The Prevalence of Adrenal Insufficiency in Individuals with Traumatic Spinal Cord Injury: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion/Exclusion Criteria
2.3. Quality Appraisal
2.4. Data Extraction
2.5. Data Synthesis and Analyses
3. Results
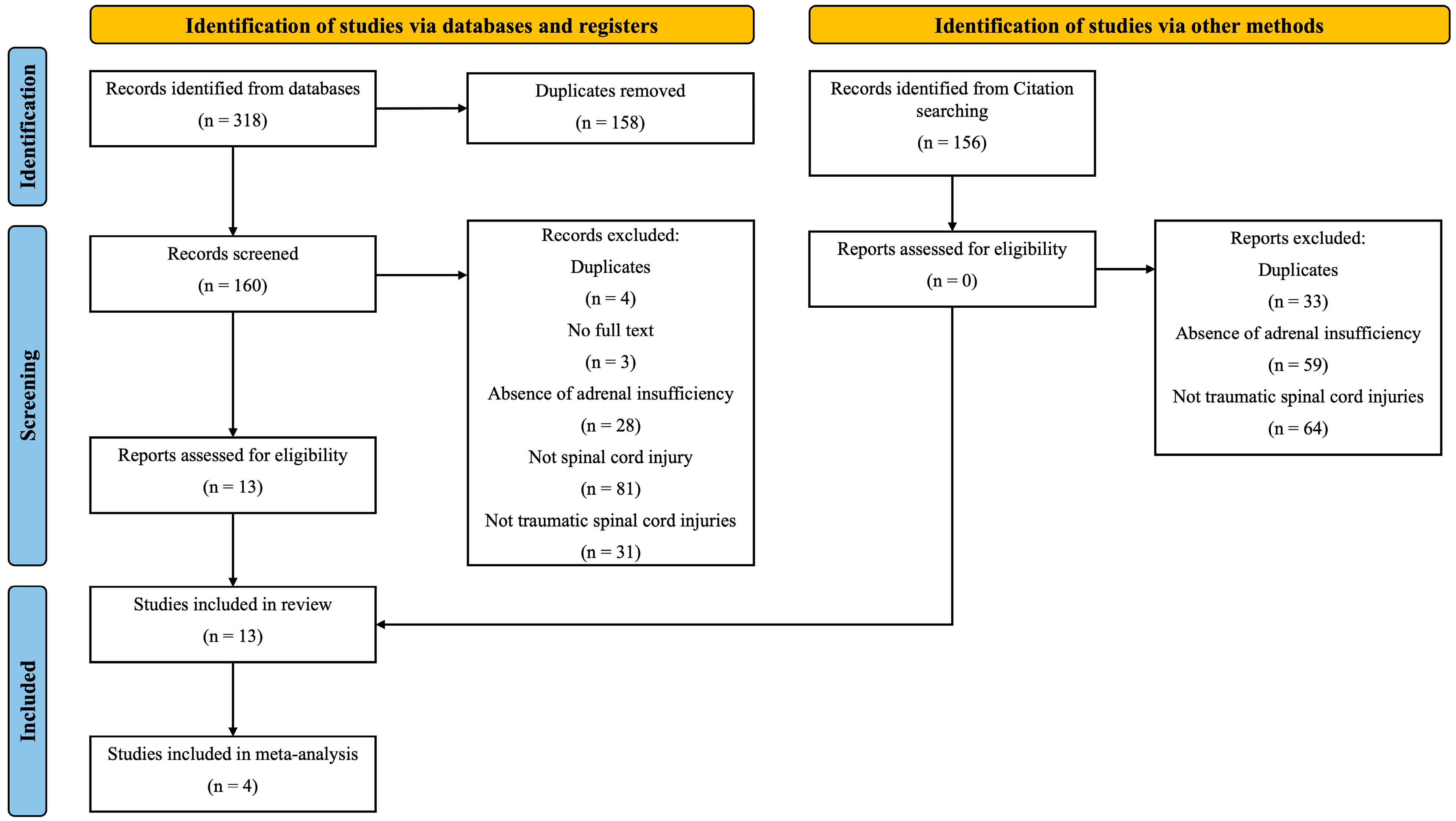
3.1. Screening
3.2. Study Quality
3.3. Participant Characteristics
3.4. Glucocorticoid Administration
3.5. Adrenal Insufficiency
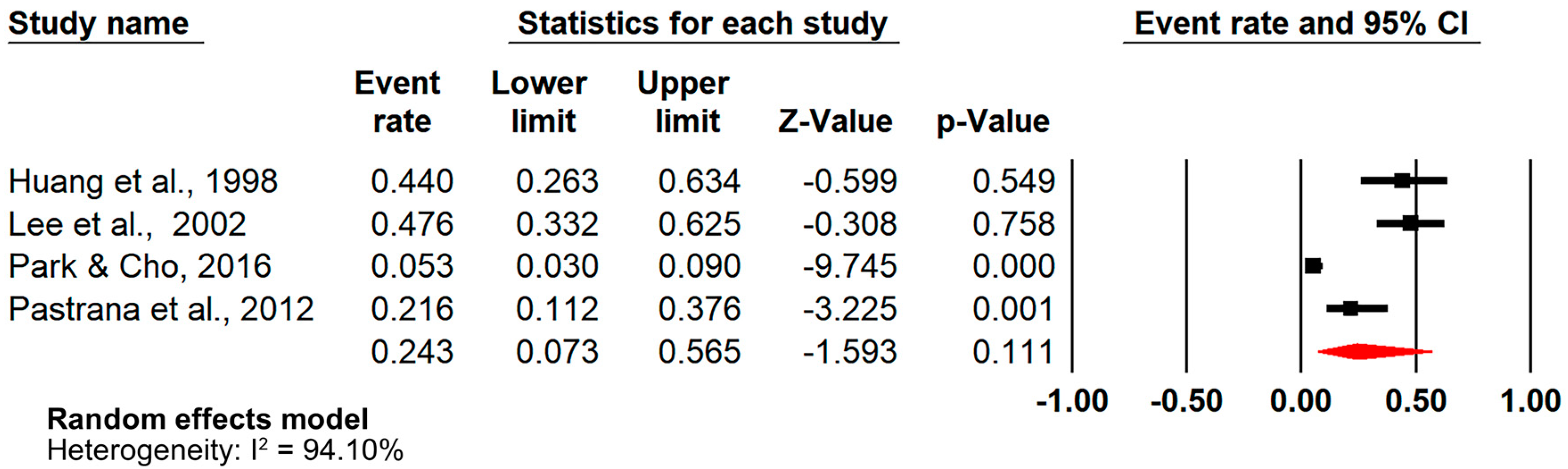
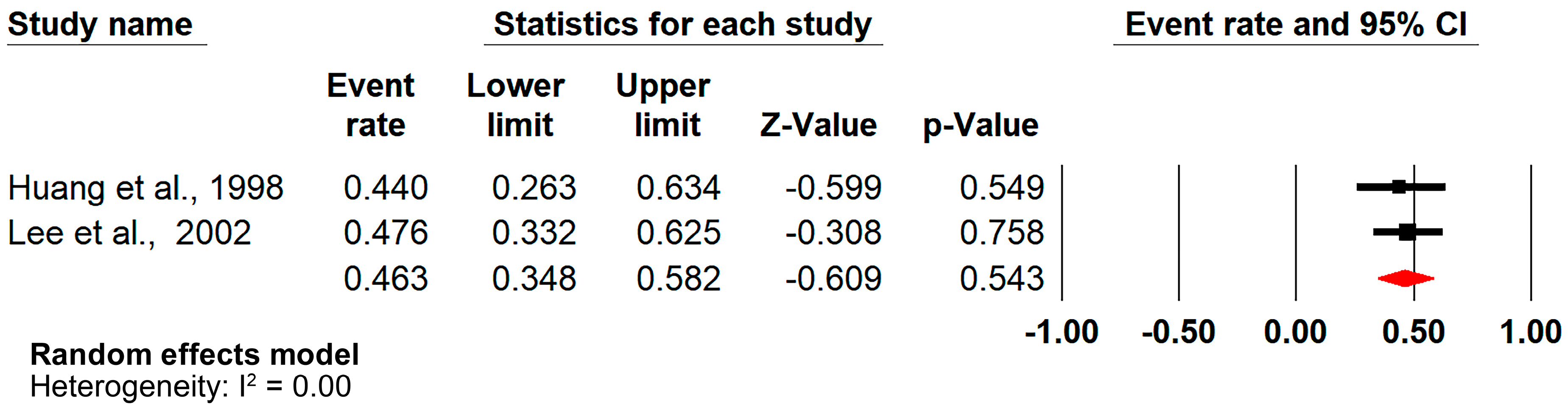
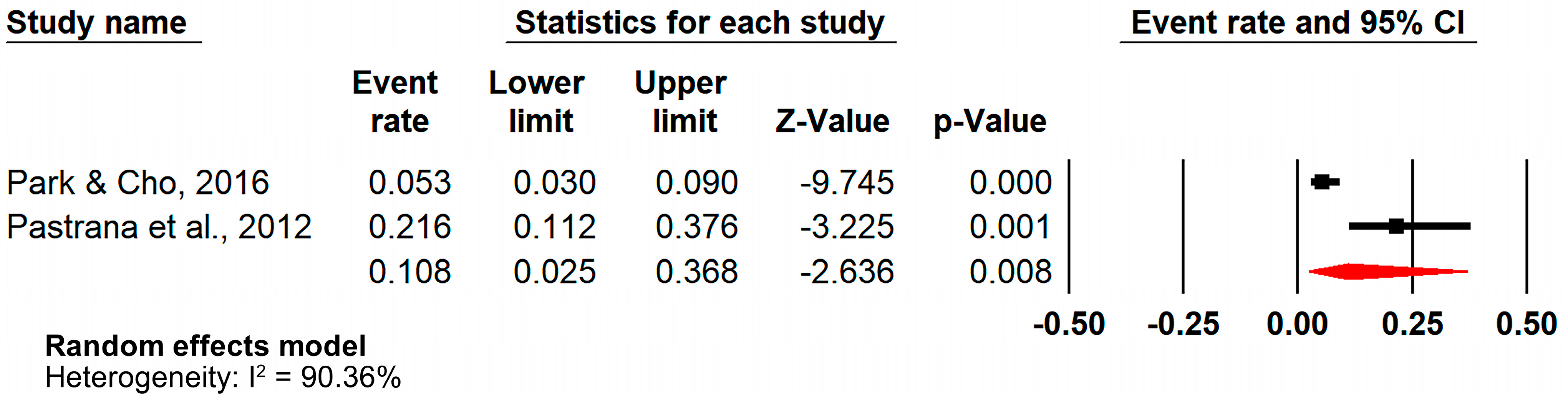
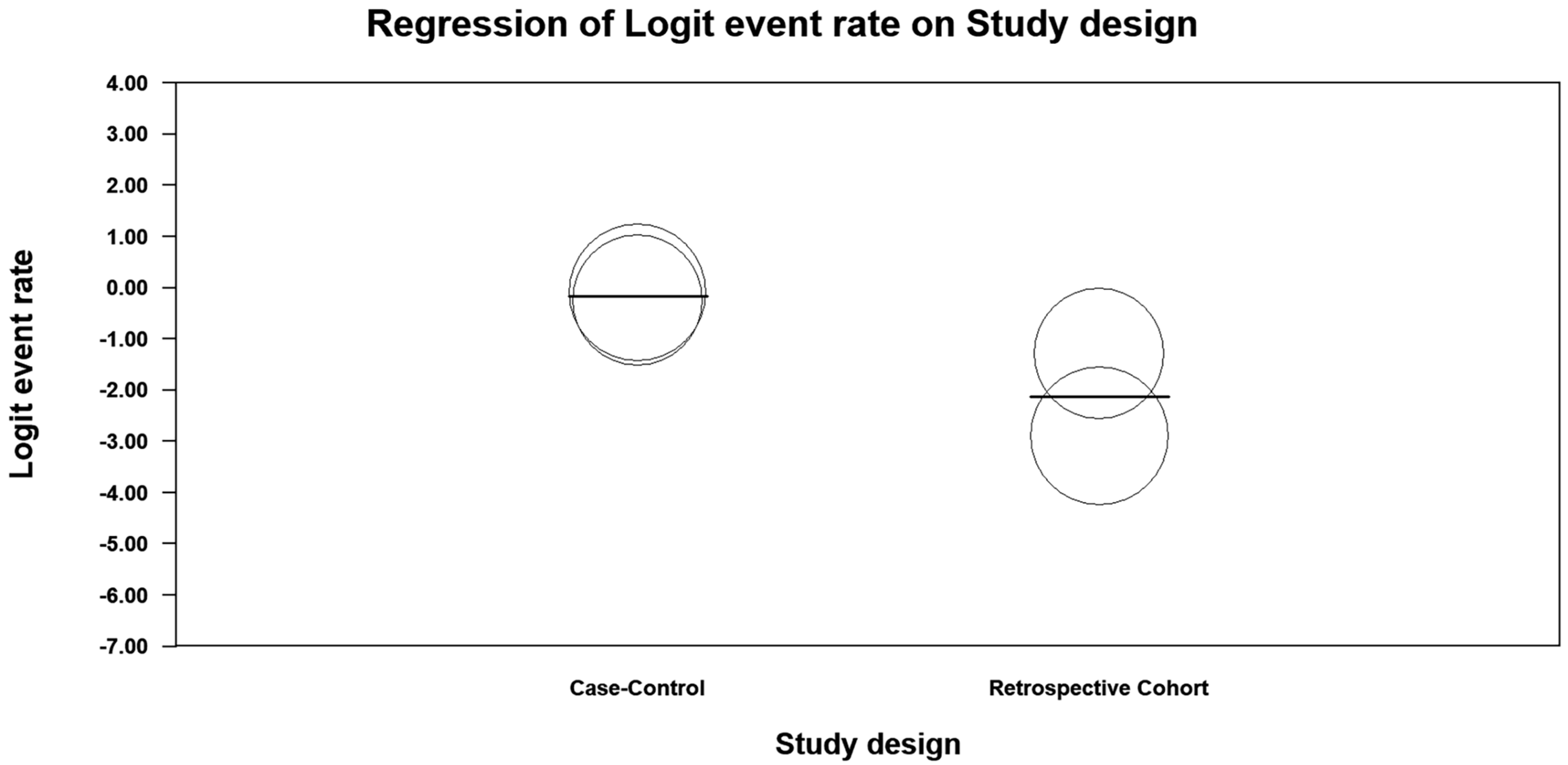
3.6. Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACTH | Adrenocorticotropic hormone |
| AI | Adrenal insufficiency |
| AIS | American Spinal Injury Association Impairment Scale |
| ALS | Amyotrophic lateral sclerosis |
| CRH | Corticotropin-releasing hormone |
| ER | Even rate |
| HPA | Hypothalamic–pituitary–adrenal |
| NLI | Neurological level of injury |
| OR | Odds ratio |
| SCI | Spinal cord injury |
| SCI-AI | Spinal cord injury-related adrenal insufficiency |
| SMA | Spinal muscular atrophy |
| TSI | Time since injury |
| 95%CI | 95% confidence intervals |
| 95%PI | 95% prediction intervals |
References
- GBD Spinal Cord Injuries Collaborators. Global, regional, and national burden of spinal cord injury, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2023, 22, 1026–1047. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Shang, Z.; Zhang, W.; Pang, M.; Hu, X.; Dai, Y.; Shen, R.; Wu, Y.; Liu, C.; Luo, T.; et al. Global incidence and characteristics of spinal cord injury since 2000–2021: A systematic review and meta-analysis. BMC Med. 2024, 22, 285. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Rabchevsky, A.G. Autonomic consequences of spinal cord injury. Compr. Physiol. 2014, 4, 1419–1453. [Google Scholar] [CrossRef]
- Calderón-Juárez, M.; Samejima, S.; Rempel, L.; Sachdeva, R.; Krassioukov, A. Autonomic dysreflexia in urological practice: Pathophysiology, prevention and treatment considerations. World J. Urol. 2024, 42, 80. [Google Scholar] [CrossRef]
- Miller, T.; Lange, D.; Kizhakkedathu, J.N.; Yu, K.; Felix, D.; Samejima, S.; Shackleton, C.; Malik, R.N.; Sachdeva, R.; Walter, M.; et al. The Microbiological Burden of Short-Term Catheter Reuse in Individuals with Spinal Cord Injury: A Prospective Study. Biomedicines 2023, 11, 1929. [Google Scholar] [CrossRef]
- Bauman, W.A.; Spungen, A.M.; Adkins, R.H.; Kemp, B.J. Metabolic and endocrine changes in persons aging with spinal cord injury. Assist. Technol. Off. J. RESNA 1999, 11, 88–96. [Google Scholar] [CrossRef]
- Ahuja, C.S.; Wilson, J.R.; Nori, S.; Kotter, M.R.N.; Druschel, C.; Curt, A.; Fehlings, M.G. Traumatic spinal cord injury. Nat. Rev. Dis. Primer. 2017, 3, 17018. [Google Scholar] [CrossRef] [PubMed]
- Krassioukov, A. Autonomic Dysreflexia: Current Evidence Related to Unstable Arterial Blood Pressure Control Among Athletes with Spinal Cord Injury. Clin. J. Sport. Med. 2012, 22, 39. [Google Scholar] [CrossRef]
- Calderón-Juárez, M.; Miller, T.; Samejima, S.; Shackleton, C.; Malik, R.N.; Sachdeva, R.; Dorey, T.W.; Krassioukov, A.V. Heart Rate Variability-Based Prediction of Autonomic Dysreflexia After Spinal Cord Injury. J. Neurotrauma. 2024, 41, 1172–1180. [Google Scholar] [CrossRef]
- Kumar, R.; Wassif, W.S. Adrenal insufficiency. J. Clin. Pathol. 2022, 75, 435–442. [Google Scholar] [CrossRef]
- Lindan, R.; Joiner, E.; Freehafer, A.A.; Hazel, C. Incidence and clinical features of autonomic dysreflexia in patients with spinal cord injury. Paraplegia 1980, 18, 285–292. [Google Scholar] [CrossRef]
- Bornstein, S.R.; Allolio, B.; Arlt, W.; Barthel, A.; Don-Wauchope, A.; Hammer, G.D.; Husebye, E.S.; Merke, D.P.; Murad, M.H.; Stratakis, C.A.; et al. Diagnosis and Treatment of Primary Adrenal Insufficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2016, 101, 364–389. [Google Scholar] [CrossRef]
- Huecker, M.R.; Bhutta, B.S.; Dominique, E. Adrenal Insufficiency. In StatPearls; StatPearls Publishing: St. Petersburg, FL, USA, 2023. Available online: http://www.ncbi.nlm.nih.gov/books/NBK441832/ (accessed on 13 January 2025).
- Herman, J.P.; McKlveen, J.M.; Ghosal, S.; Kopp, B.; Wulsin, A.; Makinson, R.; Scheimann, J.; Myers, B. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr. Physiol. 2016, 6, 603–621. [Google Scholar] [CrossRef] [PubMed]
- Davies, K. Understanding tertiary adrenal insufficiency. J. Pediatr. Nurs. 2023, 69, 121–122. [Google Scholar] [CrossRef]
- Weant, K.A.; Sasaki-Adams, D.; Kilpatrick, M.; Hadar, E.J. Relative Adrenal Insufficiency in Patients with Acute Spinal Cord Injury. Neurocrit. Care. 2008, 8, 53–56. [Google Scholar] [CrossRef]
- Yang, H.; Trbovich, M.; Harrow, J. Secondary adrenal insufficiency after glucocorticosteroid administration in acute spinal cord injury: A case report. J. Spinal Cord Med. 2014, 37, 786–790. [Google Scholar] [CrossRef]
- Claydon, V.E.; Steeves, J.D.; Krassioukov, A. Orthostatic hypotension following spinal cord injury: Understanding clinical pathophysiology. Spinal Cord 2006, 44, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Henke, A.M.; Billington, Z.J.; Gater, D.R. Autonomic Dysfunction and Management after Spinal Cord Injury: A Narrative Review. J. Pers. Med. 2022, 12, 1110. [Google Scholar] [CrossRef] [PubMed]
- Fehlings, M.G.; Tetreault, L.A.; Wilson, J.R.; Kwon, B.K.; Burns, A.S.; Martin, A.R.; Hawryluk, G.; Harrop, J.S. A Clinical Practice Guideline for the Management of Acute Spinal Cord Injury: Introduction, Rationale, and Scope. Glob. Spine J. 2017, 7 (Suppl. S3), 84S–94S. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions Version 6.5 (Updated August 2024). Cochrane. 2024. Available online: https://training.cochrane.org/handbook (accessed on 13 January 2025).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of Observational Studies in EpidemiologyA Proposal for Reporting. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef] [PubMed]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef]
- Pérez, J.; Díaz, J.; Garcia-Martin, J.; Tabuenca, B. Systematic literature reviews in software engineering—Enhancement of the study selection process using Cohen’s Kappa statistic. J. Syst. Softw. 2020, 168, 110657. [Google Scholar] [CrossRef]
- Moola, S.; Munn, Z.; Tufanaru, C.; Aromataris, E.; Sears, K.; Sfetcu, R.; Currie, M.; Lisy, K.; Qureshi, R.; Mattis, P.; et al. Chapter 7: Systematic Reviews of Etiology and Risk. In JBI Manual for Evidence Synthesis; JBI: Miami, FL, USA, 2020; Available online: https://jbi.global/critical-appraisal-tools (accessed on 13 January 2025).
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Borenstein, M.; Higgins, J.P.T.; Hedges, L.V.; Rothstein, H.R. Basics of meta-analysis: I2 is not an absolute measure of heterogeneity. Res. Synth. Methods 2017, 8, 5–18. [Google Scholar] [CrossRef] [PubMed]
- IntHout, J.; Ioannidis, J.P.A.; Borm, G.F. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med. Res. Methodol. 2014, 14, 25. [Google Scholar] [CrossRef]
- The Endocrine Society. Endocrine Facts and Figures: Adrenal, 1st ed.; The Endocrine Society: Washington, DC, USA, 2016. [Google Scholar]
- Marcucci, G.; Cianferotti, L.; Beck-Peccoz, P.; Capezzone, M.; Cetani, F.; Colao, A.; Davì, M.V.; degli Uberti, E.; Del Prato, S.; Elisei, R.; et al. Rare diseases in clinical endocrinology: A taxonomic classification system. J. Endocrinol. Investig. 2015, 38, 193–259. [Google Scholar] [CrossRef] [PubMed]
- Baird-Howell, M.A.; Wurzel, J. Fatal gastrointestinal hemorrhage in a paraplegic man with undiagnosed AA (secondary) amyloidosis. Amyloid 2011, 18, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Zozaya, I.A. Adrenal insufficiency in acute spinal cord injury. J. Spinal Cord Med. 2006, 29, 67–69. [Google Scholar] [CrossRef]
- Ishiki, Y.; Tamaki, A.; Honma, K.I.; Yonaha, K.; Yabiku, T.; Teruya, T.; Uehara, M.; Nakayama, Y.; Chinen, R.; Uema, T.; et al. Post-traumatic pituitary stalk transection syndrome (PSTS) expeditiously manifested after a fall from a height combined with acute traumatic spinal cord injury: A rare case report with review of literature. Endocr. J. 2024, 71, 817–824. [Google Scholar] [CrossRef]
- Lecamwasam, H.S.; Baboolal, H.A.; Dunn, P.F. Acute Adrenal Insufficiency After Large-Dose Glucocorticoids for Spinal Cord Injury. Anesth. Analg. 2004, 99, 1813–1814. [Google Scholar] [CrossRef]
- Lee, L.W.; Glenn, M.B. Adrenal insufficiency masquerading as sepsis in a patient with tetraparesis: A case report. Arch. Phys. Med. Rehabil. 2000, 81, 830–833. [Google Scholar] [CrossRef]
- Lee, W.J.; Wang, Y.H.; Su, C.T.; Chen, S.J.; Li, Y.W.; Huang, T.S. Adrenal Gland Volume After Spinal Cord Injury. Am. J. Phys. Med. Rehabil. 2002, 81, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Cho, K.H. Large-Dose Glucocorticoid Induced Secondary Adrenal Insufficiency in Spinal Cord Injury. Ann. Rehabil. Med. 2016, 40, 1033. [Google Scholar] [CrossRef]
- Pastrana, E.A.; Saavedra, F.M.; Murray, G.; Estronza, S.; Rolston, J.D.; Rodriguez-Vega, G. Acute Adrenal Insufficiency in Cervical Spinal Cord Injury. World Neurosurg. 2012, 77, 561–563. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, F.U.; Birge, S.J.; Cooke, N.E. Hypercalcaemia in adolescent tetraplegic patients: Case report and review. Spinal Cord 1978, 16, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Huang, T.S. Impaired adrenal reserve in men with spinal cord injury: Results of low- and high-dose adrenocorticotropin stimulation tests. Arch. Phys. Med. Rehabil. 1999, 80, 863–866. [Google Scholar] [CrossRef]
- Huang, T.S.; Wang, Y.H.; Lee, S.H.; Lai, J.S. Impaired hypothalamus-pituitary-adrenal axis in men with spinal cord injuries. Am. J. Phys. Med. Rehabil. 1998, 77, 108–112. [Google Scholar] [CrossRef]
- Gagnier, J.J.; Kienle, G.; Altman, D.G.; Moher, D.; Sox, H.; Riley, D.; CARE Group. The CARE guidelines: Consensus-based clinical case reporting guideline development. Headache 2013, 53, 1541–1547. [Google Scholar] [CrossRef]
- Munn, Z.; Barker, T.H.; Moola, S.; Tufanaru, C.; Stern, C.; McArthur, A.; Stephenson, M.; Aromataris, E. Methodological quality of case series studies: An introduction to the JBI critical appraisal tool. JBI Evid. Synth. 2020, 18, 2127. [Google Scholar] [CrossRef]
- González Villarroel, P.; Fernández Pérez, I.; Páramo, C.; González, M.G.; López, B.C.; Tuñas, M.L.V.; Álvarez, J.A.C. Megestrol acetate-induced adrenal insufficiency. Clin. Transl. Oncolog. 2008, 10, 235–237. [Google Scholar] [CrossRef] [PubMed]
- Zarra, F.; Rolando, M.; Gandhi, D.N.; Alozai, M.I.; Mercado, A.; Chaurasia, B.; Videtta, W. Glucocorticoids in Acute Spinal Cord Injury: Why are they still used nowadays? Neurosurg. Rev. 2024, 47, 658. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Wang, Y.; Qian, T. Inflammatory Response to Spinal Cord Injury and Its Treatment. World Neurosurg. 2021, 155, 19–31. [Google Scholar] [CrossRef]
- Bornstein, S.R. Predisposing factors for adrenal insufficiency. N. Engl. J. Med. 2009, 360, 2328–2339. [Google Scholar] [CrossRef] [PubMed]
- Guly, H.R.; Bouamra, O.; Lecky, F.E.; Trauma Audit and Research Network. The incidence of neurogenic shock in patients with isolated spinal cord injury in the emergency department. Resuscitation 2008, 76, 57–62. [Google Scholar] [CrossRef]
- Birtolo, M.F.; Antonini, S.; Saladino, A.; Zampetti, B.; Lavezzi, E.; Chiodini, I.; Mazziotti, G.; Lania, A.G.A.; Cozzi, R. ACTH Stimulation Test for the Diagnosis of Secondary Adrenal Insufficiency: Light and Shadow. Biomedicines 2023, 11, 904. [Google Scholar] [CrossRef]
- Ngaosuwan, K.; Johnston, D.G.; Godsland, I.F.; Cox, J.; Majeed, A.; Quint, J.K.; Oliver, N.; Robinson, S. Increased Mortality Risk in Patients With Primary and Secondary Adrenal Insufficiency. J. Clin. Endocrinol. Metab. 2021, 106, e2759–e2768. [Google Scholar] [CrossRef]
- Cragg, J.J.; Noonan, V.K.; Krassioukov, A.; Borisoff, J. Cardiovascular disease and spinal cord injury: Results from a national population health survey. Neurology 2013, 81, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res. Synth. Methods. 2010, 1, 97–111. [Google Scholar] [CrossRef]
- Dekkers, O.M.; Egger, M.; Altman, D.G.; Vandenbroucke, J.P. Distinguishing case series from cohort studies. Ann. Intern. Med. 2012, 156 Pt 1, 37–40. [Google Scholar] [CrossRef]
- Esene, I.N.; Ngu, J.; El Zoghby, M.; Solaroglu, I.; Sikod, A.M.; Kotb, A.; Dechambenoit, G.; El Husseiny, H. Case series and descriptive cohort studies in neurosurgery: The confusion and solution. Child’s Nerv. Syst. 2014, 30, 1321–1332. [Google Scholar] [CrossRef] [PubMed]
- Abu-Zidan, F.M.; Abbas, A.K.; Hefny, A.F. Clinical “case series”: A concept analysis. Afr. Health Sci. 2012, 12, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Straus, S.E.; Richardson, W.S.; Glasziou, P.; Haynes, R.B. Evidence-Based Medicine: How to Practice and Teach EBM, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
| Author (Year) | Study Design | SCI-AI Prevalence | Event Rate | Participant Characteristics | Glucocorticoids/Mineralocorticoid | Adrenal Insufficiency | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SCI (n = 545)/Control (n = 42) | Sex | Age | Neurological Level of Injury | Classification Based on AIS | Recovery Stage | Time Since Injury (Years) | Glucocorticoids or Mineralocorticoid (Before AI) | Name | Dose | Onset | Signs/Symptoms | SCI-AI Location/Level Affected | ||||
| [32] (Baird-Howell & Wurzel, 2011) | Case report | N/A | N/A | SCI (n = 1) | Male | 41 | T2 | N/A | Chronic | 20 | N/A | N/A | N/A | 20 years after SCI (Admission: urinary tract infection) | Gastrointestinal bleeding, death, renal failure, amyloidosis | Primary |
| [33] (Garcia-Zozaya, 2006) | Case report | N/A | N/A | SCI (n = 1) | Male | 21 | C6 | Grade A | Acute | N/A | Glucocorticoids | Methylprednisolone | Bolus 30 mg/kg over 15 min, with maintenance infusion of 5.4 mg/kg per hour for 23 h | 2 weeks after SCI | Hypotension resistant to vasopressor and volume resuscitation therapy | Tertiary |
| [42] (Huang et al., 1998) | Case–control study | 11/25 | 0.440 | SCI (n = 25); Control (n =25) | Male | Range: 18–55 (mean: 35.4) | C5–C8 (n = 9) T1–L2 (n = 16) | N/A | Chronic | 1.1–15.8 (mean 35.4) | N/A | N/A | N/A | N/A | N/A | Primary |
| [34] (Ishiki et al., 2024) | Case report | N/A | N/A | SCI (n = 1) | Female | 34 | C7 | Grade C | Acute | N/A | N/A | N/A | N/A | 12 days after SCI | No AI-related symptoms; hyperkalemia, slight hyponatremia | Secondary Pituitary |
| [35] (Lecamwasam et al., 2004) | Case report | N/A | N/A | SCI (n = 1) | Male | 23 | C5 | N/A | Acute | N/A | Glucocorticoids | Dexamethasone, Methylprednisolone | Dexamethasone: 560 mg followed by 100 mg per hour for 6 h intravenously Methylprednisolone: 5.4 mg/kg per hour for 23 h intravenously | 4 days after steroid cessation | fever, hypotension, low basal cortisol | Tertiary |
| [36] (Lee & Glenn, 2000) | Case report | N/A | N/A | SCI (n = 1) | Male | 51 | C5 | Grade C | Chronic | 8 | A synthetic progestin with glucocorticoid-like activity | Megestrol acetate | 200 mg by mouth, twice per day for 5 months | 8 years after SCI (Admission: difficulty with bladder catheterization and left flank pain possibly caused by a left kidney stone) | Mild hypotension, sinus tachycardia, hypoglycemia, hyponatremia | Secondary |
| [37] (Lee et al., 2002) | Case–control study | 20/42 | 0.476 | SCI (n = 42); Control (n =17) | Male | Mean (SD): 40.5 (7.8) | N/A | Grade A or B | Chronic | >1 | N/A | N/A | N/A | N/A | Relatively larger adrenal volume than healthy individuals | Secondary |
| [38] (Park & Cho, 2016) | Retrospective cohort | 12/228 (Treated with large-dose glucocorticoid = 10; not treated with large-dose glucocorticoid = 2) | 0.053 | SCI (n = 228) | Patients diagnosed with AI: Male (n = 10), Female (n = 2) | Range: 20–81 | Patients who have suspected AI: C3–C5 (n = 23) T6–T12 (n = 6) Patients diagnosed with AI: C3–C7 (n = 11) T10 (n = 1) | Patients diagnosed with AI: Grade A (n = 2), Grade C (n = 2), Grade D (n = 8) | NA | N/A | Glucocorticoids | N/A | Large dose | N/A | Fatigue, hypotension, anorexia | Secondary/Tertiary |
| [39] (Pastrana et al., 2012) | Retrospective cohort | 8/37 | 0.216 | SCI (n = 199) SCI patients with neurogenic shock (n = 37) | NA | Range: 18–66 (mean: 32.3) | All patients: Cervical level (n = 199) Patients diagnosed with AI: C4–C5 (n = 8) | Grade A (n = 8) | Acute | N/A | N/A | N/A | N/A | N/A | Low cortisol, hypotension, neurogenic shock | NA |
| [40] (Steinberg et al., 1978) | Case report | N/A | N/A | SCI (n = 1) | Male | 15 | C5 | N/A | Acute | N/A | N/A | N/A | N/A | 2 months after SCI | Hypercalcemia, orthostatic hypotension, low plasma cortisol level | Primary |
| [41] (Wang & Huang, 1999) | Case series (same data as Lee (2002), excluded from meta-analysis) | 20/42 | 0.476 | SCI (n = 42) | Male | Range: 20–60 (mean: 39.2) | All patient: Cervical level (n = 17) Thoracolumbar level (n = 25) Patients diagnosed with AI: C4–C6 (n = 7) T3–T12 (n = 13) | Grade A or B | Chronic | 1.1–35 (mean 9.4) | N/A | N/A | N/A | N/A | Decreased adrenal reserve | Secondary |
| [16] (Weant et al., 2008) | Case report | N/A | N/A | SCI (n = 2) | Male | 39, 75 | Case 1: C6 Case 2: C1 | N/A | Acute | N/A | Glucocorticoids | Case 1: Methylprednisolone; Case 2: N/A | N/A | Case 1: 23–31 days post-admission; Case 2: Day 6 | Case 1: low cortisol, fever;Case 2: hypotensive, not responsive to vasopressors | Secondary/Tertiary |
| [17] (Yang et al., 2014) | Case report | N/A | N/A | SCI (n = 1) | Female | 61 | C3 | Grade D | Acute | N/A | Glucocorticoids | Dexamethasone | Dexamethasone intravenously for 11 days: 4 mg every 6 h for 18 doses 2 mg every 6 h for 5 doses 2 mg every 12 h for 7 doses | 2 days after steroid cessation | Low basal cortisol, acute neck pain, fatigue, muscle weakness, hypotension | Tertiary |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hosseinzadeh, A.; Hou, R.; Zeng, R.R.; Calderón-Juárez, M.; Lau, B.W.M.; Fong, K.N.K.; Wong, A.Y.L.; Zhang, J.J.; Sánchez Vidaña, D.I.; Miller, T.; et al. The Prevalence of Adrenal Insufficiency in Individuals with Traumatic Spinal Cord Injury: A Systematic Review and Meta-Analysis. J. Clin. Med. 2025, 14, 2141. https://doi.org/10.3390/jcm14072141
Hosseinzadeh A, Hou R, Zeng RR, Calderón-Juárez M, Lau BWM, Fong KNK, Wong AYL, Zhang JJ, Sánchez Vidaña DI, Miller T, et al. The Prevalence of Adrenal Insufficiency in Individuals with Traumatic Spinal Cord Injury: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2025; 14(7):2141. https://doi.org/10.3390/jcm14072141
Chicago/Turabian StyleHosseinzadeh, Ali, Rangchun Hou, Roy Rongyue Zeng, Martín Calderón-Juárez, Benson Wui Man Lau, Kenneth Nai Kuen Fong, Arnold Yu Lok Wong, Jack Jiaqi Zhang, Dalinda Isabel Sánchez Vidaña, Tiev Miller, and et al. 2025. "The Prevalence of Adrenal Insufficiency in Individuals with Traumatic Spinal Cord Injury: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 14, no. 7: 2141. https://doi.org/10.3390/jcm14072141
APA StyleHosseinzadeh, A., Hou, R., Zeng, R. R., Calderón-Juárez, M., Lau, B. W. M., Fong, K. N. K., Wong, A. Y. L., Zhang, J. J., Sánchez Vidaña, D. I., Miller, T., & Kwong, P. W. H. (2025). The Prevalence of Adrenal Insufficiency in Individuals with Traumatic Spinal Cord Injury: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 14(7), 2141. https://doi.org/10.3390/jcm14072141