bars at, e.g., time segments 225–227, 235–237, etc.
The different complexity distribution of the different direction acceleration points shown in
Figure 4 points to possible multifractal BD structure(s) hidden in the actigraphy data.
2.3. Predictability
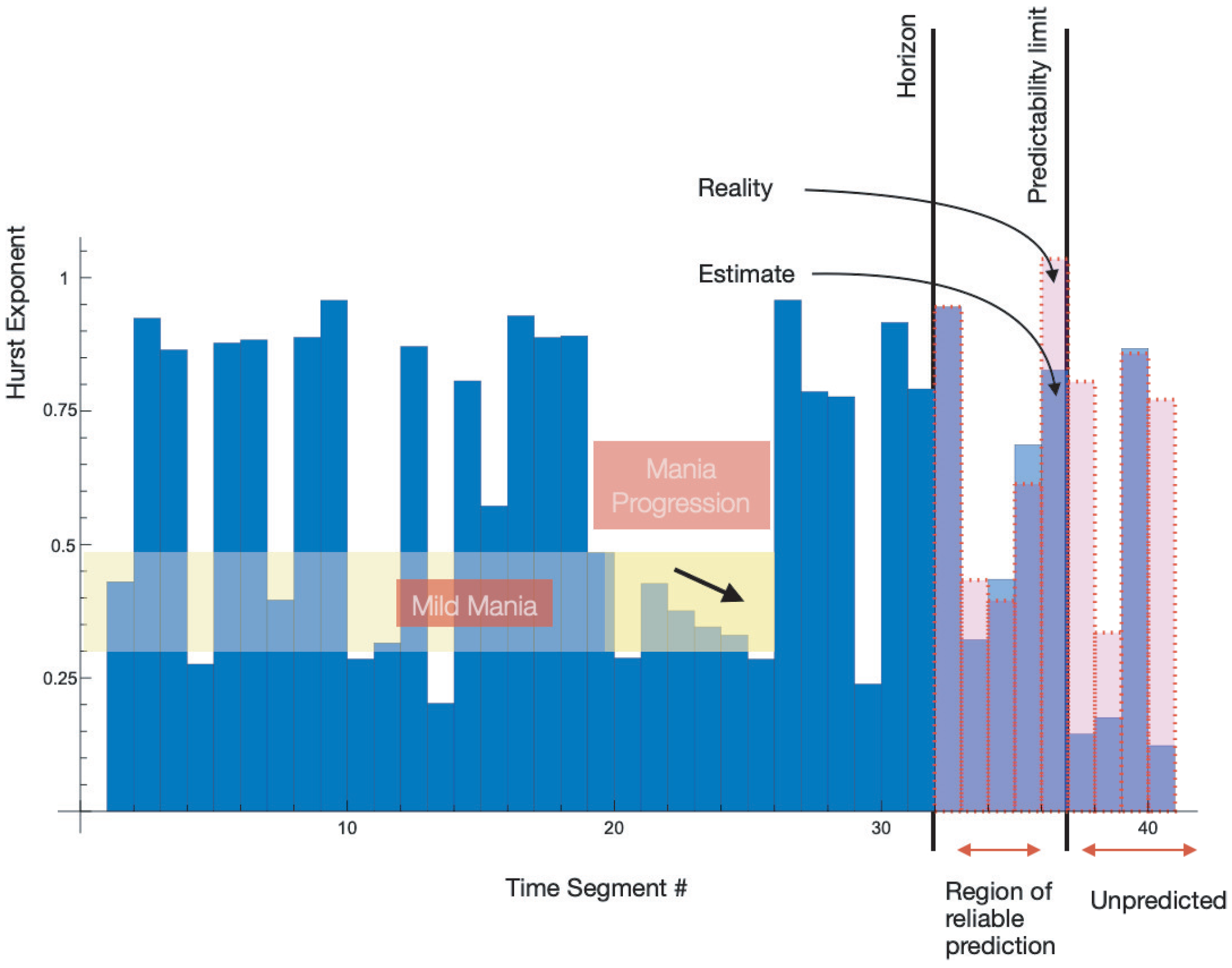
An example of the application of this stochastic optimization is shown in
Figure 5 considering a patient’s complexity data. This example can serve as a mechanism for early warning systems such as the prediction of, e.g., manic relapses.
Figure 5.
The plot shows application of the presented variational principle to predict the possible evolution of mental deceases and BD in particular. The known data are shortened, and the extrapolation is compared to the real complexity image of patient
. The Hurst Exponent evolution is shown for the dataset
and a predictability evolution of the data. The plot is based on 41 Hurst exponents related to a partition of the original data. Only 31 data points are considered when computing the extrapolation using the approach described above. The predicted and the assumed unknown dynamics of the mesoscale Hurst indices evolution are compared. The red dotted line highlights the assumed unknown Hurst data points, while the light blue rectangles indicate the computed Hurst exponent evolution prediction. The prediction errors are shown in
Table 3.
Figure 5.
The plot shows application of the presented variational principle to predict the possible evolution of mental deceases and BD in particular. The known data are shortened, and the extrapolation is compared to the real complexity image of patient
. The Hurst Exponent evolution is shown for the dataset
and a predictability evolution of the data. The plot is based on 41 Hurst exponents related to a partition of the original data. Only 31 data points are considered when computing the extrapolation using the approach described above. The predicted and the assumed unknown dynamics of the mesoscale Hurst indices evolution are compared. The red dotted line highlights the assumed unknown Hurst data points, while the light blue rectangles indicate the computed Hurst exponent evolution prediction. The prediction errors are shown in
Table 3.
The approach to predictability fundamentally relies on the coarse-graining of microscopic self-similar vital time sequences. This step that is performed by complexity mesoscale projection of microscopic signals is similar to the step from Langevin dynamics to the Fokker–Planck one.
2.4. Observable and Meta-Stable Mental Episodes
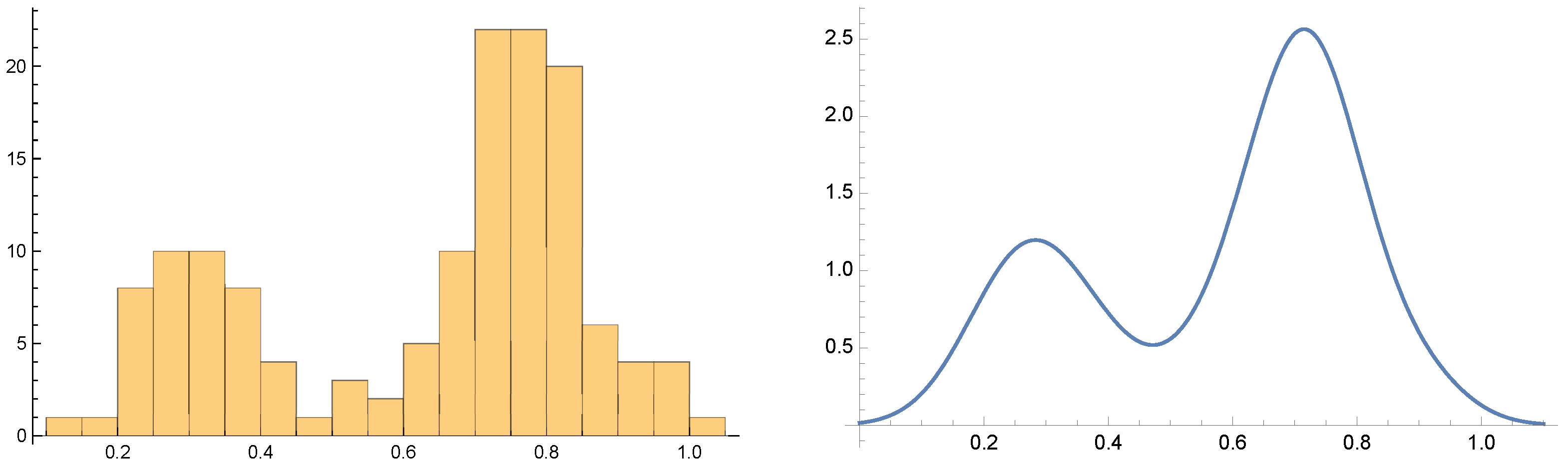
Both the histogram and the PDF shown at
Figure 6 resulting from the clinical BD
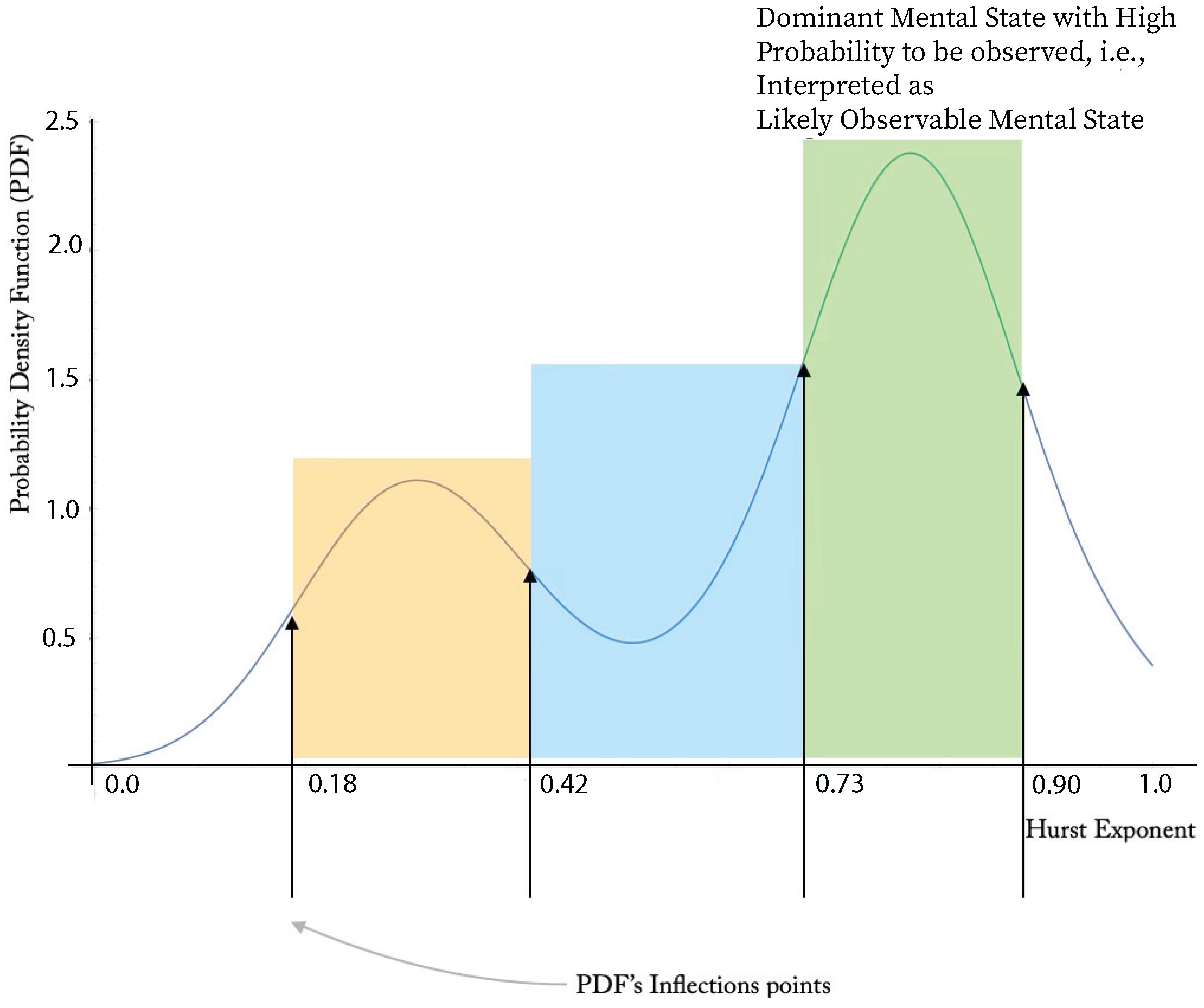
patient actigraphy data exhibit “inverted double-well” structure. We conclude that the BD evolution is a mixture of stable and meta-stable mental states. In general, the propose a stable/meta-stable distinction to be related to the type of local extrema of PDF. Analytically, the state differentiation is computed in the “dual” state of PDF. The approach is demonstrated at
Figure 7. The probabilities of the particular states are computed at
Table 4.
The probabilities shown at
Table 4 point to another important aspect of the presented semicontinuous approach. We indicate at
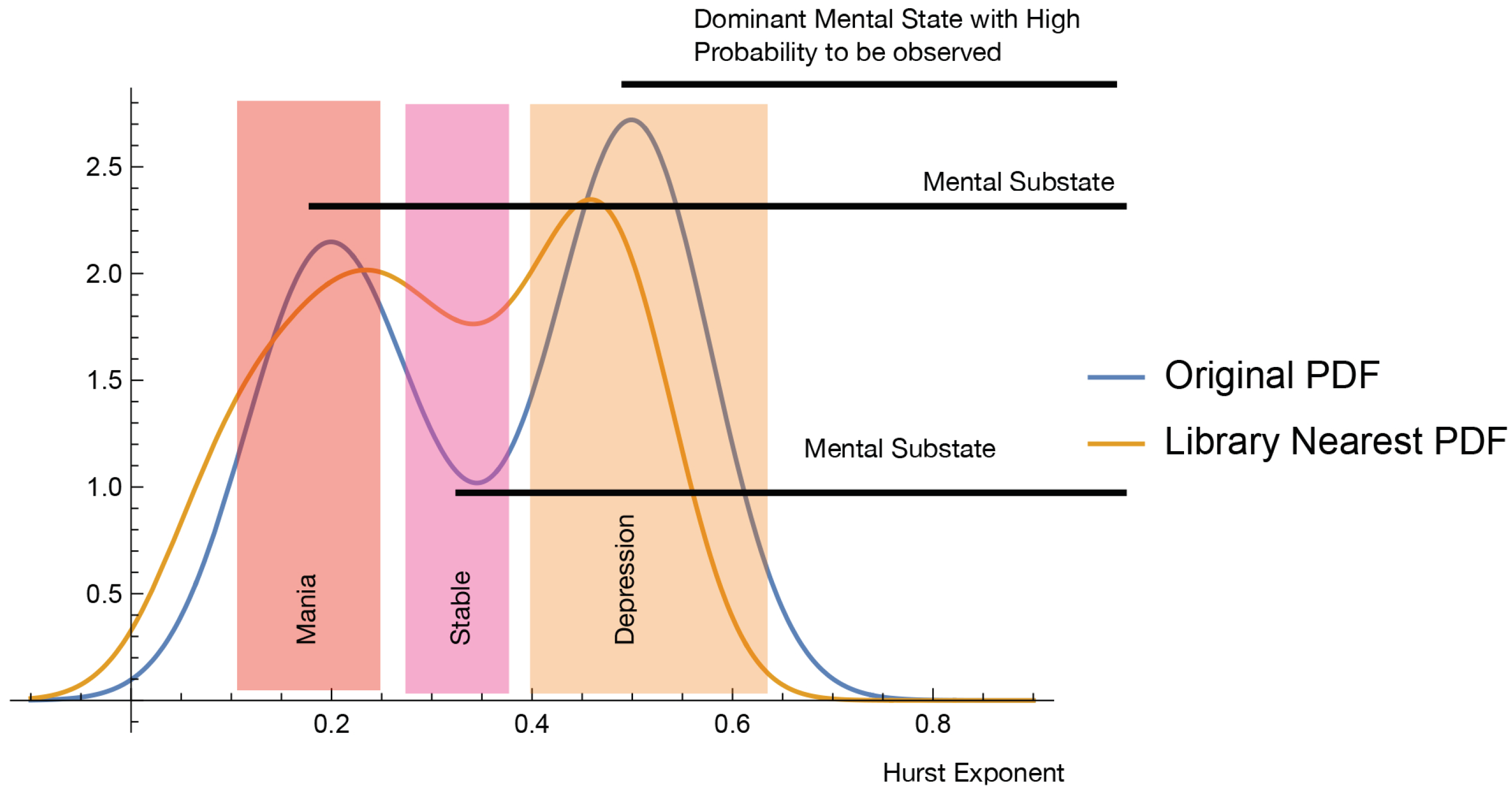
Figure 1 possible overshadowing of BD by an observable depression phase. The actigraphy data corresponding to the
patient indicate that the probability of observing the depression episode,
, is higher than the manic and stable episodes. That is likely to mean that a large-in-time dispersion of both subjective and objective evaluations would indicate depression phases, states with a higher observable probability, overlooking the other mental states.
The mathematical aspect of computation of the probabilities shown in
Table 4 are discussed in
Section 3.4.
The BD episodes probabilities shown in
Table 4 correspond to the time distribution of the Hurst exponents displayed in
Figure 5. There are about 16 depression episodes in 31 of 120-min segments and 7 manic episodes of the
patient. Thus, spared observations will most likely report depression episodes, hiding the manic ones. This conclusion is confirmed by the computed probabilities, c.f.,
Table 4.