Abstract
Background: Type 1 diabetes (T1D) is a chronic autoimmune disorder characterized by the destruction of pancreatic β-cells, leading to absolute insulin deficiency. Despite advancements in insulin therapy and glucose monitoring, achieving optimal glycemic control remains a challenge. Emerging technologies and novel therapeutic strategies are transforming the landscape of T1D management, offering new opportunities for improved outcomes. Methods: This review synthesizes recent advancements in T1D treatment, focusing on innovations in continuous glucose monitoring (CGM), automated insulin delivery systems, smart insulin formulations, telemedicine, and artificial intelligence (AI). Additionally, we explore biomedical approaches such as stem cell therapy, gene editing, immunotherapy, gut microbiota modulation, nanomedicine-based interventions, and trace element-based therapies. Results: Advances in digital health, including CGM integration with hybrid closed-loop insulin pumps and AI-driven predictive analytics, have significantly improved real-time glucose management. AI and telemedicine have enhanced personalized diabetes care and patient engagement. Furthermore, regenerative medicine strategies, including β-cell replacement, CRISPR-based gene editing, and immunomodulatory therapies, hold potential for disease modification. Probiotics and microbiome-targeted therapies have demonstrated promising effects in maintaining metabolic homeostasis, while nanomedicine-based trace elements provide additional strategies to regulate insulin sensitivity and oxidative stress. Conclusions: The future of T1D management is shifting toward precision medicine and integrated technological solutions. While these advancements present promising therapeutic avenues, challenges such as long-term efficacy, safety, accessibility, and clinical validation must be addressed. A multidisciplinary approach, combining biomedical research, artificial intelligence, and nanotechnology, will be essential to translate these innovations into clinical practice, ultimately improving the quality of life for individuals with T1D.
1. Introduction
Diabetes is a metabolic disorder with multiple causes, characterized by chronic hyperglycemia and alterations in glucose, lipid, protein, and electrolyte metabolism due to defects in insulin secretion and/or action. According to the World Health Organization (WHO), prolonged elevated blood glucose levels can cause severe damage to the heart, blood vessels, eyes, kidneys, and nerves [1]. Diabetes is classified into three major types based on its pathophysiology: type 1 diabetes (T1D), type 2 diabetes, and gestational diabetes mellitus [2,3]. Among these, T1D is the most common form in children and adolescents, although it can occur at any age [4,5]. Petersmann et al. highlighted that T1D results from autoimmune-mediated β-cell destruction, leading to absolute insulin deficiency [6]. Recent WHO data indicate a significant rise in T1D incidence, particularly among the pediatric population [7]. Furthermore, Krzewska and Ben-Skowronek reported that T1D is the second most prevalent chronic health condition affecting individuals under 18 years of age [8].
The International Diabetes Federation (IDF) estimated that in 2021, approximately 537 million people worldwide were living with diabetes, with projections reaching 643 million by 2030 and 783 million by 2045 [9]. Moreover, in 2022, there were 8.75 million individuals diagnosed with T1D globally [10]. According to The Lancet, the number of adults with diabetes has exceeded 800 million, a fourfold increase since 1990, making diabetes one of the leading causes of mortality worldwide [11].
Recent advancements in T1D treatment are increasingly embracing a multidisciplinary approach that integrates technology, immunotherapy, and personalized medicine [12]. Technology-driven innovations, such as CGM systems, hybrid closed-loop insulin delivery systems, and AI-based glucose prediction models, are significantly enhancing glycemic control and minimizing disease burden for patients [13,14]. These tools enable real-time data collection, individualized insulin dosing, and improved patient adherence, leading to better long-term outcomes [15]. At the same time, immunotherapy is emerging as a potential game-changer in T1D management, aiming to modulate the immune system to preserve or restore pancreatic β-cell function [16]. Novel immunotherapeutic strategies—including monoclonal antibodies, antigen-specific immunotherapy, and regulatory T-cell (Treg) therapy—are currently being explored in clinical trials to slow or halt the progression of β-cell destruction [17]. Additionally, personalized medicine is revolutionizing T1D care by tailoring treatments based on individual genetic, immunological, and metabolic profiles. Advances in gene-editing techniques, such as CRISPR-based β-cell regeneration and gut microbiome-targeted interventions, are paving the way for more patient-specific treatment strategies [18]. Together, these three fields—technology, immunotherapy, and personalized medicine—are driving a paradigm shift in T1D care, moving toward a future where disease management is more effective, individualized, and proactive. However, challenges such as accessibility, cost, and regulatory approval remain critical hurdles that must be addressed to make these advancements widely available.
T1D is a major chronic disease affecting children, with an estimated 651,700 cases among children and adolescents globally in 2021. By 2040, the prevalence is expected to reach 13.5–17.4 million, with the highest increases predicted in low- and middle-income countries [19]. In 2021, approximately 108,300 new cases of T1D were diagnosed among children under 15 years old, a number that rises to 149,500 when extending the age range to individuals under 20 years [20,21]. Ogle et al. noted that T1D incidence is highest among populations of Northern European descent and in several North African and Middle Eastern countries. Additionally, India and the United States report the highest number of annual incident cases among children under 15 years, with 19,194 and 15,288 cases, respectively [20].
A T1D diagnosis at a young age necessitates a significant period of adjustment for both the child and their family. Children and adolescents with T1D often face emotional challenges, particularly when diagnosed early, making parental support essential for disease management [22,23,24]. Studies indicate that adolescents with T1D have higher levels of anxiety [25], increased risk of emotional and behavioral disorders [26], and lower self-esteem and self-image [27], all of which can affect adherence to therapeutic and dietary regimens [28]. Moreover, depression and diabetes frequently coexist, with each condition potentially exacerbating the other [29].
The management of T1D primarily relies on intensive insulin therapy, which includes multiple daily injections or insulin pump therapy. Effective treatment requires ongoing education, adherence to a balanced diet, and regular physical activity to minimize cardiometabolic risks [30,31]. However, adolescence presents unique challenges due to the desire for independence and increased psychological stress, which may lead to risky behaviors such as insulin omission, resulting in complications like diabetic ketoacidosis [32]. Additionally, the rapid endocrinological changes during puberty increase insulin resistance, necessitating frequent insulin adjustments. Complications such as dermatological reactions to therapeutic devices can further impact glycemic control and psychological well-being [33,34]. Prolonged diabetes duration and suboptimal glycemic control can also contribute to early-onset chronic complications, including retinopathy, nephropathy, peripheral neuropathy, and cardiovascular autonomic neuropathy, significantly affecting both patients and caregivers [35,36].
This narrative review aims to provide a comprehensive analysis of the clinical and psychological challenges associated with T1D during adolescence. By synthesizing current evidence, this paper highlights the primary difficulties faced by young individuals with T1D and explores potential strategies to optimize disease management. The review also examines advancements in insulin therapy, technological innovations in diabetes care, and psychosocial interventions that may enhance treatment adherence and quality of life for adolescents with T1D. Through this comprehensive approach, we aim to contribute to the existing body of literature and support healthcare professionals in developing more effective, patient-centered interventions for this vulnerable population.
To conduct this narrative review, we performed a comprehensive literature search focusing on original research articles related to T1D in children and adolescents. Our selection criteria included studies on insulin therapy, insulin pumps, and advanced medical technologies for insulin infusion. We prioritized peer-reviewed publications from the past decade (2014–2025) written in English, without geographic restrictions. The initial search was conducted using Google Scholar, followed by a more refined selection from three major academic databases: PubMed®/MEDLINE, ScienceDirect, and Web of Science®. The search strategy incorporated the following keywords: type 1 diabetes technology, continuous glucose monitoring system, automated insulin delivery, continuous insulin infusion, patch insulin pump, hybrid closed-loop insulin pump, closed-loop insulin pump, artificial pancreas, and bionic pancreas. Only articles published in English were considered, and the final database search was completed on 30 January 2025.
2. The Role of Smart Technology in T1D: CGM, Insulin Pumps, and AI Systems
Advancements in diabetes technology have significantly improved disease management and clinical outcomes. The development of automated insulin delivery systems has been a game changer, aiming to enhance metabolic control and quality of life for individuals with T1D. The most effective and reliable therapy for improving HbA1c levels, reducing hypoglycemia, and maintaining glucose within the target range is CSII, commonly known as insulin pump therapy, combined with CGM systems [7]. Additionally, artificial pancreas (AP) systems, also referred to as closed-loop systems (CLS), are now a promising solution for pediatric patients, offering automated and adaptive insulin delivery based on real-time glucose levels. The integration of smartphone-connected diabetes management devices represents a significant step forward in T1D therapy, providing patients and caregivers with real-time data and remote monitoring capabilities.
These advancements, along with personalized insulin regimens and emerging digital tools, are reshaping the landscape of diabetes care, offering greater flexibility, improved adherence, and better long-term health outcomes.
However, despite these promising developments, several challenges remain. CGM and automated insulin delivery systems, while improving glycemic control, still face limitations related to high costs, insurance coverage disparities, and patient adherence. Additionally, accuracy issues in CGM devices, particularly during rapid glucose fluctuations, can lead to dosing errors that impact metabolic control [37]. Similarly, while AI-driven diabetes management provides predictive analytics for glucose trends and insulin adjustments, concerns about data privacy, algorithmic bias, and regulatory hurdles must be addressed before widespread clinical adoption [38]. Nevertheless, ensuring equitable access, addressing technological limitations, and refining regulatory pathways will be essential to maximize their potential impact on clinical practice [39].
Regarding glycemic targets and treatment guidelines, recent recommendations specify optimal glucose targets, such as the following:
- Fasting glucose levels should be maintained between 3.9 and 7.8 mmol/L (70–140 mg/dL) [40].
- For children under 7 years of age, a tighter time interval is recommended, ensuring that at least 50% of CGM readings fall within 3.9–7.8 mmol/L (70–140 mg/dL) or 70% within the range of 3.9–10 mmol/L (70–180 mg/dL) [41].
- ISPAD recommends maintaining HbA1c levels below 53 mmol/mol (<7.0%) to prevent long-term microvascular and macrovascular complications [40].
2.1. Continuous Glucose Monitoring (CGM) Systems
Over the past 15 years, advancements in accuracy, accessibility, and insurance coverage have significantly contributed to the widespread adoption of CGM in pediatric diabetes care [42]. Data from the SWEET Registry, which includes centers in Europe, North America, Australia, and the Middle East, confirm a steady increase in CGM and insulin pump usage among children [43]. A population-based study in Australia reported a remarkable rise in CGM uptake among individuals under 21 years old, increasing from 5% to 79%, following the introduction of universal subsidized funding for CGM [44]. However, despite these positive trends, CGM adoption remains inconsistent within the same country, with notable variations between clinical centers and regions [45].
Currently, there are two primary types of CGM available:
- Professional CGM: data are recorded over several days and can be accessed only by healthcare providers, typically for retrospective analysis [46].
- Personal CGM: Users can view glucose levels in real time, allowing for immediate intervention. This category includes the following:
- ○
- Intermittently scanned CGM (IS-CGM) requires manual scanning to retrieve glucose readings [47].
- ○
- Real-time CGM (RT-CGM) continuously transmits glucose data to a connected device without scanning [48].
CGM technology can be categorized based on minimally invasive [49] and non-invasive techniques [50]. Several companies have developed minimally invasive CGM systems for diabetes management, as observed in Table 1 [46].

Table 1.
Global manufacturers of CGM devices.
2.2. Continuous Subcutaneous Insulin Delivery (CSII) Systems
Continuous subcutaneous insulin infusion (CSII), commonly known as insulin pump therapy, is an advanced method for managing T1D. Compared to multiple daily injections, insulin pumps provide more precise and flexible insulin delivery, helping patients maintain optimal glycemic control [7]. These devices administer insulin in two key ways:
- Basal insulin infusion: a continuous supply of small amounts of rapid-acting insulin throughout the day and night to maintain stable glucose levels;
- Bolus insulin delivery: a single, larger dose of insulin given at mealtimes or to correct high blood glucose levels [51].
Insulin pumps offer significant advantages, including improved glucose regulation, reduced risk of hypoglycemia, and lower incidence of diabetes-related hospitalizations [52,53]. Research has shown that combining insulin pumps with CGM enhances glycemic outcomes, which has led to the FDA’s approval of integrated CGM systems. These systems can automatically adjust insulin infusion based on real-time glucose readings, suspending or increasing insulin delivery in response to hypoglycemia or hyperglycemia [53]. There are several types of insulin pumps, each offering distinct features and functionalities [52]:
- Traditional tube pumps: these pumps feature an insulin reservoir connected to the body via a thin tube, which leads to a cannula (needle or catheter) inserted under the skin.
- Patch pumps: These devices are directly attached to the skin and do not require tubing. Insulin is delivered via a short cannula that penetrates the skin.
- Closed-loop pumps (artificial pancreas systems): these fully automated systems combine an insulin pump with a CGM sensor, continuously adjusting insulin doses based on real-time glucose data.
- Integrated CGM pumps: while not entirely automated, these pumps work in conjunction with CGM sensors and can suggest insulin dose adjustments based on glucose trends.
- Mechanical pumps: these are simpler devices that provide fixed and programmable insulin delivery, often used in specific clinical scenarios [46].
2.3. Glucose-Responsive Insulin Delivery System and Artificial Intelligence (AI) in Diabetes Management
Artificial intelligence (AI) is increasingly being explored for its promising applications in data analysis, advanced computing, and predictive modeling. Among the various AI subsets, machine learning and deep learning remain the most widely utilized approaches for enhancing diabetes management. Recent breakthroughs in AI-driven algorithms have significantly improved the prevention of complications, therapeutic strategies, and personalized treatment approaches for T1D [54]. By integrating AI-based solutions into smartphone applications and clinical decision-support systems, patients and healthcare providers can achieve optimized insulin dosing, early detection of glycemic variability, and enhanced self-management strategies [54]. These AI-powered tools can also facilitate early intervention, reducing the risk of hypoglycemia and hyperglycemia-related complications. In addition to glycemic prediction, AI plays a crucial role in precision medicine, particularly through the analysis of genetic and metabolomic data. The increasing availability of digitized glucose monitoring data from T1D patients has fueled AI advancements, enabling the development of personalized treatment algorithms tailored to an individual’s genetic profile, metabolic characteristics, and lifestyle factors. This personalized medicine approach aims to enhance treatment efficacy, minimize adverse effects, and improve overall quality of life [46].
Smart insulin represents a groundbreaking innovation designed to automatically adjust insulin release in response to blood glucose levels. This technology has the potential to improve glycemic management, reduce hypoglycemia risk, and enhance patient quality of life by minimizing the need for frequent insulin injections and dose adjustments [55]. The core technology behind smart insulin involves the encapsulation of insulin molecules in nanocarriers, which function as glucose-sensitive delivery systems. The process works as follows in Figure 1:
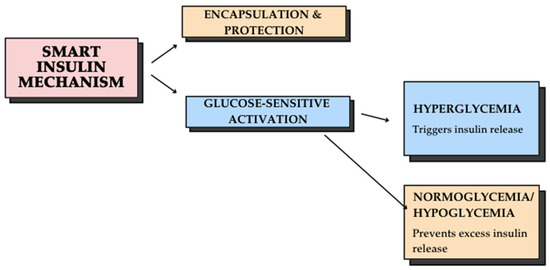
Figure 1.
Smart insulin mechanism: a glucose-responsive delivery system.
Some of the benefits of smart insulin are reducing the need for frequent injections, improving glycemic control by minimizing hyperglycemia and hypoglycemia risks, and lowering the likelihood of long-term diabetes-related complications [55]. While smart insulin technology holds tremendous potential, challenges remain in optimizing formulation stability, ensuring precise glucose response, and advancing clinical trials for widespread use. Future innovations may integrate biocompatible polymers, AI-driven glucose sensors, and advanced nanotechnology to refine insulin delivery systems. The transition toward an AI-driven framework in diabetes care represents a significant shift in clinical practice, emphasizing individualized treatment protocols and proactive disease management. This is particularly relevant for children and adolescents with T1D, who require adaptive, real-time treatment strategies to account for growth-related metabolic changes and evolving insulin needs. Despite its potential, the implementation of AI in T1D management presents several challenges, including data privacy concerns, algorithmic bias, and the need for large-scale clinical validation. Future research should focus on integrating AI-driven decision-making into routine clinical workflows while ensuring accuracy, reliability, and ethical considerations in diabetes care [54].
2.4. Supporting Adolescents in T1D Self-Management: Education and Digital Tools
T1D remains a chronic condition associated with significant morbidity [15], mortality, and reduced quality of life [56,57]. Managing T1D becomes even more complex when it coexists with other medical conditions [58,59,60,61,62]. A good long-term glycemic control can reduce the risk of acute complications (e.g., hypoglycemia, hyperglycemia) and long-term complications (e.g., retinopathy, nephropathy, neuropathy) [63]. Since T1D requires lifelong self-management, frequent consultations with healthcare professionals are essential [64]. Intensive self-care includes continuous blood glucose monitoring, carbohydrate counting, physical activity adjustments, and personalized insulin therapy [65,66].
The transition from childhood to adolescence is a critical phase for individuals with T1D, as they gradually assume greater responsibility for self-management while parents progressively relinquish control [64]. However, this period is often challenging due to the complexity of diabetes management, particularly in the context of suboptimal communication between adolescents and their caregivers. This breakdown in communication can negatively impact health outcomes [67]. Inadequate management during this transition significantly increases the risk of severe hypoglycemia, which may lead to seizures, diabetic ketoacidosis, and comas, potentially resulting in neurological damage or even fatal consequences [68]. Therefore, ensuring effective support during this transition is crucial for safeguarding the adolescent’s health and well-being. Implementing targeted interventions to assist adolescents with T1D in this phase could lead to improved glycemic control, better health outcomes, and enhanced quality of life [69]. Additionally, for young people with diabetes, self-management education can help them gain independence and understand that good diabetes management leads to the prevention of chronic and critical complications and glycemic control [70].
A fundamental aspect of T1D management is self-management education, which should be provided to all children and adolescents diagnosed with the disease [71]. Studies have explored various educational approaches to facilitate self-management in young patients, including personal training, video tutorials [72], mobile phone applications [73], and text messaging interventions [74]. However, findings indicate that while these methods can be beneficial, their effectiveness varies depending on individual learning preferences and engagement levels [71].
The emergence of digital learning tools has paved the way for innovative educational strategies in diabetes management. Among these, digital storytelling has gained attention as a particularly effective method for engaging adolescents. This approach combines narrative elements with multimedia tools, offering a structured way to convey complex medical information in an engaging and accessible manner. Digital storytelling has the potential to enhance knowledge retention and promote behavioral changes, regardless of differences in age, education level, or cognitive abilities [75].
2.4.1. The Role of Digital Storytelling in Diabetes Education
Digital storytelling is a modern educational approach that enables individuals with diabetes to better understand their condition and develop effective self-management strategies. By integrating learning with the enhancement of coping skills, this method serves as a powerful tool to motivate behavioral changes and promote a healthier lifestyle [76]. As a technology-based intervention, digital storytelling combines narrative elements with multimedia capabilities, facilitating the engaging, simplified, and widespread dissemination of educational concepts. This interactive and immersive format encourages active learning, making complex medical information more accessible and relatable [77].
Currently, digital storytelling is widely utilized in adolescent education, with numerous studies examining its effectiveness across various domains. Research highlights its positive impact on academic performance and social intelligence [77], youth achievement [78], and anxiety reduction in patients undergoing cardiac surgery [76] and children with cancer [79]. Additionally, this method has been shown to enhance social skills and cognitive awareness in children and adolescents with autism spectrum disorder [80].
2.4.2. Mobile Technology and IT Innovations in T1D Management
With over 2.7 billion smartphone users worldwide and approximately 0.5 billion individuals already utilizing mobile applications for diet tracking [81], chronic disease management [82,83,84], and physical activity monitoring [85], mobile health (mHealth) technologies have emerged as a promising tool for T1D self-management. In recent decades, advancements in IT have significantly enhanced diabetes care services, offering patients improved access to monitoring tools, personalized interventions, and real-time communication with healthcare providers [86]. Numerous studies have examined the effectiveness of mobile applications and messaging systems as digital health tools for diabetes management, demonstrating their potential to optimize glucose monitoring, treatment adherence, and patient engagement [5,22,23,87,88,89,90,91,92,93,94]. As a result, the availability of T1D-specific applications on platforms such as the iOS App Store and Google Play has expanded significantly [5,23]. These applications—commonly categorized as mobile health (mHealth) apps—are designed to enhance patient education, facilitate communication with healthcare professionals, and promote peer support in an interactive and user-friendly manner [88,95]. This technology is particularly well-suited for children and adolescents, an age group that readily embraces digital tools for daily health management [96].
Table 2 provides an overview of different smartphone applications developed to assist T1D self-management among young individuals.

Table 2.
Different smartphone apps for T1D management.
Auxiliary-style mobile applications represent a promising strategy for improving glycemic control in young patients with T1D, given the widespread use of smartphones in daily life. These apps leverage technology to support self-management, education, and adherence to treatment, offering a convenient and interactive approach to diabetes care.
Den Akker et al. introduced PERMAGON, an integrated gaming and coaching platform designed specifically for adolescents with T1D. The system combines monitoring, educational games, and motivational feedback to enhance self-management skills in both patients and their caregivers [106]. Similarly, Tidepool, a device-agnostic cloud platform, was developed by Neinstein et al. to centralize and analyze data from various diabetic devices. This innovation addresses two major challenges in T1D management: limited access to device-generated data and poor interoperability across different devices [107].
Another notable digital tool is DigiBete, a free self-management and educational application designed for children, adolescents, families (CYPFs), and healthcare professionals (HCPs). Developed in collaboration with both CYPFs and HCPs, this platform provides clinically approved and age-appropriate resources to aid in T1D management. It offers over 200 educational videos covering topics such as carbohydrate counting, sports, and exercise. Additionally, DigiBete allows users to store insulin ratios, dosage information, and pump settings, while also facilitating direct communication between patients and their diabetes care team. A key feature of this application is its multilingual accessibility, supporting British Sign Language, Bengali, Chinese, Arabic, Somali, Tamil, Polish, and Urdu, with further language expansions in progress [5].
Beyond mobile applications, text messaging services have also emerged as effective tools for diabetes education and management. Bin-Abbas et al. implemented a mobile phone messaging service for children and adolescents with T1D, where parents received three types of messages: informational, interactive, and multimedia-based [108]. The study revealed significant benefits, including enhanced communication between patients and healthcare providers during clinic visits as well as significant reductions in HbA1c levels, indicating improved glycemic control and increased parental knowledge of diabetes, leading to better disease management at home [109]. When integrated into mobile applications and patient portals, digital health interventions have proven to be highly effective in enhancing self-efficacy, promoting treatment adherence, and ultimately improving glycemic outcomes [110,111,112]. As the field of digital diabetes care continues to evolve, the incorporation of advanced IT tools and communication platforms holds great potential in optimizing the management of T1D, particularly among adolescents.
2.4.3. The Role of Telemedicine in T1D Management
The COVID-19 pandemic accelerated the adoption of telemedicine, transforming it into a primary mode of diabetes care worldwide [113,114,115]. During lockdowns, diabetes management was conducted almost entirely through virtual consultations, and both patients and healthcare providers became increasingly comfortable with this remote clinical environment [116]. Telemedicine has demonstrated significant benefits in T1D management, particularly in improving insulin adherence (patients receive real-time guidance on insulin dosing adjustments), sick day management (immediate access to medical advice prevents severe complications), and the reduction in unnecessary hospital visits (virtual care decreases emergency admissions). The positive impact of telemedicine lies in the frequent touchpoints between patients and healthcare teams, facilitated through emails, text messaging, and videoconferencing [117,118]. A recent study on children with T1D found that families perceived telehealth as beneficial, citing cost savings, increased flexibility, and more frequent insulin adjustments as major advantages [119]. This shift toward remote monitoring and intervention has led to a surge in telemedicine-focused research, with numerous studies reporting remarkable success in the remote follow-up of pediatric T1D patients during the pandemic [114,120,121]. Several limitations have been identified that may impact the quality of T1D care:
- Lack of physical examination: remote consultations limit physicians’ ability to conduct comprehensive physical assessments, which are critical for detecting diabetes-related complications [122,123].
- Reduced patient-physician interaction: personal contact is a key element in establishing trust and improving patient adherence, which may be compromised in virtual care settings [124].
- Technological barriers: some patients and healthcare providers struggle with limited digital literacy, affecting their ability to effectively utilize telemedicine platforms [123].
While telemedicine continues to evolve as an integral component of diabetes management, future advancements should focus on hybrid care models that integrate in-person consultations with digital health tools, ensuring comprehensive and personalized care for individuals with T1D. Future research should focus on integrating telemedicine with mobile applications and digital tools to enhance comprehensive diabetes care.
2.5. Expanding Diabetes Technology Worldwide: Challenges and Opportunities
The integration of AI-driven decision-support systems, CGM, and hybrid closed-loop insulin delivery has significantly advanced T1D management, enhancing glycemic control and reducing the burden of self-care [125]. These technologies enable more precise insulin dosing, leading to fewer hypoglycemic events and improved time-in-range outcomes. However, while their clinical efficacy is well-documented, the successful implementation of these innovations in real-world practice remains contingent upon several factors, including patient adherence, healthcare provider training, and long-term safety validation [126].
Despite their advantages, the high cost and limited insurance coverage continue to pose significant challenges to widespread adoption. This issue is particularly evident in resource-limited settings, where conventional insulin therapy remains the primary treatment option due to financial and accessibility constraints. The availability of specialized diabetes care infrastructure is another major barrier, as advanced diabetes technologies require specific expertise for effective implementation [125]. However, a significant number of healthcare professionals, particularly in low- and middle-income countries, have not received formal training in CGM interpretation, insulin pump therapy, and AI-driven diabetes management [127]. This knowledge gap further limits the accessibility and scalability of these advanced technologies in clinical practice. In addition, data privacy concerns and digital literacy gaps remain significant barriers. Many AI-driven diabetes management tools rely on cloud-based platforms, necessitating secure data-sharing frameworks [128]. However, in regions with weaker cybersecurity infrastructure, compliance with data protection regulations such as GDPR and HIPAA presents additional challenges to adoption [129].
To ensure equitable access to these emerging diabetes technologies, governments and healthcare systems must prioritize cost-reduction strategies, including subsidies for CGM devices and insulin pumps and the expansion of insurance coverage for digital health solutions [130]. Furthermore, regulatory agencies should expedite approval pathways for AI-powered diabetes management tools while maintaining rigorous safety and efficacy standards. Collaborative efforts between healthcare providers, policymakers, and technology developers are essential to streamline these processes and ensure that innovative solutions reach those who need them most [46]. In addition, the adoption of hybrid healthcare models—which integrate telemedicine with in-person diabetes care—may facilitate broader access to specialized diabetes management services, particularly in underserved regions [122]. Digital health literacy programs should also be developed to enhance the ability of both patients and healthcare professionals to effectively utilize emerging diabetes technologies, thereby maximizing their clinical impact and improving long-term health outcomes for individuals with T1D [130].
3. Future Solutions in the Therapy of T1D
Managing T1D in children and adolescents presents significant challenges, as it requires achieving optimal glucose control while minimizing hypoglycemia risks. Over the past two decades, diabetes technology has evolved considerably, providing advanced solutions to support glycemic management [131]. The continuous development of diabetes management technologies has led to updates in clinical recommendations, particularly for young patients, as outlined in the International Society for Pediatric and Adolescent Diabetes (ISPAD) Guidelines [30]. These guidelines establish specific glucose targets to improve outcomes and reduce long-term complications.
Emerging therapies not only improve the quality of life for individuals with T1D but also provide hope for a future where disease management becomes more efficient and less burdensome [132]. Figure 2 represents a schematic diagram of future solutions for T1D.
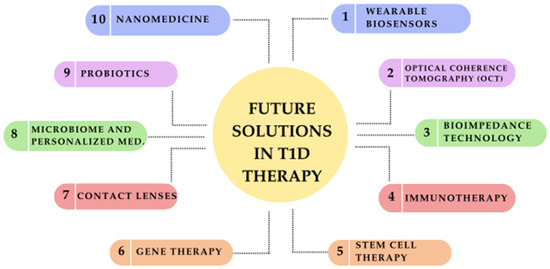
Figure 2.
Future solutions in type 1 diabetes (T1D) therapy: a schematic overview of emerging innovations.
The landscape of T1D treatment is rapidly evolving, with several innovations ranging from clinically available treatments to experimental therapies still in early research. In Table 3, we categorize advancements into two groups: emerging therapies (undergoing clinical trials with potential for near-future implementation) and experimental therapies (in early-stage research or preclinical development, requiring further validation before clinical application).

Table 3.
T1D therapy categorization.
3.1. Wearable Biosensors: Non-Invasive Continuous Monitoring
Wearable biosensors are a promising innovation in T1D management, offering a non-invasive and continuous method for monitoring glucose levels. These sensors are designed to detect glucose variations in interstitial fluid by using advanced nanomaterials or chemical biosensors that react to glucose by changing color or emitting light. This direct visual feedback provides real-time insights into glycemic fluctuations [138,139].
Wearable biosensors are typically wirelessly connected to mobile devices, enabling the following:
- Real-time glucose tracking via smartphone applications;
- Automated alerts when glucose levels exceed pre-set thresholds;
- Data storage and analysis to enhance long-term glycemic management.
Compared to traditional glucometers, wearable biosensors eliminate the need for frequent finger pricks, offering a more convenient and user-friendly alternative [140].
3.2. Optical Coherence Tomography (OCT) for Glucose Monitoring
Optical coherence tomography (OCT) is an imaging technology that uses light waves to generate high-resolution cross-sectional images of biological tissues. Recent studies have explored its potential for non-invasive CGM, leveraging its high signal-to-noise ratio and superior resolution [21,141].
The advantages of OCT-based glucose monitoring include the following:
- Completely non-invasive, eliminating the need for needles or skin punctures;
- High imaging precision enables real-time tracking of glucose fluctuations;
- Enhanced patient comfort is particularly beneficial for pediatric patients [142].
However, challenges remain, including motion artifacts, temperature sensitivity, and tissue property variations, which affect measurement accuracy. Further technological refinements are needed before OCT-based CGM can be widely implemented [142].
3.3. Bioimpedance-Based Glucose Monitoring
Bioimpedance technology measures the electrical resistance of biological tissues when an electric current passes through them. Since bioimpedance variations correlate with glucose fluctuations, researchers have explored its potential for accurate, real-time glucose monitoring [143,144].
Key findings suggest the following:
- Bioimpedance offers a stable and precise estimation of glucose levels, particularly within an optimal frequency range below 40 kHz [145].
- CGM devices based on bioimpedance include sensors, analytical algorithms, and measurement circuits, offering a potential alternative to traditional CGM systems [138].
3.4. Immunotherapy: Modulating the Immune Response in T1D
Immunotherapy represents a rapidly advancing field in T1D research, aiming to modify the immune response and prevent the destruction of insulin-producing pancreatic beta cells [146]. T1D is an autoimmune disorder in which the immune system mistakenly targets beta cells, leading to insulin deficiency and lifelong dependence on external insulin administration. Immunotherapy has emerged as a potential solution to slow disease progression or even halt autoimmune destruction [17]. In Figure 3, there is a schematic representation of different types of immunotherapies in T1D.
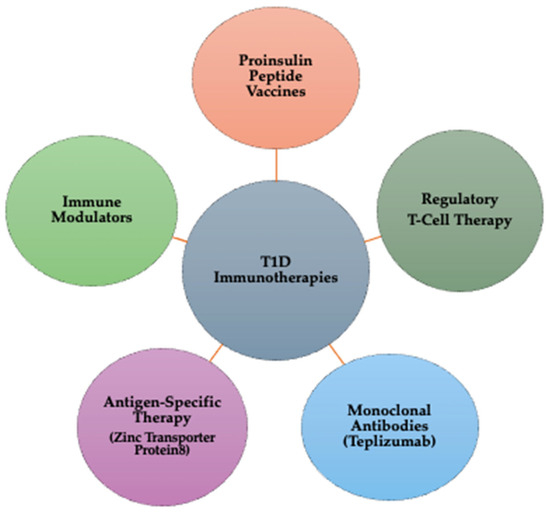
Figure 3.
Types of immunotherapies for T1D: mechanisms and targets.
The types of Immunotherapies in T1D are outlined below:
- Proinsulin peptide (PPI) vaccines: Proinsulin peptide vaccines train the immune system to tolerate beta cells instead of attacking them. By exposing the immune system to proinsulin fragments, these vaccines help induce immune tolerance, potentially preventing or delaying T1D onset. Clinical stage: phase I/II trials are ongoing to assess efficacy and safety [17].
- Regulatory T-cell (Treg) therapy: Tregs are a specialized subset of immune cells responsible for suppressing autoimmune reactions. In Treg therapy, patient-derived Tregs are extracted, expanded in a laboratory, and reinfused to restore immune balance. This approach reduces beta cell destruction and modulates immune responses, helping preserve endogenous insulin production. Clinical stage: multiple Phase I/II clinical trials are investigating Treg therapy in newly diagnosed T1D patients [147].
- Monoclonal antibodies (teplizumab therapy): Teplizumab is a monoclonal antibody therapy that targets CD3 on T-cells, effectively by blocking key immune pathways involved in the attack on beta cells, delaying disease progression in newly diagnosed individuals, and reducing inflammation and modulating the immune system’s response to beta cells. Clinical stage: Teplizumab has received FDA approval for delaying the onset of T1D in at-risk individuals. Ongoing studies continue evaluating long-term efficacy [16,148].
- Antigen-specific therapy— Zinc Transporter Protein 8 (ZnT8) targeting: ZnT8 is an autoantigen present in prediabetic and diabetic patients, making it a critical target for immunotherapy. ZnT8-based treatments help regulate immune responses, potentially protecting beta cells from further autoimmune damage. Early diagnosis strategies using ZnT8 autoantibodies can also improve predictive screening for T1D risk. Clinical stage: preclinical and early-stage Phase I trials [149,150];
- Immune modulators: Immune modulation aims to modify the immune response to maintain beta cell function and prevent or delay the onset of the disease. Abatacept, a T-cell co-stimulation blocker, reduces inflammation and lowers the likelihood of autoimmune destruction. Other immunomodulatory drugs are currently being tested to improve beta cell survival and enhance long-term glycemic control. Clinical stage: phase II/III trials are evaluating the efficacy in delaying T1D progression [151].
3.5. Stem Cell Therapy
Stem cell therapy represents a groundbreaking approach in T1D treatment, aiming to regenerate insulin-producing beta cells in the pancreas. This method holds great potential for restoring endogenous insulin production and improving long-term glycemic control. Current research focuses on utilizing different types of stem cells to replace damaged beta cells and enhance pancreatic function [152]. The types of stem cells used in T1D therapy are the following:
- Embryonic stem cells (ESCs) are pluripotent cells capable of differentiating into any cell type, including beta cells. These cells have shown great potential for beta cell regeneration but raise ethical concerns and a risk of immune rejection [153];
- Induced pluripotent stem cells (iPSCs) are reprogrammed adult cells that mimic ESCs, offering a patient-specific approach. These cells are generated from the patient’s own cells, reducing the risk of immune system rejection and provide an ethically viable alternative to embryonic stem cells [154];
- Mesenchymal stem cells (MSCs) are found in bone marrow, adipose tissue, and umbilical cord blood. These cells exhibit anti-inflammatory and immunomodulatory properties, making them valuable in autoimmune diseases like T1D. Also, they differentiate into multiple cell types, including insulin-producing beta cells [155,156].
Despite promising results in preclinical and clinical trials, challenges remain in ensuring long-term beta cell survival, optimizing differentiation protocols, and addressing immune rejection issues. Future advancements in gene editing (e.g., CRISPR) and tissue engineering could further enhance the success of stem cell-based T1D therapies [18].
3.6. Gene Therapy
Gene therapy for T1D is a cutting-edge approach aimed at correcting genetic defects or modifying gene expression to restore insulin-producing beta cell function in the pancreas. This innovative strategy involves introducing, removing, or altering genetic material within a patient’s cells, with the ultimate goal of treating or potentially curing the disease [135]. Key mechanisms of gene therapy in T1D include the following:
- Gene transfer for beta-cell regeneration uses viral vectors (e.g., adenoviruses, lentiviruses) to introduce essential transcription factors like PDX1 and MAFA. These factors play a critical role in beta cell development and regeneration, restoring insulin production in individuals with T1D [135].
- CRISPR-Cas9 gene editing is a revolutionary gene-editing technique that enables precise genetic modifications. This gene can correct mutations in the insulin gene to ensure proper insulin production. Also, it allows the introduction of protective sequences to shield beta cells from autoimmune destruction [157].
- Gene therapy for localized immunosuppression delivers therapeutic genes that produce immunosuppressive proteins (e.g., IL-10 and TGF-beta). Also, it protects beta cells from autoimmune attacks without compromising the body’s immune defense against infections [157].
- Alpha-to-beta cell conversion introduces specific genes like ARX and PDX1 to reprogram glucagon-producing alpha cells into insulin-producing beta cells. This technique offers an alternative source of functional beta cells, restoring natural insulin production [18].
Although gene therapy holds immense potential in revolutionizing T1D treatment, several challenges remain: ensuring long-term beta cell survival post treatment, minimizing immune rejection of newly regenerated beta cells, and enhancing gene delivery efficiency to improve treatment outcomes. Future advancements integrating CRISPR technology, stem cell therapy, and improved gene delivery systems could bring gene therapy closer to clinical application as a viable cure for T1D [18].
3.7. Contact Lenses for CGM
Contact lenses represent an innovative technology in the management of T1D, offering a non-invasive and continuous method for monitoring blood glucose levels. These smart lenses are designed to detect glucose concentration in tears, providing real-time data that help patients achieve better glycemic control and prevent complications associated with blood glucose fluctuations [158]. The technology behind these lenses relies on microscopic sensors embedded in the contact lens material. These sensors use electrochemical or optical detection systems to measure glucose levels in the tear film. The collected data are then wirelessly transmitted to an external device, such as a smartphone or smartwatch, enabling CGM. When glucose levels deviate from the normal range, the system sends immediate alerts, allowing patients to take timely action [158]. This emerging technology has the potential to revolutionize diabetes self-management by eliminating the need for frequent finger-prick blood tests, making glucose monitoring more convenient and user-friendly. However, challenges such as sensor accuracy, durability, and user comfort must be addressed before these lenses can be widely implemented in clinical practice.
3.8. Microbiome and Personalized Medicine
Bioinformatics is an interdisciplinary field that applies computer science and statistical methods to the study of biological data. It plays a crucial role in processing and analyzing vast amounts of biological information, including genome sequencing, proteomics, and transcriptomics data [159]. By utilizing computational tools, bioinformatics enables researchers to efficiently process large-scale datasets, extract hidden patterns, and uncover significant biological insights.
The gut microbiota represents a complex microbial ecosystem composed of bacteria, fungi, viruses, and other microorganisms residing in the human intestine. These microbes play a key role in host metabolism and immune regulation, significantly influencing the development of diabetes and insulin resistance [160]. Disruptions in gut microbiota composition, also known as dysbiosis, have been linked to altered glucose metabolism, inflammation, and insulin resistance, highlighting its importance in metabolic diseases [161].
Bioinformatics analysis offers several advantages in the study of gut microbiota and its association with diabetes and insulin resistance. It enables efficient processing of big data, facilitates multi-level integration of complex datasets, assists in the discovery of biomarkers and therapeutic targets, and enhances predictive modeling and simulation for metabolic disorders [161]. Additionally, bioinformatics tools promote data sharing and collaboration, providing researchers with powerful computational frameworks to decode the intricate relationships between microbiota diversity and metabolic diseases [162].
Since scientific evidence suggests a strong correlation between gut microbiota composition, diabetes, and insulin resistance, bioinformatics has emerged as a cutting-edge approach for gaining deeper insights into the mechanisms by which intestinal microbes influence glucose metabolism. Moreover, personalized medicine is increasingly leveraging microbiome research to develop tailored therapeutic strategies, allowing for individualized interventions based on a patient’s unique microbial composition and metabolic profile [163]. Continued research in this field may pave the way for microbiota-targeted therapies, offering new perspectives for precision medicine in diabetes management [164].
In the literature, there are studies regarding the changes in the diversity and relative abundance of gut microbes that occur in T1D. Factors that could influence the microbiome data and its implications in T1D include data related to participants—age, sex, geographical location, dietary pattern, physical activity, and disease severity—methods used for sample collection, DNA isolation methods, and microbial profiling methods (metagenomics and metabolomics), but also bioinformatics tools used for analysis. Therefore, in future studies, these factors should be taken into account [165].
3.9. The Role of Probiotics in T1D Management
Probiotics have emerged as a potential therapeutic approach to restore microbiota balance and support glucose homeostasis [166]. Table 4 provides a structured overview of the mechanisms linking gut microbiota, probiotics, and T1D management, illustrating how microbial interventions may influence diabetes progression and therapy.

Table 4.
The role of probiotics in T1D management.
Although T1D has been recognized for a long time, the specific mechanisms underlying pancreatic β-cell destruction remain incompletely understood. The most widely accepted explanation is the interaction between genetic susceptibility and environmental triggers, such as viral infections, which can initiate an autoimmune response against pancreatic β-cells [19]. Recent research has established a strong correlation between gut microbiota imbalance (dysbiosis) and T1D development [166]. Compared to healthy individuals, patients with β-cell autoimmunity exhibit a lower abundance of beneficial bacteria (e.g., Bifidobacterium and Lactobacillus) alongside a notable increase in pro-inflammatory bacteria such as Clostridium and Bacteroides. These microbial changes contribute to intestinal permeability, chronic inflammation, and immune dysregulation, factors known to play a role in T1D progression [35].
Moreover, studies have shown that children with T1D tend to have a reduced diversity of gut microbiota and lower levels of bacteria crucial for intestinal mucosal integrity. The resulting compromised intestinal epithelial barrier leads to increased permeability, which further exacerbates inflammation. This inflammatory state triggers the release of lipopolysaccharides into circulation, a process associated with higher insulin resistance and impaired glycemic control [164,172]. According to Homayouni-Rad et al., emerging evidence suggests that the use of probiotics, prebiotics, or symbiotic may help modulate gut microbiota composition in individuals with diabetes [162]. The administration of specific probiotic strains has been shown to increase the production of short-chain fatty acids (SCFAs), such as butyrate, which plays a key role in maintaining intestinal homeostasis. SCFAs exert their beneficial effects by activating free fatty acid receptors 2 and 3 (FFAR2/3), mechanisms that regulate immune function and autoimmune disease progression, including T1D [173].
Additionally, SCFA-mediated activation of FFAR2/3 has been linked to enhanced production of glucagon-like peptide-1 (GLP-1) from intestinal L cells. Since GLP-1 stimulates insulin secretion from pancreatic β-cells, this process enhances glucose regulation and insulin sensitivity, an effect commonly referred to as the “incretin effect” [174,175,176]. These findings underscore the therapeutic potential of probiotics in preventing or managing T1D by preserving gut microbiota balance and modulating immune responses. However, further clinical trials are needed to establish standardized probiotic formulations and determine their long-term efficacy in diabetes management.
3.10. Nanomedicines Based on Trace Elements in Diabetes Management
Trace elements, also known as microelements, are essential chemical substances present in the human body in small amounts, playing a crucial role in growth, development, and metabolic functions. In recent years, nanoparticles derived from trace elements have gained increasing attention as nanomedicines in antidiabetic therapies due to their ability to modulate glucose metabolism through multiple pathways [137].
The mechanisms of action of trace element nanoparticles in diabetes management are outlined below:
- Blood glucose regulation: trace element nanoparticles help lower blood glucose levels, reducing hyperglycemia.
- Improved insulin sensitivity: these nanoparticles enhance cellular responsiveness to insulin, allowing for better glucose uptake and utilization.
- Enhanced insulin secretion: certain trace element-based nanomedicines stimulate pancreatic β-cells to produce and release insulin more efficiently.
- Alleviation of glucose intolerance: by modulating glucose absorption and utilization, these nanoparticles help prevent postprandial glucose spikes.
- Lipid profile improvement: studies have shown that trace element nanoparticles can positively influence lipid metabolism, reducing the risk of dyslipidemia in diabetes.
- Anti-inflammatory and antioxidant properties: nanoparticles derived from trace elements exhibit anti-inflammatory and antioxidant effects, which are crucial in preventing β-cell damage and mitigating diabetes-related oxidative stress [137].
Due to their broad spectrum of benefits, trace element nanoparticles have significant potential as nanomedicines or dietary supplements for effective diabetes management. However, further clinical research and safety evaluations are required to establish optimal formulations, dosages, and long-term effects in diabetes therapy.
4. Conclusions
The management of T1D has significantly evolved due to advances in technology, biomedical research, and personalized medicine. This review underscores the role of emerging therapeutic strategies in improving glycemic control, patient adherence, and quality of life. From CGM systems and automated insulin delivery technologies to nanomedicine-based solutions and gene therapy, the future of diabetes care is shifting toward precision medicine and curative interventions.
Technological advancements, such as artificial pancreas systems, smart insulin formulations, and mobile health applications, have facilitated real-time glucose monitoring and personalized insulin adjustments. AI-based algorithms and machine learning models have further enhanced predictive glucose analytics, allowing for proactive management of blood sugar levels. Additionally, telemedicine platforms have expanded access to diabetes education and remote patient monitoring, particularly benefiting children and adolescents with T1D who require adaptive, real-time treatment strategies. Future perspectives include non-invasive glucose monitoring technologies, advanced biosensors, and AI-driven closed-loop insulin delivery systems, which aim to further optimize metabolic control.
Beyond digital innovations, regenerative medicine and immunotherapies are reshaping the long-term therapeutic landscape of T1D. Stem cell-derived β-cell replacement therapies and CRISPR-based gene editing hold promise for restoring insulin production and modulating autoimmune responses. Next-generation immunotherapies, such as antigen-specific monoclonal antibodies, regulatory T-cell (Treg) therapies, and immune tolerance induction strategies, aim to halt disease progression and promote sustained remission. Future research will focus on combining immunotherapy with regenerative approaches, potentially leading to curative solutions for T1D. Additionally, gut microbiota-targeted therapies, including probiotics, prebiotics, and microbiome-based interventions, have also demonstrated potential in reducing inflammation and improving metabolic homeostasis. Nanomedicine-based solutions, such as trace element nanoparticles for insulin delivery and immune modulation, may provide non-invasive, highly effective therapies for diabetes management. Advancements in gene therapy, epigenetic editing, and synthetic biology could open new possibilities for reprogramming immune responses and regenerating pancreatic function.
Despite these scientific breakthroughs, several challenges remain. The long-term efficacy and safety of gene therapies, nanomedicines, and β-cell transplantation require further clinical validation. Additionally, barriers to accessibility, including cost, healthcare infrastructure, and patient adherence, must be addressed to ensure widespread adoption of advanced diabetes treatments. Moving forward, a multidisciplinary and patient-centered approach will be essential in integrating biomedical engineering, artificial intelligence, regenerative medicine, and nanotechnology into clinical practice. Collaborations between researchers, clinicians, biotechnologists, and regulatory agencies will be critical in translating emerging therapies from clinical trials to widespread clinical implementation. By embracing technology-driven solutions, precision medicine, and immune-based interventions, the future of T1D care is moving toward disease modification, prevention, and ultimately, a cure.
Author Contributions
Conceptualization, D.B.-M. and N.-M.M.; methodology, M.B.; software, C.S.Ș.; validation, G.V.P.; formal analysis, M.N.M. and A.N.; investigation, G.G.; resources, M.U.; data curation, C.S.Ș.; writing—original draft preparation, N.-M.M.; writing—review and editing, D.B.-M.; visualization, M.B.; supervision, M.N.M.; project administration, A.N.; funding acquisition, D.B.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the “Dunarea de Jos” University of Galati, VAT number RO27232142.
Data Availability Statement
No new data were created.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| T1D | Type 1 diabetes |
| CGM | Continuous glucose monitoring |
| AI | Artificial intelligence |
| CRISPR | Clustered Regularly Interspaced Short Palindromic Repeats |
| WHO | World Health Organization |
| IDF | International Diabetes Federation |
| ISPAD | International Society for Pediatric and Adolescent Diabetes |
| CSII | Continuous subcutaneous insulin infusion |
| FDA | Food and Drug Administration |
| IT | Information technology |
| MAFA | Musculoaponeurotic fibrosarcoma oncogene homolog A |
| PDX1 | Pancreatic and duodenal homeobox 1 |
| ARX | Aristaless-related homeobox |
| DKA | Diabetic ketoacidosis |
| SGLT2 | Sodium-Glucose Co-Transporter 2 |
| TREG | Regulatory T-cell |
| GDPR | General Data Protection Regulation |
| HIPAA | Health Insurance Portability and Accountability Act |
References
- World Health Organization. Global Report on Diabetes; World Health Organization: Geneva, Switzerland, 2016; ISBN 9789241565257. [Google Scholar]
- Kavakiotis, I.; Tsave, O.; Salifoglou, A.; Maglaveras, N.; Vlahavas, I.; Chouvarda, I. Machine Learning and Data Mining Methods in Diabetes Research. Comput. Struct. Biotechnol. J. 2017, 15, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Aldaghi, T.; Muzik, J. Multicriteria Decision-Making in Diabetes Management and Decision Support: Systematic Review. JMIR Med. Inform. 2024, 12, e47701. [Google Scholar] [CrossRef]
- Chiang, Y.; Tsay, P.; Chen, C.; Hsu, C.; Yu, H.; Chang, C.; Lo, F.; Moons, P. A Delphi Study on the Healthcare Needs of Patients with Type 1 Diabetes during the Transition from Adolescence to Adulthood: Consensus among Patients, Primary Caregivers, and Healthcare Providers. Int. J. Environ. Res. Public Health 2021, 18, 7149. [Google Scholar] [CrossRef]
- Goyal, S.; Nunn, C.A.; Rotondi, M.; Couperthwaite, A.B.; Reiser, S.; Simone, A.; Katzman, D.K.; Cafazzo, J.A.; Palmert, M.R. A Mobile App for the Self-Management of Type 1 Diabetes Among Adolescents: A Randomized Controlled Trial. JMIR mHealth uHealth 2017, 5, e82. [Google Scholar] [CrossRef] [PubMed]
- Petersmann, A.; Nauck, M.; Müller-Wieland, D.; Kerner, W.; Müller, U.; Landgraf, R.; Freckmann, G.; Heinemann, L. Definition, Classification and Diagnosis of Diabetes Mellitus. Exp. Clin. Endocrinol. Diabetes 2018, 126, 406–410. [Google Scholar] [CrossRef]
- Urbano, F.; Farella, I.; Brunetti, G.; Faienza, M.F. Pediatric Type 1 Diabetes: Mechanisms and Impact of Technologies on Comorbidities and Life Expectancy. Int. J. Mol. Sci. 2023, 24, 11980. [Google Scholar] [CrossRef]
- Krzewska, A.; Ben-Skowronek, I. Effect of Associated Autoimmune Diseases on Type 1 Diabetes Mellitus Incidence and Metabolic Control in Children and Adolescents. Biomed. Res. Int. 2016, 2016, 6219730. [Google Scholar] [CrossRef]
- García-Chapa, E.G.; Leal-Ugarte, E.; Peralta-Leal, V.; Durán-González, J.; Meza-Espinoza, J.P. Genetic Epidemiology of Type 2 Diabetes in Mexican Mestizos. Biomed. Res. Int. 2017, 2017, 3937893. [Google Scholar] [CrossRef]
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed; International Diabetes Federation: Brussels, Belgium, 2021. [Google Scholar]
- Atkinson, M.A.; Eisenbarth, G.S.; Michels, A.W. Type 1 Diabetes. Lancet 2014, 383, 69–82. [Google Scholar] [CrossRef]
- Weisman, A.; Bai, J.-W.; Cardinez, M.; Kramer, C.K.; Perkins, B.A. Effect of Artificial Pancreas Systems on Glycaemic Control in Patients with Type 1 Diabetes: A Systematic Review and Meta-Analysis of Outpatient Randomised Controlled Trials. Lancet Diabetes Endocrinol. 2017, 5, 501–512. [Google Scholar] [CrossRef]
- Pescovitz, M.D.; Greenbaum, C.J.; Bundy, B.; Becker, D.J.; Gitelman, S.E.; Goland, R.; Gottlieb, P.A.; Marks, J.B.; Moran, A.; Raskin, P.; et al. B-Lymphocyte Depletion with Rituximab and β-Cell Function: Two-Year Results. Diabetes Care 2014, 37, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Sui, T.; Song, Y.; Liu, Z.; Chen, M.; Deng, J.; Xu, Y.; Lai, L.; Li, Z. CRISPR-Induced Exon Skipping Is Dependent on Premature Termination Codon Mutations. Genome Biol. 2018, 19, 2–6. [Google Scholar] [CrossRef]
- Herkert, D.; Vijayakumar, P.; Luo, J.; Schwartz, J.I.; Rabin, T.L.; DeFilippo, E.; Lipska, K.J. Cost-Related Insulin Underuse Among Patients with Diabetes. JAMA Intern. Med. 2019, 179, 112–114. [Google Scholar] [CrossRef] [PubMed]
- Herold, K.C.; Gitelman, S.E.; Ehlers, M.R.; Gottlieb, P.A.; Greenbaum, C.J.; Hagopian, W.; Boyle, K.D.; Keyes-Elstein, L.; Aggarwal, S.; Phippard, D.; et al. Teplizumab (Anti-CD3 MAb) Treatment Preserves C-Peptide Responses in Patients with New-Onset Type 1 Diabetes in a Randomized Controlled Trial. Diabetes 2013, 62, 3766–3774. [Google Scholar] [CrossRef]
- Huang, M.; Chen, W.; Wang, M.; Huang, Y.; Liu, H.; Ming, Y.; Chen, Y.; Tang, Z.; Jia, B. Advanced Delivery Strategies for Immunotherapy in Type I Diabetes Mellitus. BioDrugs 2023, 37, 331–352. [Google Scholar] [CrossRef]
- Allemailem, K.S.; Alsahli, M.A.; Almatroudi, A.; Alrumaihi, F.; Alkhaleefah, F.K.; Rahmani, A.H.; Khan, A.A. Current Updates of CRISPR/Cas9-mediated Genome Editing and Targeting within Tumor Cells: An Innovative Strategy of Cancer Management. Cancer Commun. 2022, 42, 1257–1287. [Google Scholar] [CrossRef]
- Gregory, G.A.; Robinson, T.I.G.; Linklater, S.E.; Wang, F.; Colagiuri, S.; de Beaufort, C.; Donaghue, K.C.; Magliano, D.J.; Maniam, J.; Orchard, T.J.; et al. Global Incidence, Prevalence, and Mortality of Type 1 Diabetes in 2021 with Projection to 2040: A Modelling Study. Lancet Diabetes Endocrinol. 2022, 10, 741–760. [Google Scholar] [CrossRef]
- Ogle, G.D.; James, S.; Dabelea, D.; Pihoker, C.; Svennson, J.; Maniam, J.; Klatman, E.L.; Patterson, C.C. Global Estimates of Incidence of Type 1 Diabetes in Children and Adolescents: Results from the International Diabetes Federation Atlas, 10th Edition. Diabetes Res. Clin. Pract. 2022, 183, 109083. [Google Scholar] [CrossRef]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, Regional and Country-Level Diabetes Prevalence Estimates for 2021 and Projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef]
- Klaassen, R.; Bul, K.; Op den Akker, R.; Van der Burg, G.; Kato, P.; Di Bitonto, P. Design and Evaluation of a Pervasive Coaching and Gamification Platform for Young Diabetes Patients. Sensors 2018, 18, 402. [Google Scholar] [CrossRef]
- Holtz, B.; Mitchell, K.M.; Holmstrom, A.J.; Cotten, S.R.; Dunneback, J.K.; Jimenez-Vega, J.; Ellis, D.A.; Wood, M.A. An MHealth-Based Intervention for Adolescents with Type 1 Diabetes and Their Parents: Pilot Feasibility and Efficacy Single-Arm Study. JMIR mHealth uHealth 2021, 9, e23916. [Google Scholar] [CrossRef] [PubMed]
- Bitar, H.; Alfahid, A.; Alrige, M.; Abogazah, W.; Alsanbi, N. Ana Alsukary: An Android Mobile Application to Support Diabetic Children and Parents in Saudi Arabia. Rev. Română Informatică Autom. 2022, 32, 73–86. [Google Scholar] [CrossRef]
- Bashir, M.; Ahluwalia, H.; Sayeed, S.I.; Kapoor, R. A Comparative Study of Anxiety Levels and Its Relation with Heart Rate Variability (HRV) Indices in Adolescents with Type 1 Diabetes Mellitus. Medeni. Med. J. 2018, 33, 22–27. [Google Scholar] [CrossRef]
- Ashraff, S.; Siddiqui, M.A.; Carline, T.E. The Psychosocial Impact of Diabetes in Adolescents: A Review. Oman Med. J. 2013, 28, 159–162. [Google Scholar] [CrossRef]
- Christie, D.; Thompson, R.; Sawtell, M.; Allen, E.; Cairns, J.; Smith, F.; Jamieson, E.; Hargreaves, K.; Ingold, A.; Brooks, L.; et al. Structured, Intensive Education Maximising Engagement, Motivation and Long-Term Change for Children and Young People with Diabetes: A Cluster Randomised Controlled Trial with Integral Process and Economic Evaluation—The CASCADE Study. Health Technol. Assess. 2014, 18, 1–202. [Google Scholar] [CrossRef]
- Datye, K.; Bonnet, K.; Schlundt, D.; Jaser, S. Experiences of Adolescents and Emerging Adults Living with Type 1 Diabetes. Diabetes Educ. 2019, 45, 194–202. [Google Scholar] [CrossRef]
- Anghel, L.; Boev, M.; Stanescu, C.; Caramfil, S.M.; Luca, L.; Musat, C.L.; Ciubara, A. Depression in the Diabetic Patient. BRAIN Broad Res. Artif. Intell. Neurosci. 2023, 14, 658–672. [Google Scholar] [CrossRef]
- Cengiz, E.; Danne, T.; Ahmad, T.; Ayyavoo, A.; Beran, D.; Ehtisham, S.; Fairchild, J.; Jarosz-Chobot, P.; Ng, S.M.; Paterson, M.; et al. ISPAD: Clinical Practice Consensus Guidelines 2022. Insulin Treatment in Children and Adolescents with Diabetes. Pediatr. Diabetes 2022, 23, 1277–1296. [Google Scholar] [CrossRef]
- Adolfsson, P.; Taplin, C.E.; Zaharieva, D.P.; Pemberton, J.; Davis, E.A.; Riddell, M.C.; McGavock, J.; Moser, O.; Szadkowska, A.; Lopez, P.; et al. ISPAD Clinical Practice Consensus Guidelines 2022: Exercise in Children and Adolescents with Diabetes. Pediatr. Diabetes 2022, 23, 1341–1372. [Google Scholar] [CrossRef]
- Passanisi, S.; Salzano, G.; Basile, P.; Bombaci, B.; Caime, F.; Rulli, I.; Valenzise, M.; Gitto, E.; Lombardo, F. Prevalence and Clinical Features of Severe Diabetic Ketoacidosis Treated in Pediatric Intensive Care Unit: A 5-Year Monocentric Experience. Ital. J. Pediatr. 2023, 49, 58. [Google Scholar] [CrossRef]
- Passanisi, S.; Galletta, F.; Bombaci, B.; Cherubini, V.; Tiberi, V.; Minuto, N.; Bassi, M.; Iafusco, D.; Piscopo, A.; Mozzillo, E.; et al. Device-Related Skin Reactions Increase Emotional Burden in Youths with Type 1 Diabetes and Their Parents. J. Diabetes Sci. Technol. 2024, 18, 1293–1299. [Google Scholar] [CrossRef]
- Ledwoń, E.; Zemła-Szten, P.; von dem Berge, T.; Nalewajko, K.; Passanisi, S.; Piona, C.; dos Santos, T.; Svensson, J.; Korsgaard Berg, A.; Chobot, A. Skin Reactions in Children with Type 1 Diabetes Associated with the Use of New Diabetes Technologies—An Observational Study from a Regional Polish Pediatric Diabetes Center. Children 2024, 11, 740. [Google Scholar] [CrossRef]
- Gregory, J.W.; Cameron, F.J.; Joshi, K.; Eiswirth, M.; Garrett, C.; Garvey, K.; Agarwal, S.; Codner, E. ISPAD Clinical Practice Consensus Guidelines 2022: Diabetes in Adolescence. Pediatr. Diabetes 2022, 23, 857–871. [Google Scholar] [CrossRef]
- Gomes, M.B.; Calliari, L.E.; Conte, D.; Correa, C.L.; Drummond, K.R.G.; Mallmann, F.; Pinheiro, A.A.; Muniz, L.H.; Leal, F.S.L.; Morales, P.H.; et al. Diabetes-Related Chronic Complications in Brazilian Adolescents with Type 1 Diabetes. A Multicenter Cross-Sectional Study. Diabetes Res. Clin. Pract. 2021, 177, 108895. [Google Scholar] [CrossRef]
- Mayya, V.; Kandala, R.N.V.P.S.; Gurupur, V.; King, C.; Vu, G.T.; Wan, T.T.H. Need for an Artificial Intelligence-Based Diabetes Care Management System in India and the United States. Health Serv. Res. Manag. Epidemiol. 2024, 11, 23333928241275292. [Google Scholar] [CrossRef]
- Anandhakrishnan, A.; Hussain, S. Automating Insulin Delivery through Pump and Continuous Glucose Monitoring Connectivity: Maximizing Opportunities to Improve Outcomes. Diabetes Obes. Metab. 2024, 26, 27–46. [Google Scholar] [CrossRef]
- Mackenzie, S.C.; Sainsbury, C.A.R.; Wake, D.J. Diabetes and Artificial Intelligence beyond the Closed Loop: A Review of the Landscape, Promise and Challenges. Diabetologia 2024, 67, 223–235. [Google Scholar] [CrossRef]
- de Bock, M.; Codner, E.; Craig, M.E.; Huynh, T.; Maahs, D.M.; Mahmud, F.H.; Marcovecchio, L.; DiMeglio, L.A. ISPAD: Clinical Practice Consensus Guidelines 2022: Glycemic Targets and Glucose Monitoring for Children, Adolescents, and Young People with Diabetes. Pediatr. Diabetes 2022, 23, 1270–1276. [Google Scholar] [CrossRef]
- Sundberg, F.; DeBeaufort, C.; Krogvold, L.; Patton, S.; Piloya, T.; Smart, C.; Van Name, M.; Weissberg-Benchell, J.; Silva, J.; DiMeglio, L.A. ISPAD Clinical Practice Consensus Guidelines 2022: Managing Diabetes in Preschoolers. Pediatr. Diabetes 2022, 23, 1496–1511. [Google Scholar] [CrossRef]
- Schoelwer, M.J.; DeBoer, M.D.; Breton, M.D. Use of Diabetes Technology in Children. Diabetologia 2024, 67, 2075–2084. [Google Scholar] [CrossRef]
- Cardona-Hernandez, R.; Schwandt, A.; Alkandari, H.; Bratke, H.; Chobot, A.; Coles, N.; Corathers, S.; Goksen, D.; Goss, P.; Imane, Z.; et al. Glycemic Outcome Associated with Insulin Pump and Glucose Sensor Use in Children and Adolescents with Type 1 Diabetes. Data From the International Pediatric Registry SWEET. Diabetes Care 2021, 44, 1176–1184. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.R.; Holmes-Walker, D.J.; Chee, M.; Earnest, A.; Jones, T.W. Universal Subsidized Continuous Glucose Monitoring Funding for Young People with Type 1 Diabetes: Uptake and Outcomes Over 2 Years, a Population-Based Study. Diabetes Care 2022, 45, 391–397. [Google Scholar] [CrossRef]
- Prahalad, P.; Hardison, H.; Odugbesan, O.; Lyons, S.; Alwazeer, M.; Neyman, A.; Miyazaki, B.; Cossen, K.; Hsieh, S.; Eng, D.; et al. Benchmarking Diabetes Technology Use Among 21 U.S. Pediatric Diabetes Centers. Clin. Diabetes 2024, 42, 27–33. [Google Scholar] [CrossRef]
- Elian, V.; Popovici, V.; Ozon, E.A.; Musuc, A.M.; Fița, A.C.; Rusu, E.; Radulian, G.; Lupuliasa, D. Current Technologies for Managing Type 1 Diabetes Mellitus and Their Impact on Quality of Life—A Narrative Review. Life 2023, 13, 1663. [Google Scholar] [CrossRef]
- Franceschi, R.; Micheli, F.; Mozzillo, E.; Cauvin, V.; Liguori, A.; Soffiati, M.; Giani, E. Intermittently Scanned and Continuous Glucose Monitor Systems: A Systematic Review on Psychological Outcomes in Pediatric Patients. Front. Pediatr. 2021, 9, 660173. [Google Scholar] [CrossRef]
- Visser, M.M.; Charleer, S.; Fieuws, S.; De Block, C.; Hilbrands, R.; Van Huffel, L.; Maes, T.; Vanhaverbeke, G.; Dirinck, E.; Myngheer, N.; et al. Comparing Real-Time and Intermittently Scanned Continuous Glucose Monitoring in Adults with Type 1 Diabetes (ALERTT1): A 6-Month, Prospective, Multicentre, Randomised Controlled Trial. Lancet 2021, 397, 2275–2283. [Google Scholar] [CrossRef]
- Cappon, G.; Acciaroli, G.; Vettoretti, M.; Facchinetti, A.; Sparacino, G. Wearable Continuous Glucose Monitoring Sensors: A Revolution in Diabetes Treatment. Electronics 2017, 6, 65. [Google Scholar] [CrossRef]
- Zafar, H.; Channa, A.; Jeoti, V.; Stojanović, G.M. Comprehensive Review on Wearable Sweat-Glucose Sensors for Continuous Glucose Monitoring. Sensors 2022, 22, 638. [Google Scholar] [CrossRef]
- Nagy, G.; Szekely, T.E.; Somogyi, A.; Herold, M.; Herold, Z. New Therapeutic Approaches for Type 1 Diabetes: Disease-Modifying Therapies. World J. Diabetes 2022, 13, 835–850. [Google Scholar] [CrossRef]
- Sperling, M.A.; Laffel, L.M. Current Management of Glycemia in Children with Type 1 Diabetes Mellitus. N. Engl. J. Med. 2022, 386, 1155–1164. [Google Scholar] [CrossRef]
- Bailey, T.S.; Alva, S. Landscape of Continuous Glucose Monitoring (CGM) and Integrated CGM: Accuracy Considerations. Diabetes Technol. Ther. 2021, 23, S5–S11. [Google Scholar] [CrossRef]
- Mansour, M.; Saeed Darweesh, M.; Soltan, A. Wearable Devices for Glucose Monitoring: A Review of State-of-the-Art Technologies and Emerging Trends. Alex. Eng. J. 2024, 89, 224–243. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Yu, J.; Kahkoska, A.R.; Buse, J.B.; Gu, Z. Glucose-Responsive Insulin and Delivery Systems: Innovation and Translation. Adv. Mater. 2020, 32, e1902004. [Google Scholar] [CrossRef]
- Moroșan, E.; Popovici, V.; Elian, V.; Dărăban, A.M.; Rusu, A.I.; Licu, M.; Mititelu, M.; Karampelas, O. The Impact of Medical Nutrition Intervention on the Management of Hyperphosphatemia in Hemodialysis Patients with Stage 5 Chronic Kidney Disease: A Case Series. Int. J. Environ. Res. Public Health 2023, 20, 5049. [Google Scholar] [CrossRef]
- Stankute, I.; Radzeviciene, L.; Monstaviciene, A.; Dobrovolskiene, R.; Danyte, E.; Verkauskiene, R. Serum Cystatin C as a Biomarker for Early Diabetic Kidney Disease and Dyslipidemia in Young Type 1 Diabetes Patients. Medicina 2022, 58, 218. [Google Scholar] [CrossRef]
- Eliasson, B.; Lyngfelt, L.; Strömblad, S.-O.; Franzén, S.; Eeg-Olofsson, K. The Significance of Chronic Kidney Disease, Heart Failure and Cardiovascular Disease for Mortality in Type 1 Diabetes: Nationwide Observational Study. Sci. Rep. 2022, 12, 17950. [Google Scholar] [CrossRef]
- Da Silva de Sousa, G.G.; Yamamura, M.; Moura de Araújo, M.F.; Vieira Ramos, A.C.; Arcêncio, R.A.; Pereira de Jesus Costa, A.C.; Maia Pascoal, L.; Stabnow Santos, F.; Alves de Oliveira Serra, M.A.; Graepp Fontoura, I.; et al. Vulnerable Territories to Tuberculosis-Diabetes Mellitus Comorbidity in a Northeastern Brazilian Scenario. J. Infect. Dev. Ctries. 2022, 16, 813–820. [Google Scholar] [CrossRef]
- Ingley, M.; Quebedeaux, P.; Schmidbauer, K.; Amghaiab, I.A.; Chan, J. PMON315 Hyperglycemia Dilemma: Concomitant Type 1 Diabetes Mellitus and Cushing’s Disease. J. Endocr. Soc. 2022, 6, A624–A625. [Google Scholar] [CrossRef]
- Ilie, I. The Multifarious Cushing’s—Lessons from a Case Series. Acta Endocrinol. 2019, 15, 261–269. [Google Scholar] [CrossRef]
- Kaur, N.; Bhadada, S.K.; Minz, R.W.; Dayal, D.; Kochhar, R. Interplay between Type 1 Diabetes Mellitus and Celiac Disease: Implications in Treatment. Dig. Dis. 2018, 36, 399–408. [Google Scholar] [CrossRef]
- O’Donnell, H.K.; Vigers, T.; Johnson, S.B.; Pyle, L.; Wright, N.; Deeb, L.C.; Driscoll, K.A. Pump It Up! A Randomized Clinical Trial to Optimize Insulin Pump Self-Management Behaviors in Adolescents with Type 1 Diabetes. Contemp. Clin. Trials 2021, 102, 106279. [Google Scholar] [CrossRef]
- Ersig, A.L.; Tsalikian, E.; Coffey, J.; Williams, J.K. Stressors in Teens with Type 1 Diabetes and Their Parents: Immediate and Long-Term Implications for Transition to Self-Management. J. Pediatr. Nurs. 2016, 31, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Downing, J.; Gleeson, H.K.; Clayton, P.E.; Davis, J.R.E.; Wales, J.K.; Callery, P. Transition in Endocrinology: The Challenge of Maintaining Continuity. Clin. Endocrinol. 2013, 78, 29–35. [Google Scholar] [CrossRef]
- Herbert, L.; Owen, V.; Pascarella, L.; Streisand, R. Text Message Interventions for Children and Adolescents with Type 1 Diabetes: A Systematic Review. Diabetes Technol. Ther. 2013, 15, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Monaghan, M.; Hilliard, M.; Sweenie, R.; Riekert, K. Transition Readiness in Adolescents and Emerging Adults with Diabetes: The Role of Patient-Provider Communication. Curr. Diab. Rep. 2013, 13, 900–908. [Google Scholar] [CrossRef]
- Glycemic Targets: Standards of Medical Care in Diabetes—2021. Diabetes Care 2021, 44, S73–S84. [CrossRef]
- Chen, L.; Chuang, L.-M.; Chang, C.-H.; Wang, C.-S.; Wang, I.-C.; Chung, Y.; Peng, H.-Y.; Chen, H.-C.; Hsu, Y.-L.; Lin, Y.-S.; et al. Evaluating Self-Management Behaviors of Diabetic Patients in a Telehealthcare Program: Longitudinal Study Over 18 Months. J. Med. Internet Res. 2013, 15, e266. [Google Scholar] [CrossRef]
- Jackson, C.C.; Albanese-O’Neill, A. Supporting the Student’s Graduated Independence in Diabetes Care. NASN Sch. Nurse 2016, 31, 202–204. [Google Scholar] [CrossRef]
- Zarifsaniey, N.; Shirazi, M.O.; Mehrabi, M.; Bagheri, Z. Promoting Self-Management Behaviors in Adolescents with Type 1 Diabetes, Using Digital Storytelling: A Pilot Randomized Controlled Trial. BMC Endocr. Disord. 2022, 22, 74. [Google Scholar] [CrossRef]
- Abrar, E.A.; Yusuf, S.; Sjattar, E.L.; Rachmawaty, R. Development and Evaluation Educational Videos of Diabetic Foot Care in Traditional Languages to Enhance Knowledge of Patients Diagnosed with Diabetes and Risk for Diabetic Foot Ulcers. Prim. Care Diabetes 2020, 14, 104–110. [Google Scholar] [CrossRef]
- Jahanbakhsh, M.; Ehteshami, A.; Afkhami, S. Developing “Aryan”: Diabetes Self-Care Mobile Application. Int. J. Prev. Med. 2019, 10, 59. [Google Scholar] [CrossRef] [PubMed]
- Samimi, Z.; Talakoub, S.; Ghazavi, Z. Effect of Telephone Follow-up by Nurses on Self-Care in Children with Diabetes. Iran. J. Nurs. Midwifery Res. 2018, 23, 26. [Google Scholar] [CrossRef] [PubMed]
- Swift, A.; Etherton, J.; Twycross, A. Keeping the Patient Front and Central: The Role of Storytelling. Evid. Based Nurs. 2019, 22, 31–32. [Google Scholar] [CrossRef]
- Moghimian, M.; Akbari, M.; Moghaddasi, J.; Niknajad, R. Effect of Digital Storytelling on Anxiety in Patients Who Are Candidates for Open-Heart Surgery. J. Cardiovasc. Nurs. 2019, 34, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Lisenbee, P.S.; Ford, C.M. Engaging Students in Traditional and Digital Storytelling to Make Connections Between Pedagogy and Children’s Experiences. Early Child. Educ. J. 2018, 46, 129–139. [Google Scholar] [CrossRef]
- Kory-Westlund, J.M.; Jeong, S.; Park, H.W.; Ronfard, S.; Adhikari, A.; Harris, P.L.; DeSteno, D.; Breazeal, C.L. Flat vs. Expressive Storytelling: Young Children’s Learning and Retention of a Social Robot’s Narrative. Front. Hum. Neurosci. 2017, 11, 295. [Google Scholar] [CrossRef]
- Wilson, D.K.; Hutson, S.P.; Wyatt, T.H. Exploring the Role of Digital Storytelling in Pediatric Oncology Patients’ Perspectives Regarding Diagnosis. Sage Open 2015, 5, 2158244015572099. [Google Scholar] [CrossRef]
- Papacharissi, Z. Affective Publics and Structures of Storytelling: Sentiment, Events and Mediality. Inf. Commun. Soc. 2016, 19, 307–324. [Google Scholar] [CrossRef]
- Järvelä-Reijonen, E.; Karhunen, L.; Sairanen, E.; Muotka, J.; Lindroos, S.; Laitinen, J.; Puttonen, S.; Peuhkuri, K.; Hallikainen, M.; Pihlajamäki, J.; et al. The Effects of Acceptance and Commitment Therapy on Eating Behavior and Diet Delivered through Face-to-Face Contact and a Mobile App: A Randomized Controlled Trial. Int. J. Behav. Nutr. Phys. Act. 2018, 15, 22. [Google Scholar] [CrossRef]
- Morawski, K.; Ghazinouri, R.; Krumme, A.; Lauffenburger, J.C.; Lu, Z.; Durfee, E.; Oley, L.; Lee, J.; Mohta, N.; Haff, N.; et al. Association of a Smartphone Application with Medication Adherence and Blood Pressure Control. JAMA Intern. Med. 2018, 178, 802. [Google Scholar] [CrossRef]
- Patel, M.L.; Hopkins, C.M.; Brooks, T.L.; Bennett, G.G. Comparing Self-Monitoring Strategies for Weight Loss in a Smartphone App: Randomized Controlled Trial. JMIR mHealth uHealth 2019, 7, e12209. [Google Scholar] [CrossRef]
- Greer, J.A.; Jacobs, J.M.; Pensak, N.; Nisotel, L.E.; Fishbein, J.N.; MacDonald, J.J.; Ream, M.E.; Walsh, E.A.; Buzaglo, J.; Muzikansky, A.; et al. Randomized Trial of a Smartphone Mobile App to Improve Symptoms and Adherence to Oral Therapy for Cancer. J. Natl. Compr. Canc. Netw. 2020, 18, 133–141. [Google Scholar] [PubMed]
- Mascarenhas, M.N.; Chan, J.M.; Vittinghoff, E.; Van Blarigan, E.L.; Hecht, F. Increasing Physical Activity in Mothers Using Video Exercise Groups and Exercise Mobile Apps: Randomized Controlled Trial. J. Med. Internet Res. 2018, 20, e179. [Google Scholar] [CrossRef] [PubMed]
- Berndt, R.-D.; Takenga, C.; Preik, P.; Kuehn, S.; Berndt, L.; Mayer, H.; Kaps, A.; Schiel, R. Impact of Information Technology on the Therapy of Type-1 Diabetes: A Case Study of Children and Adolescents in Germany. J. Pers. Med. 2014, 4, 200–217. [Google Scholar] [CrossRef]
- Klee, P.; Bussien, C.; Castellsague, M.; Combescure, C.; Dirlewanger, M.; Girardin, C.; Mando, J.-L.; Perrenoud, L.; Salomon, C.; Schneider, F.; et al. An Intervention by a Patient-Designed Do-It-Yourself Mobile Device App Reduces HbA1c in Children and Adolescents with Type 1 Diabetes: A Randomized Double-Crossover Study. Diabetes Technol. Ther. 2018, 20, 797–805. [Google Scholar] [CrossRef]
- Castensøe-Seidenfaden, P.; Reventlov Husted, G.; Teilmann, G.; Hommel, E.; Olsen, B.S.; Kensing, F. Designing a Self-Management App for Young People with Type 1 Diabetes: Methodological Challenges, Experiences, and Recommendations. JMIR mHealth uHealth 2017, 5, e124. [Google Scholar] [CrossRef] [PubMed]
- Albanese-O’Neill, A.; Schatz, D.A.; Thomas, N.; Bernhardt, J.M.; Cook, C.L.; Haller, M.J.; Bernier, A.V.; Silverstein, J.H.; Westen, S.C.; Elder, J.H. Designing Online and Mobile Diabetes Education for Fathers of Children with Type 1 Diabetes: Mixed Methods Study. JMIR Diabetes 2019, 4, e13724. [Google Scholar] [CrossRef]
- Andersen, N.S.; Haugaard, L.H.; Pedersen, S.B.; Pedersen, M.S.; Bygholm, A. Digital Support for Self-Management in Children with Diabetes: Understanding Their Needs and Developing a Design Concept. In Digital Personalized Health and Medicine; IOS Press: Amsterdam, The Netherlands, 2020. [Google Scholar]
- McCulloch, V.; Hope, S.; Loranger, B.; Rea, P. How to Effectively Design and Create a Concept Mobile Application to Aid in the Management of Type 1 Diabetes in Adolescents. J. Vis. Commun. Med. 2017, 40, 101–108. [Google Scholar] [CrossRef]
- Castensøe-Seidenfaden, P.; Husted, G.R.; Jensen, A.K.; Hommel, E.; Olsen, B.; Pedersen-Bjergaard, U.; Kensing, F.; Teilmann, G. Testing a Smartphone App (Young with Diabetes) to Improve Self-Management of Diabetes Over 12 Months: Randomized Controlled Trial. JMIR mHealth uHealth 2018, 6, e141. [Google Scholar] [CrossRef]
- Schmidt, M.; Lu, J.; Luo, W.; Cheng, L.; Lee, M.; Huang, R.; Weng, Y.; Kichler, J.C.; Corathers, S.D.; Jacobsen, L.M.; et al. Learning Experience Design of an MHealth Self-Management Intervention for Adolescents with Type 1 Diabetes. Educ. Technol. Res. Dev. 2022, 70, 2171–2209. [Google Scholar] [CrossRef]
- Alsalman, D.M.; Ali, Z.B.; Alnosaier, Z.; Alotaibi, N.; Alanzi, T.M. Caregiver’s Opinions on the Design of the Screens of a Future Gamified Mobile Application for Self-Management of Type 1 Diabetes in Children in Saudi Arabia. Int. J. Telemed. Appl. 2021, 2021, 8822676. [Google Scholar] [CrossRef] [PubMed]
- Kitsiou, S.; Paré, G.; Jaana, M.; Gerber, B. Effectiveness of MHealth Interventions for Patients with Diabetes: An Overview of Systematic Reviews. PLoS ONE 2017, 12, e0173160. [Google Scholar] [CrossRef]
- Markowitz, J.T.; Cousineau, T.; Franko, D.L.; Schultz, A.T.; Trant, M.; Rodgers, R.; Laffel, L.M.B. Text Messaging Intervention for Teens and Young Adults with Diabetes. J. Diabetes Sci. Technol. 2014, 8, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Cafazzo, J.A.; Casselman, M.; Hamming, N.; Katzman, D.K.; Palmert, M.R. Design of an MHealth App for the Self-Management of Adolescent Type 1 Diabetes: A Pilot Study. J. Med. Internet Res. 2012, 14, e70. [Google Scholar] [CrossRef]
- Holtz, B.E.; Murray, K.M.; Hershey, D.D.; Dunneback, J.K.; Cotten, S.R.; Holmstrom, A.J.; Vyas, A.; Kaiser, M.K.; Wood, M.A. Developing a Patient-Centered MHealth App: A Tool for Adolescents with Type 1 Diabetes and Their Parents. JMIR mHealth uHealth 2017, 5, e53. [Google Scholar] [CrossRef]
- Husted, G.R.; Weis, J.; Teilmann, G.; Castensøe-Seidenfaden, P. Exploring the Influence of a Smartphone App (Young with Diabetes) on Young People’s Self-Management: Qualitative Study. JMIR mHealth uHealth 2018, 6, e43. [Google Scholar] [CrossRef]
- Ledderer, L.; Møller, A.; Fage-Butler, A. Adolescents’ Participation in Their Healthcare: A Sociomaterial Investigation of a Diabetes App. Digit. Health 2019, 5, 2055207619845448. [Google Scholar] [CrossRef]
- Stanger, C.; Kowatsch, T.; Xie, H.; Nahum-Shani, I.; Lim-Liberty, F.; Anderson, M.; Santhanam, P.; Kaden, S.; Rosenberg, B. A Digital Health Intervention (SweetGoals) for Young Adults with Type 1 Diabetes: Protocol for a Factorial Randomized Trial. JMIR Res. Protoc. 2021, 10, e27109. [Google Scholar] [CrossRef]
- Frøisland, D.H.; Årsand, E. Integrating Visual Dietary Documentation in Mobile-Phone-Based Self-Management Application for Adolescents with Type 1 Diabetes. J. Diabetes Sci. Technol. 2015, 9, 541–548. [Google Scholar] [CrossRef]
- Krmpotic, K.; Gallant, J.R.; Zufelt, K.; Zuijdwijk, C. User-Centred Development of an MHealth App for Youth with Type 1 Diabetes: The Challenge of Operationalizing Desired Features and Feasibility of Offering Financial Incentives. Health Technol. 2022, 12, 499–513. [Google Scholar] [CrossRef]
- Alfonsi, J.E.; Choi, E.E.Y.; Arshad, T.; Sammott, S.-A.S.; Pais, V.; Nguyen, C.; Maguire, B.R.; Stinson, J.N.; Palmert, M.R. Carbohydrate Counting App Using Image Recognition for Youth with Type 1 Diabetes: Pilot Randomized Control Trial. JMIR mHealth uHealth 2020, 8, e22074. [Google Scholar] [CrossRef] [PubMed]
- Chatzakis, C.; Floros, D.; Papagianni, M.; Tsiroukidou, K.; Kosta, K.; Vamvakis, A.; Koletsos, N.; Hatziagorou, E.; Tsanakas, I.; Mastorakos, G. The Beneficial Effect of the Mobile Application Euglyca in Children and Adolescents with Type 1 Diabetes Mellitus: A Randomized Controlled Trial. Diabetes Technol. Ther. 2019, 21, 627–634. [Google Scholar] [CrossRef] [PubMed]
- den Akker, R.O.; Klaassen, R.; Bul, K.; Kato, P.M.; van der Burg, G.-J.; di Bitonto, P. Let Them Play. In Proceedings of the 11th EAI International Conference on Pervasive Computing Technologies for Healthcare, New York, NY, USA, 23 May 2017; pp. 409–418. [Google Scholar]
- Neinstein, A.; Wong, J.; Look, H.; Arbiter, B.; Quirk, K.; McCanne, S.; Sun, Y.; Blum, M.; Adi, S. A Case Study in Open Source Innovation: Developing the Tidepool Platform for Interoperability in Type 1 Diabetes Management. J. Am. Med. Inform. Assoc. 2016, 23, 324–332. [Google Scholar] [CrossRef]
- Bin-Abbas, B.; Jabbari, M.; Al-Fares, A.; El-Dali, A.; Al-Orifi, F. Effect of Mobile Phone Short Text Messages on Glycaemic Control in Children with Type 1 Diabetes. J. Telemed. Telecare 2014, 20, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Trnka, P.; Aldaghi, T.; Muzik, J. Categorization of MHealth Coaching Technologies for Children or Adolescents with Type 1 Diabetes: Systematic Review. JMIR Pediatr. Parent. 2024, 7, e50370. [Google Scholar] [CrossRef]
- Nkhoma, D.E.; Soko, C.J.; Bowrin, P.; Manga, Y.B.; Greenfield, D.; Househ, M.; Li, Y.-C.; Iqbal, U. Digital Interventions Self-Management Education for Type 1 and 2 Diabetes: A Systematic Review and Meta-Analysis. Comput. Methods Programs Biomed. 2021, 210, 106370. [Google Scholar] [CrossRef]
- Garner, K.; Boggiss, A.; Jefferies, C.; Serlachius, A. Digital Health Interventions for Improving Mental Health Outcomes and Wellbeing for Youth with Type 1 Diabetes: A Systematic Review. Pediatr. Diabetes 2022, 23, 258–269. [Google Scholar] [CrossRef]
- Stevens, S.; Gallagher, S.; Andrews, T.; Ashall-Payne, L.; Humphreys, L.; Leigh, S. The Effectiveness of Digital Health Technologies for Patients with Diabetes Mellitus: A Systematic Review. Front. Clin. Diabetes Healthc. 2022, 3, 936752. [Google Scholar] [CrossRef]
- Giani, E.; Dovc, K.; Dos Santos, T.J.; Chobot, A.; Braune, K.; Cardona-Hernandez, R.; De Beaufort, C.; Scaramuzza, A.E. Telemedicine and COVID 19 Pandemic: The Perfect Storm to Mark a Change in Diabetes Care. Results from a World-wide Cross-sectional Web-based Survey. Pediatr. Diabetes 2021, 22, 1115–1119. [Google Scholar] [CrossRef]
- Buggs-Saxton, C. Care of Pediatric Patients with Diabetes During the Coronavirus Disease 2019 (COVID-19) Pandemic. Pediatr. Clin. N. Am. 2021, 68, 1093–1101. [Google Scholar] [CrossRef]
- Cornea, D. Local Therapy Management of Oral Pathology in Patients with Local Therapy Management of Oral Pathology in Patients with Fixed Orthodontic Appliances During the COVID-19 Pandemic. Rom. J. Oral Rehabil. 2022, 14, 200–206. [Google Scholar]
- Crossen, S.S.; Bruggeman, B.S.; Haller, M.J.; Raymond, J.K. Challenges and Opportunities in Using Telehealth for Diabetes Care. Diabetes Spectr. 2022, 35, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, D.A.; Gee, P.M.; Fatkin, K.J.; Peeples, M. A Systematic Review of Reviews Evaluating Technology-Enabled Diabetes Self-Management Education and Support. J. Diabetes Sci. Technol. 2017, 11, 1015–1027. [Google Scholar] [CrossRef] [PubMed]
- Guljas, R.; Ahmed, A.; Chang, K.; Whitlock, A. Impact of Telemedicine in Managing Type 1 Diabetes Among School-Age Children and Adolescents: An Integrative Review. J. Pediatr. Nurs. 2014, 29, 198–204. [Google Scholar] [CrossRef]
- von Sengbusch, S.; Eisemann, N.; Mueller-Godeffroy, E.; Lange, K.; Doerdelmann, J.; Erdem, A.; Menrath, I.; Bokelmann, J.; Krasmann, M.; Kaczmarczyk, P.; et al. Outcomes of Monthly Video Consultations as an Add-on to Regular Care for Children with Type 1 Diabetes: A 6-Month Quasi-Randomized Clinical Trial Followed by an Extension Phase. Pediatr. Diabetes 2020, 21, 1502–1515. [Google Scholar] [CrossRef]
- Negreiros, F.D.d.S.; Araújo, A.L.d.; Mattos, S.M.; Moreira, T.R.; Cestari, V.R.F.; da Silva, L.M.S.; Moreira, T.M.M. Digital Technologies in the Care of People with Diabetes during the COVID-19 Pandemic: A Scoping Review. Rev. Esc. Enferm. USP 2021, 55, e20210295. [Google Scholar] [CrossRef]
- d’Annunzio, G.; Maffeis, C.; Cherubini, V.; Rabbone, I.; Scaramuzza, A.; Schiaffini, R.; Minuto, N.; Piccolo, G.; Maghnie, M. Caring for Children and Adolescents with Type 1 Diabetes Mellitus: Italian Society for Pediatric Endocrinology and Diabetology (ISPED) Statements during COVID-19 Pandemia. Diabetes Res. Clin. Pract. 2020, 168, 108372. [Google Scholar] [CrossRef]
- Plachy, L.; Neuman, V.; Velichova, K.; Slavenko, M.G.; Santova, A.; Anne Amaratunga, S.; Obermannova, B.; Kolouskova, S.; Pruhova, S.; Sumnik, Z.; et al. Telemedicine Maintains Good Glucose Control in Children with Type 1 Diabetes but Is Not Time Saving for Healthcare Professionals: KITES Randomized Study. Diabetes Res. Clin. Pract. 2024, 209, 111602. [Google Scholar] [CrossRef]
- Gajarawala, S.N.; Pelkowski, J.N. Telehealth Benefits and Barriers. J. Nurse Pract. 2021, 17, 218–221. [Google Scholar] [CrossRef]
- Taras, M.A.; Tonolo, G. Psychological Aspects in the Use of Telemedicine in Diabetes Mellitus. J. Diabetes Treat. 2022, 7, 1098. [Google Scholar] [CrossRef]
- Tyler, N.S.; Jacobs, P.G. Artificial Intelligence in Decision Support Systems for Type 1 Diabetes. Sensors 2020, 20, 3214. [Google Scholar] [CrossRef]
- Tanenbaum, M.L.; Commissariat, P.V. Barriers and Facilitators to Diabetes Device Adoption for People with Type 1 Diabetes. Curr. Diab. Rep. 2022, 22, 291–299. [Google Scholar] [CrossRef]
- Addala, A.; Suttiratana, S.C.; Wong, J.J.; Lanning, M.S.; Barnard, K.D.; Weissberg-Benchell, J.; Laffel, L.M.; Hood, K.K.; Naranjo, D. Cost Considerations for Adoption of Diabetes Technology Are Pervasive: A Qualitative Study of Persons Living with Type 1 Diabetes and Their Families. Diabet. Med. 2021, 38, e14575. [Google Scholar] [CrossRef]
- Forlenza, G.P. Use of Artificial Intelligence to Improve Diabetes Outcomes in Patients Using Multiple Daily Injections Therapy. Diabetes Technol. Ther. 2019, 21, S24–S28. [Google Scholar] [CrossRef]
- Bertolazzi, A.; Marzęda-Młynarska, K.; Kięczkowska, J.; Zanier, M.L. Datafication of Care: Security and Privacy Issues with Health Technology for People with Diabetes. Societies 2024, 14, 163. [Google Scholar] [CrossRef]
- Ware, J.; Hovorka, R. Closed-Loop Insulin Delivery: Update on the State of the Field and Emerging Technologies. Expert Rev. Med. Devices 2022, 19, 859–875. [Google Scholar] [CrossRef] [PubMed]
- Abrahamson, M.J.; Barzilay, J.I.; Blonde, L.; Bloomgarden, Z.T.; Bush, M.A.; Dagogo-Jack, S.; Davidson, M.B.; Einhorn, D.; Timothy Garvey, W.; Grunberger, G.; et al. Aace Comprehensive Diabetes Management Algorithm 2013. Endocr. Pract. 2013, 19, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Children and Adolescents: Standards of Medical Care in Diabetes—2021. Diabetes Care 2021, 44, S180–S199. [CrossRef]
- Keymeulen, B.; De Groot, K.; Jacobs-Tulleneers-Thevissen, D.; Thompson, D.M.; Bellin, M.D.; Kroon, E.J.; Daniels, M.; Wang, R.; Jaiman, M.; Kieffer, T.J.; et al. Encapsulated Stem Cell–Derived β Cells Exert Glucose Control in Patients with Type 1 Diabetes. Nat. Biotechnol. 2023, 42, 1507–1514. [Google Scholar] [CrossRef]
- Karpov, D.S.; Sosnovtseva, A.O.; Pylina, S.V.; Bastrich, A.N.; Petrova, D.A.; Kovalev, M.A.; Shuvalova, A.I.; Eremkina, A.K.; Mokrysheva, N.G. Challenges of CRISPR/Cas-Based Cell Therapy for Type 1 Diabetes: How Not to Engineer a “Trojan Horse”. Int. J. Mol. Sci. 2023, 24, 17320. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, H.; Li, M. The Promise of CRISPR/Cas9 Technology in Diabetes Mellitus Therapy: How Gene Editing Is Revolutionizing Diabetes Research and Treatment. J. Diabetes Complicat. 2023, 37, 108524. [Google Scholar] [CrossRef]
- Basile, G.; Qadir, M.M.F.; Mauvais-Jarvis, F.; Vetere, A.; Shoba, V.; Modell, A.E.; Pastori, R.L.; Russ, H.A.; Wagner, B.K.; Dominguez-Bendala, J. Emerging Diabetes Therapies: Bringing Back the β-Cells. Mol. Metab. 2022, 60, 101477. [Google Scholar] [CrossRef] [PubMed]
- Ruan, S.; Guo, X.; Ren, Y.; Cao, G.; Xing, H.; Zhang, X. Nanomedicines Based on Trace Elements for Intervention of Diabetes Mellitus. Biomed. Pharmacother. 2023, 168, 115684. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, Y.; Wu, J. Review of Non-Invasive Continuous Glucose Monitoring Based on Impedance Spectroscopy. Sens. Actuators A Phys. 2020, 311, 112103. [Google Scholar] [CrossRef]
- Humulescu, I.; Flutur, M.M.; Cioancă, O.; Mircea, C.; Robu, S.; Marin-Batir, D.; Spac, A.; Corciova, A.; Hăncianu, M. Comparative Chemical and Biological Activity of Selective Herbal Extracts. Farmacia 2021, 69, 861–866. [Google Scholar] [CrossRef]
- Wang, M.; Yang, Y.; Min, J.; Song, Y.; Tu, J.; Mukasa, D.; Ye, C.; Xu, C.; Heflin, N.; McCune, J.S.; et al. A Wearable Electrochemical Biosensor for the Monitoring of Metabolites and Nutrients. Nat. Biomed. Eng. 2022, 6, 1225–1235. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Yan, Y.-X.; Liu, H. On the Use of Fiber Lasers in Non-Invasive Blood Glucose Monitoring. Opt. Fiber Technol. 2022, 68, 102822. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, G.; Wei, H.; Guo, Z.; Yang, H.; He, Y.; Xie, S.; Liu, Y. Continuous Noninvasive Monitoring of Changes in Human Skin Optical Properties during Oral Intake of Different Sugars with Optical Coherence Tomography. Biomed. Opt. Express 2014, 5, 990. [Google Scholar] [CrossRef]
- Zhu, F.; Abbas, S.R.; Bologa, R.; Levin, N.W.; Kotanko, P. Effect of Decrease in Glucose Gradient on Change in Intraperitoneal Volume Using Segmental Bioimpedance During PET. Nephrol. Dial. Transplant. 2020, 35 (Suppl. S3), gfaa142-P1183. [Google Scholar] [CrossRef]
- Jose, P.S.H.; Rajasekaran, K.; Rajalakshmy, P.; Jebastina, B. A Non-Invasive Method for Measurement of Blood Glucose Using Bio Impedance Technique. In Proceedings of the 2019 2nd International Conference on Signal Processing and Communication (ICSPC), Coimbatore, India, 29–30 March 2019; pp. 138–142. [Google Scholar]
- Takamatsu, R.; Higuchi, K.; Muramatsu, D. Measurement Frequency Evaluation for Bioimpedance-Based Blood-Glucose Estimation. In Proceedings of the 2021 IEEE 3rd Global Conference on Life Sciences and Technologies (LifeTech), Nara, Japan, 9–11 March 2021; pp. 309–310. [Google Scholar]
- Pearson, J.A.; McKinney, E.F.; Walker, L.S.K. 100 Years Post-Insulin: Immunotherapy as the next Frontier in Type 1 Diabetes. Immunother. Adv. 2021, 1, ltab024. [Google Scholar] [CrossRef]
- Bluestone, J.A.; Buckner, J.H.; Fitch, M.; Gitelman, S.E.; Gupta, S.; Hellerstein, M.K.; Herold, K.C.; Lares, A.; Lee, M.R.; Li, K.; et al. Type 1 Diabetes Immunotherapy Using Polyclonal Regulatory T Cells. Sci. Transl. Med. 2015, 7, 315ra189. [Google Scholar] [CrossRef]
- Liu, Y.; Li, W.; Chen, Y.; Wang, X. Anti-CD3 Monoclonal Antibodies in Treatment of Type 1 Diabetes: A Systematic Review and Meta-Analysis. Endocrine 2023, 83, 322–329. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, Y.; Liu, D.; Yang, L.; Xu, J.; Wang, Q. Antigen-Specific Immunotherapies in Type 1 Diabetes. J. Trace Elem. Med. Biol. 2022, 73, 127040. [Google Scholar] [CrossRef] [PubMed]
- Lungu, I.-I. Catechin-Zinc-Complex: Synthesis, Characterization and Biological Assessment. Farmacia 2023, 71, 755–763. [Google Scholar] [CrossRef]
- Zarei, M.; Sheikholeslami, M.A.; Mozaffari, M.; Mortada, Y. Innovative Immunotherapies and Emerging Treatments in Type 1 Diabetes Management. Diabetes Epidemiol. Manag. 2025, 17, 100247. [Google Scholar] [CrossRef]
- Pagliuca, F.W.; Millman, J.R.; Gürtler, M.; Segel, M.; Van Dervort, A.; Ryu, J.H.; Peterson, Q.P.; Greiner, D.; Melton, D.A. Generation of Functional Human Pancreatic β Cells in vitro. Cell 2014, 159, 428–439. [Google Scholar] [CrossRef] [PubMed]
- Diane, A.; Mu-U-Min, R.B.A.; Al-Siddiqi, H.H. Epigenetic Memory as Crucial Contributing Factor in Directing the Differentiation of Human IPSC into Pancreatic β-Cells in vitro. Cell Tissue Res. 2025, 399, 267–276. [Google Scholar] [CrossRef]
- Silva, I.B.B.; Kimura, C.H.; Colantoni, V.P.; Sogayar, M.C. Stem Cells Differentiation into Insulin-Producing Cells (IPCs): Recent Advances and Current Challenges. Stem Cell Res. Ther. 2022, 13, 309. [Google Scholar] [CrossRef]
- von Scholten, B.J.; Kreiner, F.F.; Gough, S.C.L.; von Herrath, M. Current and Future Therapies for Type 1 Diabetes. Diabetologia 2021, 64, 1037–1048. [Google Scholar] [CrossRef]
- Rezania, A.; Bruin, J.E.; Arora, P.; Rubin, A.; Batushansky, I.; Asadi, A.; O’Dwyer, S.; Quiskamp, N.; Mojibian, M.; Albrecht, T.; et al. Reversal of Diabetes with Insulin-Producing Cells Derived in vitro from Human Pluripotent Stem Cells. Nat. Biotechnol. 2014, 32, 1121–1133. [Google Scholar] [CrossRef]
- Yin, H.; Xue, W.; Chen, S.; Bogorad, R.L.; Benedetti, E.; Grompe, M.; Koteliansky, V.; Sharp, P.A.; Jacks, T.; Anderson, D.G. Genome Editing with Cas9 in Adult Mice Corrects a Disease Mutation and Phenotype. Nat. Biotechnol. 2014, 32, 551–553. [Google Scholar] [CrossRef]
- Kazanskiy, N.L.; Khonina, S.N.; Butt, M.A. Smart Contact Lenses—A Step towards Non-Invasive Continuous Eye Health Monitoring. Biosensors 2023, 13, 933. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yang, K.; Fan, H.; Wei, M.; Xiong, Q. Targeting the Gut Microbiota and Its Metabolites for Type 2 Diabetes Mellitus. Front. Endocrinol. 2023, 14, 1114424. [Google Scholar] [CrossRef]
- Takeuchi, T.; Kubota, T.; Nakanishi, Y.; Tsugawa, H.; Suda, W.; Kwon, A.T.J.; Yazaki, J.; Ikeda, K.; Nemoto, S.; Mochizuki, Y.; et al. Gut Microbial Carbohydrate Metabolism Contributes to Insulin Resistance. Nature 2023, 621, 389–395. [Google Scholar] [CrossRef]
- Guo, Q. Bioinformatics Analysis of the Diversity of Gut Microbiota and Different Microbiota on Insulin Resistance in Diabetes Mellitus Patients. Heliyon 2023, 9, e22117. [Google Scholar] [CrossRef]
- Homayouni-Rad, A.; Soroush, A.-R.; Khalili, L.; Norouzi-Panahi, L.; Kasaie, Z.; Ejtahed, H.-S. Diabetes Management by Probiotics: Current Knowledge and Future Pespective. Int. J. Vitam. Nutr. Res. 2016, 86, 216–228. [Google Scholar] [CrossRef]
- Popoviciu, M.S.; Kaka, N.; Sethi, Y.; Patel, N.; Chopra, H.; Cavalu, S. Type 1 Diabetes Mellitus and Autoimmune Diseases: A Critical Review of the Association and the Application of Personalized Medicine. J. Pers. Med. 2023, 13, 422. [Google Scholar] [CrossRef]
- Bibbò, S.; Dore, M.P.; Pes, G.M.; Delitala, G.; Delitala, A.P. Is There a Role for Gut Microbiota in Type 1 Diabetes Pathogenesis? Ann. Med. 2017, 49, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, P.; Metos, J.; Anandh Babu, P.V. Impact of Type 1 Diabetes on the Composition and Functional Potential of Gut Microbiome in Children and Adolescents: Possible Mechanisms, Current Knowledge, and Challenges. Gut Microbes 2021, 13, 1–18. [Google Scholar] [CrossRef]
- Neiva, L.P.; Lopez, L.C.; Pasiani, R.O.; Serra, M.J.R.; Rullo, V.E.V. Use of Probiotics and Similar in Pediatric Patients with Type 1 Diabetes Mellitus: A Systematic Review. Rev. Paul. Pediatr. 2024, 42, e2023097. [Google Scholar] [CrossRef]
- Zikou, E.; Dovrolis, N.; Dimosthenopoulos, C.; Gazouli, M.; Makrilakis, K. The Effect of Probiotic Supplements on Metabolic Parameters of People with Type 2 Diabetes in Greece—A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2023, 15, 4663. [Google Scholar] [CrossRef]
- Sahhaf Ebrahimi, F.; Homayouni Rad, A.; Mosen, M.; Abbasalizadeh, F.; Tabrizi, A.; Khalili, L. Effect of L. Acidophilus and B. Lactis on Blood Glucose in Women with Gestational Diabetes Mellitus: A Randomized Placebo-Controlled Trial. Diabetol. Metab. Syndr. 2019, 11, 75. [Google Scholar] [CrossRef] [PubMed]
- Groele, L.; Szajewska, H.; Szalecki, M.; Świderska, J.; Wysocka-Mincewicz, M.; Ochocińska, A.; Stelmaszczyk-Emmel, A.; Demkow, U.; Szypowska, A. Lack of Effect of Lactobacillus Rhamnosus GG and Bifidobacterium Lactis Bb12 on Beta-Cell Function in Children with Newly Diagnosed Type 1 Diabetes: A Randomised Controlled Trial. BMJ Open Diabetes Res. Care 2021, 9, e001523. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Y.; Luo, L.; Le, Y.; Li, Y.; Yuan, F.; Wu, Y.; Xu, P. The Beneficial Effects of a Multispecies Probiotic Supplement on Glycaemic Control and Metabolic Profile in Adults with Type 1 Diabetes: A Randomised, Double-Blinded, Placebo-Controlled Pilot-Study. Diabetes Metab. Syndr. Obes. 2023, 16, 829–840. [Google Scholar] [CrossRef]
- Moravejolahkami, A.R.; Shakibaei, M.; Fairley, A.M.; Sharma, M. Probiotics, Prebiotics, and Synbiotics in Type 1 Diabetes Mellitus: A Systematic Review and Meta-analysis of Clinical Trials. Diabetes. Metab. Res. Rev. 2024, 40, e3655. [Google Scholar] [CrossRef]
- Li, X.; Atkinson, M.A. The Role for Gut Permeability in the Pathogenesis of Type 1 Diabetes—A Solid or Leaky Concept? Pediatr. Diabetes 2015, 16, 485–492. [Google Scholar] [CrossRef]
- Ang, Z.; Ding, J.L. GPR41 and GPR43 in Obesity and Inflammation—Protective or Causative? Front. Immunol. 2016, 7, 28. [Google Scholar] [CrossRef]
- Priyadarshini, M.; Navarro, G.; Layden, B.T. Gut Microbiota: FFAR Reaching Effects on Islets. Endocrinology 2018, 159, 2495–2505. [Google Scholar] [CrossRef]
- Psichas, A.; Sleeth, M.L.; Murphy, K.G.; Brooks, L.; Bewick, G.A.; Hanyaloglu, A.C.; Ghatei, M.A.; Bloom, S.R.; Frost, G. The Short Chain Fatty Acid Propionate Stimulates GLP-1 and PYY Secretion via Free Fatty Acid Receptor 2 in Rodents. Int. J. Obes. 2015, 39, 424–429. [Google Scholar] [CrossRef]
- Christiansen, C.B.; Gabe, M.B.N.; Svendsen, B.; Dragsted, L.O.; Rosenkilde, M.M.; Holst, J.J. The Impact of Short-Chain Fatty Acids on GLP-1 and PYY Secretion from the Isolated Perfused Rat Colon. Am. J. Physiol. Liver Physiol. 2018, 315, G53–G65. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).