Mechanisms of Endometrioma-Mediated Ovarian Damage: Myths and Facts
Abstract
1. Introduction
2. Materials and Methods
3. The Impact of Endometriomas on Ovarian Function
3.1. The Effect of Endometrioma on the “Two-Cell-Two-Gonadotropin Theory” and Folliculogenesis
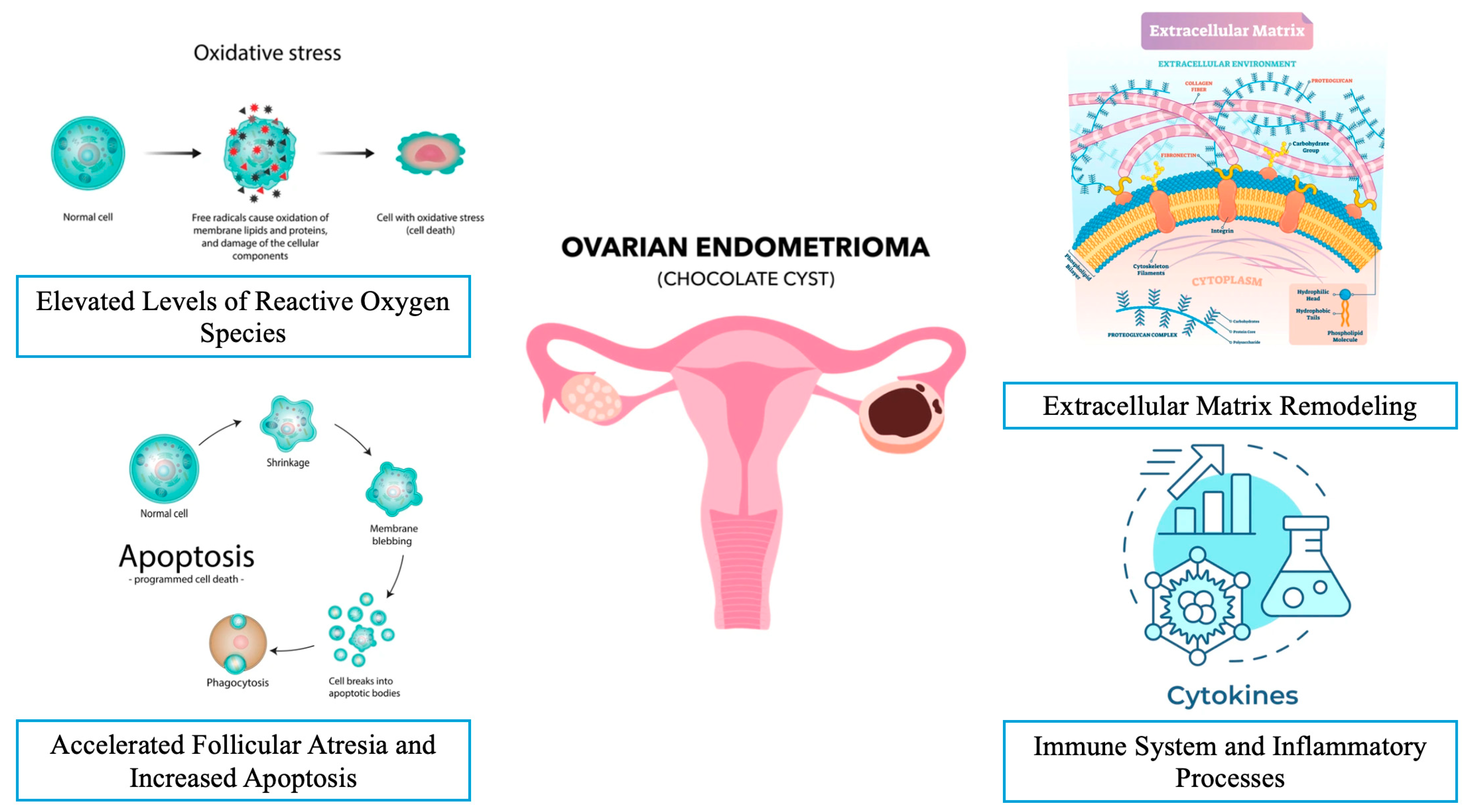
3.2. Endometriomas and Ovarian Microenvironment
3.2.1. The Effect of Elevated Levels of Reactive Oxygen Species (ROS)
| Pathophysiology | Effects on Ovarian Function and Microenvironment |
|---|---|
| Elevated levels of ROS [38,39,40,49,50] |
|
| Accelerated follicular atresia and increased apoptosis [51,52,53,54,55,56,57,58] |
|
| Activation of the plasminogen system and matrix metalloproteinases [59,60,61,62,63,64] |
|
| Chronic inflammation and increased levels of cytokines [65,66,67,68,69,70,71,72,73,74,75] |
|
3.2.2. Accelerated Follicular Atresia and Increased Apoptosis in the Presence of Endometrioma
3.2.3. Extracellular Matrix Remodeling and Fibrosis in the Presence of Endometrioma
3.2.4. The Immune System and Inflammatory Processes in the Presence of Endometrioma
4. The Impact of Endometriomas on Assisted Reproductive Technology Outcomes
| Myths | Facts |
|---|---|
| Myth: Endometriomas do not significantly affect the ovarian reserve. | Fact: Endometriomas are associated with a reduction in the ovarian reserve, evidenced by decreased AFC and AMH levels [15,16,61,62,63]. |
| Myth: Endometriomas do not affect the oocyte or embryo quality. | Fact: Endometriomas are linked to reduced oocyte and embryo quality due to oxidative stress, inflammation, and altered follicular fluid composition [21,43,50,89,90,91]. |
| Myth: Endometriomas do not influence IVF outcomes. | Fact: While live birth rates are not significantly affected, endometriomas are associated with a lower number of retrieved oocytes and poor ovarian response during IVF [84,85]. |
| Myth: Oxidative stress in endometriomas has no significant impact on ovarian function. | Fact: Elevated ROS in endometriomas cause mitochondrial DNA damage, ATP depletion, chromosomal aberrations, and impaired folliculogenesis [40,41,42,43,45]. |
| Myth: Endometriomas do not accelerate follicular atresia. | Fact: Endometriomas promote the premature activation of primordial follicles and accelerate follicular atresia, leading to a decline in the ovarian reserve [55,56,57]. |
| Myth: Endometriomas do not alter the ovarian microenvironment. | Fact: Endometriomas induce chronic inflammation, fibrosis, and extracellular matrix remodeling, disrupting the ovarian microenvironment [65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82]. |
| Myth: Endometriomas do not affect granulosa cell function. | Fact: Endometriomas impair granulosa cell steroidogenesis, induce apoptosis, and disrupt energy metabolism, negatively impacting follicular development [19,20,21,22,25,30,31]. |
| Myth: Endometriomas do not influence theca cell function. | Fact: Endometriomas alter theca cell hormone production and the secretion of growth factors, such as BMP-15, which may impact follicular health [32,33,34,35,36]. |
| Myth: Endometriomas do not trigger immune dysregulation. | Fact: Endometriomas are associated with elevated levels of pro-inflammatory cytokines (e.g., IL-6, IL-8, IL-1β) and immune dysfunction, contributing to disease progression [71,72,73,74,75,76,77,78,79,80,81,82]. |
| Myth: Endometriomas do not cause fibrosis in the ovarian tissue. | Fact: Endometriomas are surrounded by fibrotic tissue due to excessive extracellular matrix remodeling and the activation of fibrogenic pathways (e.g., TGF-β, PDGF) [65,66,67,68,69,70]. |
| Myth: Endometriomas do not affect the follicular fluid composition. | Fact: Endometriomas alter follicular fluid by increasing iron levels, ROS, and pro-inflammatory cytokines, which negatively impacts oocyte maturation and quality [21,37,45,46,47,48,49,50,51,52,53,54]. |
| Myth: Endometriomas do not activate signaling pathways that harm ovarian function. | Fact: Endometriomas activate pathways such as PI3K/AKT/mTOR, YAP, and TAZ, leading to aberrant follicular activation and ovarian damage [56,57,58,59,60]. |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Donnez, J. Endometriosis: Enigmatic in the pathogenesis and controversial in its therapy. Fertil. Steril. 2012, 98, 509–510. [Google Scholar] [CrossRef] [PubMed]
- Working group of ESGE, ESHRE and WES; Saridogan, E.; Becker, C.M.; Feki, A.; Grimbizis, G.F.; Hummelshoj, L.; Keckstein, J.; Nisolle, M.; Tanos, V.; Ulrich, U.A.; et al. Recommendations for the Surgical Treatment of Endometriosis. Part 1: Ovarian Endometrioma. Hum. Reprod. Open 2017, 19, hox016. [Google Scholar] [CrossRef]
- Hughesdon, P.E. The structure of endometrial cysts of the ovary. J. Obs. Gynaecol. Br. Emp. 1957, 64, 481–487. [Google Scholar] [CrossRef]
- Brosens, I.A.; Puttemans, P.J.; Deprest, J. The endoscopic localization of endometrial implants in the ovarian chocolate cyst. Fertil. Steril. 1994, 61, 1034–1038. [Google Scholar] [CrossRef]
- Nezhat, F.; Nezhat, C.; Allan, C.J.; Metzger, D.A.; Sears, D.L. Clinical and histologic classification of endometriomas. Implications for a mechanism of pathogenesis. J. Reprod. Med. 1992, 37, 771–776. [Google Scholar]
- Nisolle, M.; Donnez, J. Peritoneal endometriosis, ovarian endometriosis, and adenomyotic nodules of the rectovaginal septum are three different entities. Fertil. Steril. 1997, 68, 585–596. [Google Scholar] [CrossRef]
- Zondervan, K.T.; Becker, C.M.; Missmer, S.A. Endometriosis. N. Engl. J. Med. 2020, 26, 1244–1256. [Google Scholar] [CrossRef]
- Kalra, R.; McDonnell, R.; Stewart, F.; Hart, R.J.; Hickey, M.; Farquhar, C. Excisional surgery versus ablative surgery for ovarian endometrioma. Cochrane. Database Syst. Rev. 2024, 26, CD004992. [Google Scholar] [CrossRef]
- Prescott, J.; Farland, L.V.; Tobias, D.K.; Gaskins, A.J.; Spiegelman, D.; Chavarro, J.E.; Rich-Edwards, J.W.; Barbieri, R.L.; Missmer, S.A. A prospective cohort study of endometriosis and subsequent risk of infertility. Hum. Reprod. 2016, 31, 1475–1482. [Google Scholar] [CrossRef]
- Somigliana, E.; Infantino, M.; Benedetti, F.; Arnoldi, M.; Calanna, G.; Ragni, G. The presence of ovarian endometriomas is associated with a reduced responsiveness to gonadotropins. Fertil. Steril. 2006, 86, 192–196. [Google Scholar] [CrossRef]
- Raffi, F.; Metwally, M.; Amer, S. The impact of excision of ovarian endometrioma on ovarian reserve: A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2012, 97, 3146–3154. [Google Scholar] [CrossRef] [PubMed]
- Coccia, M.E.; Rizzello, F.; Mariani, G.; Bulletti, C.; Palagiano, A.; Scarselli, G. Ovarian surgery for bilateral endometriomas influences age at menopause. Hum. Reprod. 2011, 26, 3000–3007. [Google Scholar] [CrossRef] [PubMed]
- Latif, S.; Khanjani, S.; Saridogan, E. Endometriosis and In Vitro Fertilization. Medicina 2024, 21, 1358. [Google Scholar] [CrossRef]
- Harb, H.M.; Gallos, I.D.; Chu, J.; Harb, M.; Coomarasamy, A. The effect of endometriosis on in vitro fertilisation outcome: A systematic review and meta-analysis. BJOG 2013, 120, 1308–1320. [Google Scholar] [CrossRef]
- Hamdan, M.; Dunselman, G.; Li, T.C.; Cheong, Y. The impact of endometrioma on IVF/ICSI outcomes: A systematic review and meta-analysis. Hum. Reprod. Update 2015, 21, 809–825. [Google Scholar] [CrossRef]
- Latif, S.; Saridogan, E. Endometriosis, Oocyte, and Embryo Quality. J. Clin. Med. 2023, 21, 4186. [Google Scholar] [CrossRef]
- Fan, W.; Yuan, Z.; Li, M.; Zhang, Y.; Nan, F. Decreased oocyte quality in patients with endometriosis is closely related to abnormal granulosa cells. Front. Endocrinol. 2023, 16, 1226687. [Google Scholar] [CrossRef]
- Bonavina, G.; Taylor, H.S. Endometriosis-associated infertility: From pathophysiology to tailored treatment. Front. Endocrinol. 2022, 26, 1020827. [Google Scholar] [CrossRef]
- Sanchez, A.M.; Somigliana, E.; Vercellini, P.; Pagliardini, L.; Candiani, M.; Vigano, P. Endometriosis as a detrimental condition for granulosa cell steroidogenesis and development: From molecular alterations to clinical impact. J. Steroid. Biochem. Mol. Biol. 2016, 155, 35–46. [Google Scholar] [CrossRef]
- Ding, Y.; Zhu, Q.; He, Y.; Lu, Y.; Wang, Y.; Qi, J.; Wu, H.; Xu, R.; Li, J.; Li, X.; et al. Induction of autophagy by Beclin-1 in granulosa cells contributes to follicular progesterone elevation in ovarian endometriosis. Transl. Res. 2021, 227, 15–29. [Google Scholar] [CrossRef]
- Yland, J.; Carvalho, L.F.P.; Beste, M.; Bailey, A.; Thomas, C.; Abrão, M.S.; Racowsky, C.; Griffith, L.; Missmer, S.A. Endometrioma, the follicular fluid inflammatory network and its association with oocyte and embryo characteristics. Reprod. Biomed. Online 2020, 40, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, S.; Schubert, B. Oxidative stress status in normal ovarian cortex surrounding ovarian endometriosis. Fertil. Steril. 2010, 1, 2431–2432. [Google Scholar] [CrossRef] [PubMed]
- González-Fernández, R.; Peña, Ó.; Hernández, J.; Martín-Vasallo, P.; Palumbo, A.; Ávila, J. Patients with endometriosis and patients with poor ovarian reserve have abnormal follicle-stimulating hormone receptor signaling pathways. Fertil. Steril. 2011, 95, 2373–2378. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shen, X.X.; Huang, X.H.; Zhao, Z.M. Follicular fluid levels of prostaglandin E2 and the effect of prostaglandin E2 on steroidogenesis in granulosa-lutein cells in women with moderate and severe endometriosis undergoing in vitro fertilization and embryo transfer. Chin. Med. J. 2012, 125, 3985–3990. [Google Scholar]
- Sreerangaraja Urs, D.B.; Wu, W.H.; Komrskova, K.; Postlerova, P.; Lin, Y.F.; Tzeng, C.R.; Kao, S.H. Mitochondrial Function in Modulating Human Granulosa Cell Steroidogenesis and Female Fertility. Int. J. Mol. Sci. 2020, 19, 3592. [Google Scholar] [CrossRef]
- Wunder, D.M.; Mueller, M.D.; Birkhäuser, M.H.; Bersinger, N.A. Steroids and protein markers in the follicular fluid as indicators of oocyte quality in patients with and without endometriosis. J. Assist. Reprod. Genet. 2005, 22, 257–264. [Google Scholar] [CrossRef]
- Garrido, N.; Navarro, J.; Remohí, J.; Simón, C.; Pellicer, A. Follicular hormonal environment and embryo quality in women with endometriosis. Hum. Reprod. Update 2000, 6, 67–74. [Google Scholar] [CrossRef]
- Pellicer, A.; Valbuena, D.; Bauset, C.; Albert, C.; Bonilla-Musoles, F.; Remohí, J.; Simón, C. The follicular endocrine environment in stimulated cycles of women with endometriosis: Steroid levels and embryo quality. Fertil. Steril. 1998, 69, 1135–1141. [Google Scholar] [CrossRef]
- Garrido, N.; Krüssel, J.S.; Remohí, J.; Simón, C.; Pellicer, A. Expression and function of 3beta hydroxisteroid dehydrogenase (3beta HSD) type II and corticosteroid binding globulin (CBG) in granulosa cells from ovaries of women with and without endometriosis. J. Assist. Reprod. Genet. 2002, 19, 24–30. [Google Scholar] [CrossRef]
- Huo, P.; Zhang, N.; Zhang, P.; Wu, X. The levels of follicular fluid cell-free mitochondrial DNA could serve as a biomarker for pregnancy success in patients with small ovarian endometriosis cysts: A case-control study. Medicine 2020, 25, e23348. [Google Scholar] [CrossRef]
- Kunitomi, C.; Harada, M.; Takahashi, N.; Azhary, J.M.K.; Kusamoto, A.; Nose, E.; Oi, N.; Takeuchi, A.; Wada-Hiraike, O.; Hirata, T.; et al. Activation of endoplasmic reticulum stress mediates oxidative stress-induced apoptosis of granulosa cells in ovaries affected by endometrioma. Mol. Hum. Reprod. 2020, 1, 40–52. [Google Scholar] [CrossRef]
- Young, J.M.; McNeilly, A.S. Theca: The forgotten cell of the ovarian follicle. Reproduction 2010, 140, 489–504. [Google Scholar] [CrossRef]
- Richards, J.S.; Ren, Y.A.; Candelaria, N.; Adams, J.E.; Rajkovic, A. Ovarian Follicular Theca Cell Recruitment, Differentiation, and Impact on Fertility: 2017 Update. Endocr. Rev. 2018, 1, 1–20. [Google Scholar] [CrossRef]
- Casalechi, M.; Di Stefano, G.; Fornelli, G.; Somigliana, E.; Viganò, P. Impact of endometriosis on the ovarian follicles. Best. Pract. Res. Clin. Obstet. Gynaecol. 2024, 92, 102430. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, R.B.; Lane, M.; Thompson, J.G. Oocyte-secreted factors: Regulators of cumulus cell function and oocyte quality. Hum. Reprod. Update 2008, 14, 159–177. [Google Scholar] [CrossRef]
- Rasulzade, A.; Akalın, M.; Demirdağ, E.; Erdem, M.; Erdem, A. Comparing serum and follicle fluid GDF-9 and BMP-15 parameters in IVF patients with ovarian endometrioma. Reprod. BioMedicine Online 2023, 47, 103459. [Google Scholar]
- Yan, S.; Dong, X.; Ding, D.; Xue, J.; Wang, X.; Huang, Y.; Pan, Z.; Sun, H.; Ren, Q.; Dou, W.; et al. Iron deposition in ovarian endometriosis evaluated by magnetic resonance imaging R2* correlates with ovarian function. Reprod. Biomed. Online 2023, 47, 103231. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Mandai, M.; Toyokuni, S.; Hamanishi, J.; Higuchi, T.; Takakura, K.; Fujii, S. Contents of endometriotic cysts, especially the high concentration of free iron, are a possible cause of carcinogenesis in the cysts through the iron-induced persistent oxidative stress. Clin. Cancer. Res. 2008, 14, 32–40. [Google Scholar]
- Aramouni, K.; Assaf, R.; Shaito, A.; Fardoun, M.; Al-Asmakh, M.; Sahebkar, A.; Eid, A.H. Biochemical and cellular basis of oxidative stress: Implications for disease onset. J. Cell. Physiol. 2023, 238, 1951–1963. [Google Scholar] [CrossRef]
- Shields, H.J.; Traa, A.; Van Raamsdonk, J.M. Beneficial and Detrimental Effects of Reactive Oxygen Species on Lifespan: A Comprehensive Review of Comparative and Experimental Studies. Front. Cell Dev. Biol. 2021, 11, 628157. [Google Scholar] [CrossRef]
- Drum, B.M.; Yuan, C.; Li, L.; Liu, Q.; Wordeman, L.; Santana, L.F. Oxidative stress decreases microtubule growth and stability in ventricular myocytes. J. Mol. Cell Cardiol. 2016, 93, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Limoli, C.L.; Giedzinski, E. Induction of chromosomal instability by chronic oxidative stress. Neoplasia 2003, 5, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 28, 4642. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, Z.; Lv, L.; Liu, C.; Wang, L.; Sun, Y.N.; Zhao, Z.; Shi, B.; Li, Q.; Hao, G.M. Molecular regulation of DNA damage and repair in female infertility: A systematic review. Reprod. Biol. Endocrinol. 2024, 14, 103. [Google Scholar] [CrossRef]
- Agarwal, A.; Aponte-Mellado, A.; Premkumar, B.J.; Shaman, A.; Gupta, S. The effects of oxidative stress on female reproduction: A review. Reprod. Biol. Endocrinol. 2012, 29, 49. [Google Scholar] [CrossRef]
- Giacomini, E.; Sanchez, A.M.; Sarais, V.; Beitawi, S.A.; Candiani, M.; Viganò, P. Characteristics of follicular fluid in ovaries with endometriomas. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 209, 34–38. [Google Scholar] [CrossRef]
- Wyatt, J.; Fernando, S.M.; Powell, S.G.; Hill, C.J.; Arshad, I.; Probert, C.; Ahmed, S.; Hapangama, D.K. The role of iron in the pathogenesis of endometriosis: A systematic review. Hum. Reprod. Open 2023, 2023, hoad033. [Google Scholar] [CrossRef]
- Li, A.; Ni, Z.; Zhang, J.; Cai, Z.; Kuang, Y.; Yu, C. Transferrin Insufficiency and Iron Overload in Follicular Fluid Contribute to Oocyte Dysmaturity in Infertile Women with Advanced Endometriosis. Front. Endocrinol. 2020, 19, 391. [Google Scholar] [CrossRef]
- Scutiero, G.; Iannone, P.; Bernardi, G.; Bonaccorsi, G.; Spadaro, S.; Volta, C.A.; Greco, P.; Nappi, L. Oxidative Stress and Endometriosis: A Systematic Review of the Literature. Oxid. Med. Cell. Longev. 2017, 2017, 7265238. [Google Scholar] [CrossRef]
- Singh, A.K.; Chattopadhyay, R.; Chakravarty, B.; Chaudhury, K. Markers of oxidative stress in follicular fluid of women with endometriosis and tubal infertility undergoing IVF. Reprod. Toxicol. 2013, 42, 116–124. [Google Scholar] [CrossRef]
- Nakagawa, K.; Hisano, M.; Sugiyama, R.; Yamaguchi, K. Measurement of oxidative stress in the follicular fluid of infertility patients with an endometrioma. Arch. Gynecol. Obstet. 2016, 293, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Regiani, T.; Cordeiro, F.B.; da Costa Ldo, V.; Salgueiro, J.; Cardozo, K.; Carvalho, V.M.; Perkel, K.J.; Zylbersztejn, D.S.; Cedenho, A.P.; Lo Turco, E.G. Follicular fluid alterations in endometriosis: Label-free proteomics by MS(E) as a functional tool for endometriosis. Syst. Biol. Reprod. Med. 2015, 61, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Jafari, R.; Taghavi, S.A.; Amirchaghmaghi, E.; Yazdi, R.S.; Karimian, L.; Ashrafi, M.; Aflatoonian, R. Detailed Investigation of Downstream TLR Signaling in the Follicular Cells of Women with Endometriosis. J. Reprod. Infertil. 2020, 21, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Homer, H.A. The Role of Oocyte Quality in Explaining "Unexplained" Infertility. Semin. Reprod. Med. 2020, 38, 21–28. [Google Scholar] [CrossRef]
- Dolmans, M.M.; Martinez-Madrid, B.; Gadisseux, E.; Guiot, Y.; Yuan, W.Y.; Torre, A.; Camboni, A.; Van Langendonckt, A.; Donnez, J. Short-term transplantation of isolated human ovarian follicles and cortical tissue into nude mice. Reproduction 2007, 134, 253–262. [Google Scholar] [CrossRef]
- David, A.; Van Langendonckt, A.; Gilliaux, S.; Dolmans, M.M.; Donnez, J.; Amorim, C.A. Effect of cryopreservation and transplantation on the expression of kit ligand and anti-Mullerian hormone in human ovarian tissue. Hum. Reprod. 2012, 27, 1088–1095. [Google Scholar] [CrossRef]
- Kitajima, M.; Dolmans, M.M.; Donnez, O.; Masuzaki, H.; Soares, M.; Donnez, J. Enhanced follicular recruitment and atresia in cortex derived from ovaries with endometriomas. Fertil. Steril. 2014, 101, 1031–1037. [Google Scholar] [CrossRef]
- McKinnon, B.D.; Kocbek, V.; Nirgianakis, K.; Bersinger, N.A.; Mueller, M.D. Kinase signalling pathways in endometriosis: Potential targets for non-hormonal therapeutics. Hum. Reprod. Update 2016, 22, 382–403. [Google Scholar] [CrossRef]
- Takeuchi, A.; Koga, K.; Satake, E.; Makabe, T.; Taguchi, A.; Miyashita, M.; Takamura, M.; Harada, M.; Hirata, T.; Hirota, Y.; et al. Endometriosis Triggers Excessive Activation of Primordial Follicles via PI3K-PTEN-Akt-Foxo3 Pathway. J. Clin. Endocrinol. Metab. 2019, 104, 5547–5554. [Google Scholar] [CrossRef]
- Matsuzaki, S.; Pankhurst, M.W. Hyperactivation of dormant primordial follicles in ovarian endometrioma patients. Reproduction 2020, 160, R145–R153. [Google Scholar] [CrossRef]
- Kasapoglu, I.; Ata, B.; Uyaniklar, O.; Seyhan, A.; Orhan, A.; Yildiz Oguz, S.; Uncu, G. Endometrioma-related reduction in ovarian reserve (ERROR): A prospective longitudinal study. Fertil. Steril. [CrossRef]
- Muzii, L.; Di Tucci, C.; Di Feliciantonio, M.; Galati, G.; Di Donato, V.; Musella, A.; Palaia, I.; Panici, P.B. Antimüllerian hormone is reduced in the presence of ovarian endometriomas: A systematic review and meta-analysis. Fertil. Steril. 2018, 110, 932–940.e1. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Zhang, Y.; Zhang, C.; Wang, Y.; Zhu, H.L. Antral follicle count is reduced in the presence of endometriosis: A systematic review and meta-analysis. Reprod. Biomed. Online 2021, 42, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Samarakoon, R.; Overstreet, J.M.; Higgins, P.J. TGF-β signaling in tissue fibrosis: Redox controls, target genes and therapeutic opportunities. Cell. Signal. 2013, 25, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Ramón, L.; Gilabert-Estellés, J.; Castelló, R.; Gilabert, J.; España, F.; Romeu, A.; Chirivella, M.; Aznar, J.; Estellés, A. mRNA analysis of several components of the plasminogen activator and matrix metalloproteinase systems in endometriosis using a real-time quantitative RT-PCR assay. Hum. Reprod. 2005, 20, 272–278. [Google Scholar] [CrossRef]
- Boss, E.A.; Massuger, L.F.; Thomas, C.M.; Geurts-Moespot, A.; van Schaik, J.H.; Boonstra, H.; Sweep, C.G. Clinical value of components of the plasminogen activation system in ovarian cyst fluid. Anticancer. Res. 2002, 22, 275–282. [Google Scholar]
- Chung, H.W.; Wen, Y.; Chun, S.H.; Nezhat, C.; Woo, B.H.; Lake Polan, M. Matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-3 mRNA expression in ectopic and eutopic endometrium in women with endometriosis: A rationale for endometriotic invasiveness. Fertil. Steril. 2001, 75, 152–159. [Google Scholar] [CrossRef]
- Kokorine, I.; Nisolle, M.; Donnez, J.; Eeckhout, Y.; Courtoy, P.J.; Marbaix, E. Expression of interstitial collagenase (matrix metalloproteinase-1) is related to the activity of human endometriotic lesions. Fertil. Steril. 1997, 68, 246–251. [Google Scholar] [CrossRef]
- Luddi, A.; Marrocco, C.; Governini, L.; Semplici, B.; Pavone, V.; Luisi, S.; Petraglia, F.; Piomboni, P. Expression of Matrix Metalloproteinases and Their Inhibitors in Endometrium: High Levels in Endometriotic Lesions. Int. J. Mol. Sci. 2020, 18, 2840. [Google Scholar] [CrossRef]
- Garcia Garcia, J.M.; Vannuzzi, V.; Donati, C.; Bernacchioni, C.; Bruni, P.; Petraglia, F. Endometriosis: Cellular and Molecular Mechanisms Leading to Fibrosis. Reprod. Sci. 2023, 30, 1453–1461. [Google Scholar] [CrossRef]
- Dymanowska-Dyjak, I.; Terpiłowska, B.; Morawska-Michalska, I.; Michalski, A.; Polak, G.; Terpiłowski, M.; Rahnama-Hezavah, M.; Grywalska, E. Immune Dysregulation in Endometriomas: Implications for Inflammation. Int. J. Mol. Sci. 2024, 28, 4802. [Google Scholar] [CrossRef]
- Carmona, F.; Chapron, C.; Martínez-Zamora, M.Á.; Santulli, P.; Rabanal, A.; Martínez-Florensa, M.; Lozano, F.; Balasch, J. Ovarian endometrioma but not deep infiltrating endometriosis is associated with increased serum levels of interleukin-8 and interleukin-6. J. Reprod. Immunol. 2012, 95, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Werdel, R.; Mabie, A.; Evans, T.L.; Coté, R.D.; Schlundt, A.; Doehrman, P.; Dilsaver, D.; Coté, J.J. Serum Levels of Interleukins in Endometriosis Patients: A Systematic Review and Meta-analysis. J. Minim. Invasive. Gynecol. 2024, 31, 387–396.e11. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.J.; Jeung, I.C.; Park, A.; Park, Y.J.; Jung, H.; Kim, T.D.; Lee, H.G.; Choi, I.; Yoon, S.R. An increased level of IL-6 suppresses NK cell activity in peritoneal fluid of patients with endometriosis via regulation of SHP-2 expression. Hum. Reprod. 2014, 10, 2176–2189. [Google Scholar] [CrossRef] [PubMed]
- Gabay, C. Interleukin-6 and chronic inflammation. Arthritis. Res. Ther. 2006, 8, S3. [Google Scholar] [CrossRef]
- Demir, B.; Guven, S.; Guven, E.S.; Atamer, Y.; Gul, T. Serum IL-6 level may have role in the pathophysiology of unexplained infertility. Am. J. Reprod. Immunol. 2009, 62, 261–267. [Google Scholar] [CrossRef]
- Javvaji, P.K.; Francis, J.R.; Dhali, A.; Kolte, A.P.; Mech, A.; Roy, S.C.; Mishra, A. Interleukin-6 stimulates in vitro development of late-stage ovine embryos. J. Reprod. Immunol. 2023, 159, 104133. [Google Scholar] [CrossRef]
- Daraï, E.; Detchev, R.; Hugol, D.; Quang, N.T. Serum and cyst fluid levels of interleukin (IL)-6, IL-8 and tumour necrosis factor-alpha in women with endometriomas and benign and malignant cystic ovarian tumours. Hum. Reprod. 2003, 18, 1681–1685. [Google Scholar] [CrossRef]
- Vilotić, A.; Nacka-Aleksić, M.; Pirković, A.; Bojić-Trbojević, Ž.; Dekanski, D.; Jovanović Krivokuća, M. IL-6 and IL-8: An Overview of Their Roles in Healthy and Pathological Pregnancies. Int. J. Mol. Sci. 2022, 23, 14574. [Google Scholar] [CrossRef]
- Iwabe, T.; Harada, T.; Tsudo, T.; Nagano, Y.; Yoshida, S.; Tanikawa, M.; Terakawa, N. Tumor necrosis factor-alpha promotes proliferation of endometriotic stromal cells by inducing interleukin-8 gene and protein expression. J. Clin. Endocrinol. Metab. 2000, 85, 824–829. [Google Scholar] [CrossRef]
- Bilotas, M.; Meresman, G.; Buquet, R.; Sueldo, C.; Barañao, R.I. Effect of vascular endothelial growth factor and interleukin-1beta on apoptosis in endometrial cell cultures from patients with endometriosis and controls. J. Reprod. Immunol. 2010, 84, 193–198. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Zhang, X. An Update on the Multifaceted Role of NF-kappaB in Endometriosis. Int. J. Biol. Sci. 2022, 18, 4400–4413. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, A.M.; Vanni, V.S.; Bartiromo, L.; Papaleo, E.; Zilberberg, E.; Candiani, M.; Orvieto, R.; Viganò, P. Is the oocyte quality affected by endometriosis? A review of the literature. J. Ovarian Res. 2017, 12, 43. [Google Scholar] [CrossRef] [PubMed]
- Invernici, D.; Reschini, M.; Benaglia, L.; Somigliana, E.; Galati, G.; La Vecchia, I.; Vigano’, P.; Vercellini, P. The impact of endometriosis on IVF efficacy: Qualitative and quantitative assessment of ovarian response and embryo development. Reprod. Biomed. Online 2022, 45, 275–281. [Google Scholar] [CrossRef] [PubMed]
- González-Comadran, M.; Schwarze, J.E.; Zegers-Hochschild, F.; Souza, M.D.; Carreras, R.; Checa, M.Á. The impact of endometriosis on the outcome of Assisted Reproductive Technology. Reprod. Biol. Endocrinol. 2017, 24, 8. [Google Scholar] [CrossRef]
- Senapati, S.; Sammel, M.D.; Morse, C.; Barnhart, K.T. Impact of endometriosis on in vitro fertilization outcomes: An evaluation of the Society for Assisted Reproductive Technologies Database. Fertil. Steril. 2016, 106, 164–171.e1. [Google Scholar] [CrossRef]
- Muteshi, C.M.; Ohuma, E.O.; Child, T.; Becker, C.M. The effect of endometriosis on live birth rate and other reproductive outcomes in ART cycles: A cohort study. Hum. Reprod. Open. 2018, 2018, hoy016. [Google Scholar] [CrossRef]
- Gayete-Lafuente, S.; Vilà Famada, A.; Albayrak, N.; Espinós Gómez, J.J.; Checa Vizcaíno, M.Á.; Moreno-Sepulveda, J. Indirect markers of oocyte quality in patients with ovarian endometriosis undergoing IVF/ICSI: A systematic review and meta-analysis. Reprod. Biomed. Online 2024, 49, 104075. [Google Scholar] [CrossRef]
- Goud, P.T.; Goud, A.P.; Joshi, N.; Puscheck, E.; Diamond, M.P.; Abu-Soud, H.M. Dynamics of nitric oxide, altered follicular microenvironment, and oocyte quality in women with endometriosis. Fertil. Steril. 2014, 102, 151–159.e5. [Google Scholar] [CrossRef]
- Kasapoglu, I.; Kuspinar, G.; Saribal, S.; Turk, P.; Avcı, B.; Uncu, G. Detrimental effects of endometriosis on oocyte morphology in intracytoplasmic sperm injection cycles: A retrospective cohort study. Gynecol. Endocrinol. 2018, 34, 206–211. [Google Scholar] [CrossRef]
- Ferrero, H.; Corachán, A.; Aguilar, A.; Quiñonero, A.; Carbajo-García, M.C.; Alamá, P.; Tejera, A.; Taboas, E.; Muñoz, E.; Pellicer, A.; et al. Single-cell RNA sequencing of oocytes from ovarian endometriosis patients reveals a differential transcriptomic profile associated with lower quality. Hum. Reprod. 2019, 34, 1302–1312. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Özcan, P.; Varlı, B.; Sarıdoğan, E.; Oral, E.; Mabrouk, M.; Usta, T.; Constantin, A.S. Mechanisms of Endometrioma-Mediated Ovarian Damage: Myths and Facts. J. Clin. Med. 2025, 14, 2147. https://doi.org/10.3390/jcm14072147
Özcan P, Varlı B, Sarıdoğan E, Oral E, Mabrouk M, Usta T, Constantin AS. Mechanisms of Endometrioma-Mediated Ovarian Damage: Myths and Facts. Journal of Clinical Medicine. 2025; 14(7):2147. https://doi.org/10.3390/jcm14072147
Chicago/Turabian StyleÖzcan, Pınar, Bulut Varlı, Ertan Sarıdoğan, Engin Oral, Muhammed Mabrouk, Taner Usta, and Alin Stefan Constantin. 2025. "Mechanisms of Endometrioma-Mediated Ovarian Damage: Myths and Facts" Journal of Clinical Medicine 14, no. 7: 2147. https://doi.org/10.3390/jcm14072147
APA StyleÖzcan, P., Varlı, B., Sarıdoğan, E., Oral, E., Mabrouk, M., Usta, T., & Constantin, A. S. (2025). Mechanisms of Endometrioma-Mediated Ovarian Damage: Myths and Facts. Journal of Clinical Medicine, 14(7), 2147. https://doi.org/10.3390/jcm14072147






