Physiotherapy Intervention in the Immediate Postoperative Phase of Lipedema Surgery—Observational Study
Abstract
1. Introduction
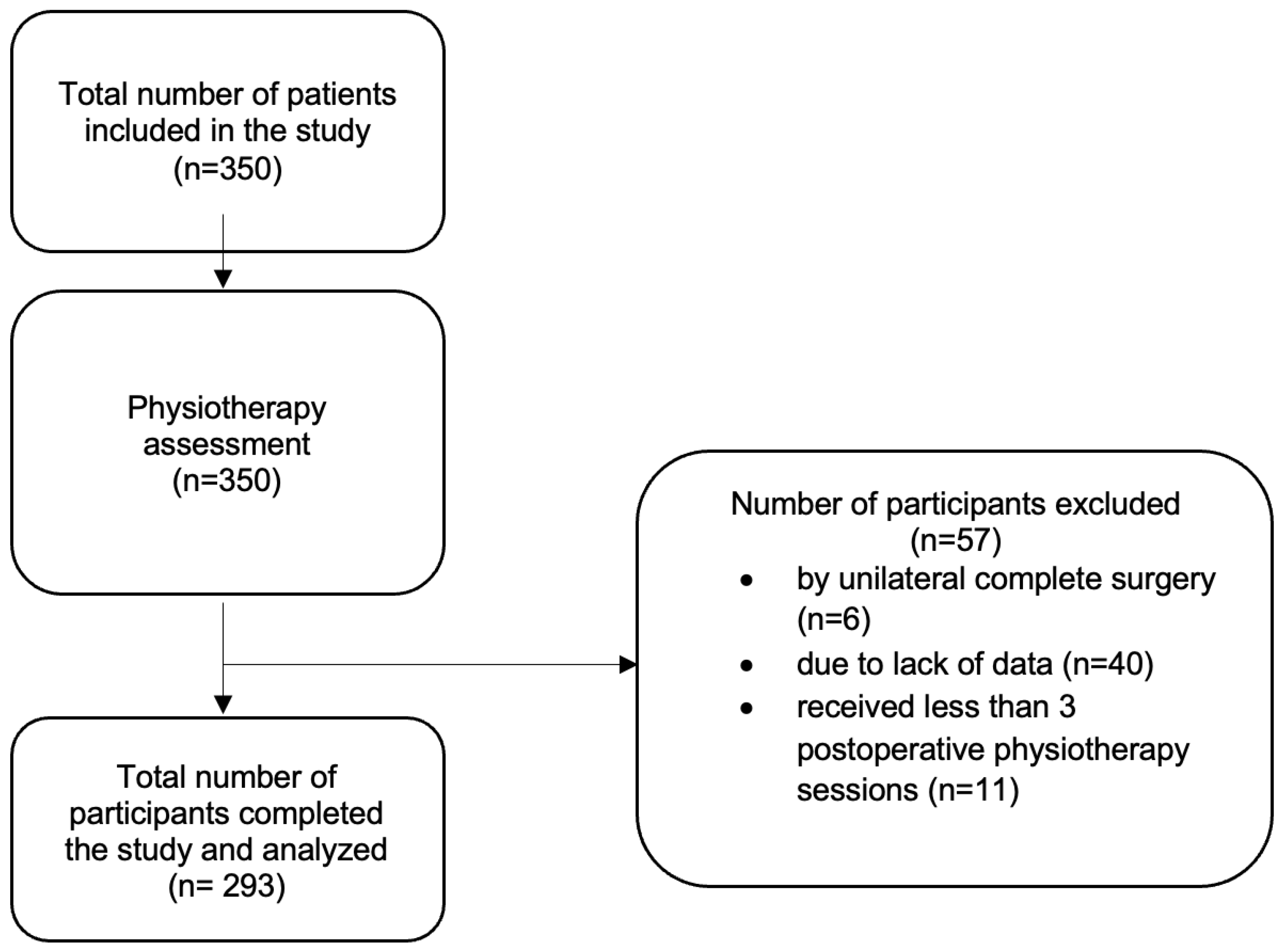
2. Materials and Methods
2.1. Design
2.2. Intervention and Assessment
2.3. Outcomes
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LiDo | Lipohyperplasia dolorosa |
| CDT | Complete Decongestive Therapy |
| MLD | Manual Lymphatic Drainage |
| PO | Postoperative |
| BMI | Body mass index |
| VAS | Visual Analogue Scale |
References
- Gensior, M.H.L.; Cornely, M. Pain in lipoedema, fat in lipoedema and its consequences: Results of a patient survey based on a pain questionnaire. Handchir. Mikrochir. Plast. Chir. 2019, 51, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Kruppa, P.; Georgiou, I.; Ghods, M.; Biermann, N.; Prantl, L.; Klein-Weigel, P. Lipedema—Pathogenesis, diagnosis, and treatment options. Dtsch. Arztebl. Int. 2020, 117, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Herpertz, U. Lipedema—Pathogenesis, diagnosis and treatment options. Dtsch. Ärzteblatt Int. 2020, 118, 39. [Google Scholar]
- Herbst, K.L.; Kahn, L.A.; Iker, E.; Ehrlich, C.; Wright, T.; McHutchison, L.; Schwartz, J.; Sleigh, M.; Donahue, P.M.; Lisson, K.H.; et al. Standard of care for lipedema in the United States. Phlebology 2021, 36, 779–796. [Google Scholar] [CrossRef]
- Forner-Cordero, I.; Forner-Cordero, A.; Szolnoky, G. Update in the management of lipedema. Int. Angiol. 2021, 40, 345–357. [Google Scholar] [CrossRef]
- Herbst, K.L. Rare adipose disorders (RADs) masquerading as obesity. Acta Pharmacol. Sin. 2012, 33, 155–172. [Google Scholar] [CrossRef]
- Alcolea, J.M.; Alonso Álvarez, B.; Arroyo, A.; Domingo, P.; Galindo, A.; Gracias, M.; Iglesias Urraca, C.; Insua Nipoti, E.; Martín Castillo, E.; Martínez Álvarez, J.R.; et al. Documento de Consenso de Lipedema; Spanish Association of Lymphedema and Lipedema: Vilagarcía de Arousa, Spain, 2019. [Google Scholar]
- Coppel, T. Best Practice Guidelines. The Management of Lipedema; Wounds: London, UK, 2017. [Google Scholar]
- Hardy, D.; Williams, A. Best practice guidelines for the management of lipoedema. Br. J. Community Nurs. 2017, 22 (Suppl. S10), S44–S48. [Google Scholar] [CrossRef]
- Stutz, J.J.; am Wald, S. Liposuction of Lipedema to Prevent Later Joint Complications. Vasomed 2011, 23, 6. [Google Scholar]
- Nicolás, P.C. Lipedema: More than a problem of “fat legs”. Update in the pathophysiology, diagnosis and surgical treatment. Rev. Cir. 2021, 73, 370–377. [Google Scholar]
- Bauer, A.T.; von Lukowicz, D.; Lossagk, K.; Aitzetmueller, M.; Moog, P.; Cerny, M.; Erne, H.; Schmauss, D.; Duscher, D.; Machens, H.-G. New Insights on Lipedema: The Enigmatic Disease of the Peripheral Fat. Plast. Reconstr. Surg. 2019, 144, 1475–1484. [Google Scholar] [CrossRef]
- Reich-Schupke, S.; Schmeller, W.; Brauer, W.J.; Cornely, M.E.; Faerber, G.; Ludwig, M.; Lulay, G.; Miller, A.; Rapprich, S.; Richter, D.F.; et al. S1 guidelines: Lipedema. JDDG J. Dtsch. Dermatol. Ges. 2017, 15, 758–767. [Google Scholar] [CrossRef] [PubMed]
- Wollina, U.; Heinig, B. Treatment of lipedema by low-volume micro-cannular liposuction in tumescent anesthesia: Results in 111 patients. Dermatol. Ther. 2019, 32, e12820. [Google Scholar] [CrossRef] [PubMed]
- Szolnoky, G.; Szendi-Horváth, K.; Seres, L.; Boda, K.; Kemény, L. Manual lymph drainage efficiently reduces postoperative facial swelling and discomfort after removal of impacted third molars. Lymphology 2007, 40, 138–142. [Google Scholar] [PubMed]
- Bjork, R.; Ehmann, S. STRIDE Professional Guide to Compression Garment Selection for the Lower Extremity. J. Wound Care 2019, 28, 1–44. [Google Scholar] [CrossRef]
- Cornely, M.; Gensior, M. Complications and their management in the surgical treatment of Lipohyperplasia dolorosa. English version. Der Hautarzt 2022, 75, 63–68. [Google Scholar] [CrossRef]
- Peprah, K.; Danielle, M. Liposuction for the Treatment of Lipedema: A Review of Clinical Effectiveness and Guidelines; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, Canada, 2019. [Google Scholar] [PubMed]
- Sandhofer, M.; Hanke, C.W.; Habbema, L.; Podda, M.; Rapprich, S.; Schmeller, W.; Herbst, K.; Anderhuber, F.; Pilsl, U.; Sattler, G.; et al. Prevention of Progression of Lipedema with Liposuction Using Tumescent Local Anesthesia: Results of an International Consensus Conference. Dermatol. Surg. 2020, 46, 220–228. [Google Scholar] [CrossRef]
- Klein, I.; Tidhar, D.; Kalichman, L. Lymphatic treatments after orthopedic surgery or injury: A systematic review. J. Bodyw. Mov. Ther. 2020, 24, 109–117. [Google Scholar] [CrossRef]
- Novoa, M. Cirugía Plástica Ibero-Latinoamericana. Cir Plást Iberolatinoam. 48. Available online: https://scielo.isciii.es/scielo.php?script=sci_arttext&pid=S0376-78922022000100001&lng=es (accessed on 14 March 2025). [CrossRef]
- Földi, E.; Földi, M.; Rockson, S.G. Complete Decongestive Physiotherapy. In Lymphedema; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; pp. 403–411. [Google Scholar] [CrossRef]
- Dadras, M.; Mallinger, P.J.; Corterier, C.C.; Theodosiadi, S.; Ghods, M. Liposuction in the treatment of lipedema: A longitudinal study. Arch Plast. Surg. 2017, 44, 324–331. [Google Scholar] [CrossRef]
- Paling, I.; Macintyre, L. Survey of lipoedema symptoms and experience with compression garments. Br. J. Community Nurs. 2020, 25 (Suppl. S4), S17–S22. [Google Scholar] [CrossRef]
- Wollina, U.; Heinig, B.; Nowak, A. Treatment of elderly patients with advanced lipedema: A combination of laser-assisted liposuction, medial thigh lift, and lower partial abdominoplasty. Clin. Cosmet. Investig. Dermatol. 2014, 7, 35–42. [Google Scholar] [CrossRef]
- de Godoy, J.M.P.; Godoy, M.d.F.G.; de Godoy, H.J.P. Mechanical Lymphatic Drainage (RAGodoy®): Literature Review. Cureus 2022, 14, e21263. [Google Scholar] [CrossRef]
- de Godoy, J.M.; de Godoy, A.C.; Guimarães, T.D.; de Godoy, M.D. The Godoy & Godoy cervical stimulation technique in the treatment of primary congenital lymphedema. Pediatr. Rep. 2012, 4, e31. [Google Scholar] [CrossRef] [PubMed]
- Kanapathy, M.; Pacifico, M.; Yassin, A.M.; Bollen, E.; Mosahebi, A. Safety of Large-Volume Liposuction in Aesthetic Surgery: A Systematic Review and Meta-Analysis. Aesthetic Surg. J. 2020, 41, 1040–1053. [Google Scholar] [CrossRef] [PubMed]
- de Godoy, J.M.; de Godoy, M.D. Godoy & Godoy technique in the treatment of lymphedema for under-privileged populations. Int. J. Med. Sci. 2010, 7, 68–71. [Google Scholar] [CrossRef][Green Version]
- Thong, I.S.K.; Jensen, M.P.; Miró, J.; Tan, G. The validity of pain intensity measures: What do the NRS, VAS, VRS, and FPS-R measure? Scand. J. Pain 2018, 18, 99–107. [Google Scholar] [CrossRef]
- Aksoy, H.; Karadag, A.S.; Wollina, U. Cause and management of lipedema-associated pain. Dermatol. Ther. 2020, 34, e14364. [Google Scholar] [CrossRef]
- Herbst, K.L.; Hansen, E.A.; Cobos Salinas, L.M.; Wright, T.F.; Larson, E.E.; Schwartz, J.S. Survey Outcomes of Lipedema Reduction Surgery in the United States. In Plastic and Reconstructive Surgery—Global Open; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2021. [Google Scholar] [CrossRef]
- Greene, A.K.; Sudduth, C.L. Lower extremity lymphatic function predicted by body mass index: A lymphoscintigraphic study of obesity and lipedema. Int. J. Obes. 2021, 45, 369–373. [Google Scholar] [CrossRef]
| Outcome Measure | N | (SD) | ||
|---|---|---|---|---|
| Age | 292 | 37.79 | 7.59 | |
| Extracted liters | 293 | 4.43 | 1.33 | |
| Physiotherapy sessions per surgery | 290 | 6.68 | 0.23 | |
| Week 1 | 3.64 | 1.41 | ||
| Week 2 | 1.87 | 1.71 | ||
| Week 3 | 0.72 | 1.12 | ||
| Week 4 | 0.39 | 0.75 | ||
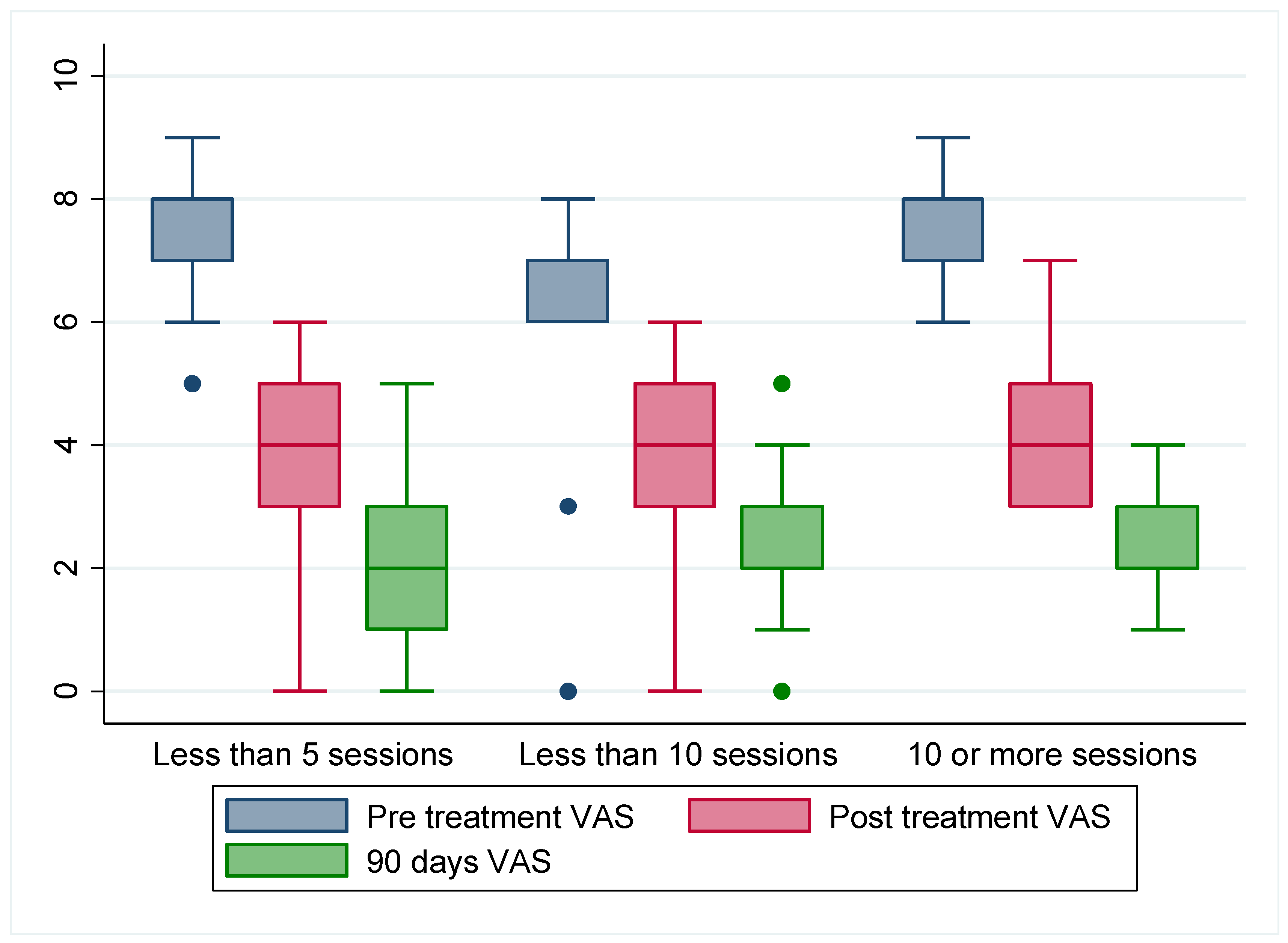
| Pre-treatment VAS | 293 | 7.04 | 0.92 | |
| Post-treatment VAS | 293 | 3.98 | 0.95 | |
| 90 days post-treatment VAS | 293 | 2.34 | 0.95 | |
| Outcome measure | N | |||
| Body mass index | Normal weight | 286 | 49 | 17.13 |
| Overweight | 121 | 42.31 | ||
| Obesity | 116 | 40.56 | ||
| Year of intervention | 2019 | 293 | 70 | 23.89 |
| 2020 | 105 | 35.83 | ||
| 2021 | 118 | 40.27 | ||
| Hot month | No | 293 | 156 | 53.24 |
| Yes | 137 | 46.76 | ||
| Kind of surgery | Calf | 293 | 139 | 47.60 |
| Front thigh | 73 | 25 | ||
| Hind thigh | 28 | 9.59 | ||
| Arms | 11 | 3.77 | ||
| Whole thigh | 41 | 14.04 | ||
| Psychological treatment | 293 | 70 | 23.89 | |
| Mobility day 1 | Independent | 292 | 0 | 0 |
| Somewhat dependent | 11 | 3.77 | ||
| Dependent | 281 | 96.23 | ||
| Mobility day 3 | Independent | 290 | 137 | 47.24 |
| Somewhat dependent | 151 | 52.07 | ||
| Dependent | 2 | 0.69 | ||
| Complications | 293 | 120 | 40.96 | |
| Seroma | 60 | 20.48 | ||
| Wound infection | 33 | 11.26 | ||
| Chafing or risk of ulcer | 55 | 18.77 | ||
| Persistent pain | 51 | 17.41 | ||
| Asymmetrical pain | 36 | 12.29 | ||
| Fibrosis | 155 | 52.9 | ||
| Genital edema | 43 | 14.68 | ||
| Smoking | 293 | 31 | 10.58 | |
| Inadequate compression | 293 | 107 | 36.52 | |
| Less Than 5 Sessions | Less Than 10 Sessions | More Than 10 Sessions | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (SD) | Mean Difference (95% CI) | p-Value | (SD) | Mean Difference (95% CI) | p | (SD) | Mean Difference (95% CI) | p-Value | ||
| Complications | Yes | 3.152 (0.788) | −0.108 (−0.445–0.229) | 0.526 | 6.789 (1.277) | −0.509 (−1.040–0.023) | 0.060 | 12 (1.515) | −0.0217 (−0.833–0.790) | 0.957 |
| No | 3.044 (0.953) | 6.28 (1.278) | 11.978 (2.016) | |||||||
| Seroma | Yes | 3.292 (0.888) | −0.258 (−0.661–0.145) | 0.207 | 6.947 (1.268) | −0.579 (−1.232–0.073) | 0.081 | 12.118(1.654) | −0.165 (−1.151–0.822) | 0.740 |
| No | 3.033 (0.954) | 6.368 (1.284) | 11.953 (1.855) | |||||||
| Wound infection | Yes | 3.333 (0.779) | −0.275 (8–0.8115–0.2629) | 0.313 | 6.7 (1.338) | −0.241 (−1.104–0.622) | 0.580 | 11.546 (1.573) | 0.512 (−0.656–1.680) | 0.385 |
| No | 3.059 (80.8999) | 6.459 (1.296) | 12.057 (1.841) | |||||||
| Chafing or risk of ulcer | Yes | 3.227 (0.685) | −0.173 (−0.591–0.245) | 0.414 | 6.625 (5.983) | −0.169 (−0.877–0.539) | 0.636 | 12.125(1.708) | −0.1715 (−1.180–0.838) | 0.736 |
| No | 3.054 (0.930) | 6.456 (1.318) | 11.954 (1.841) | |||||||
| Persistent pain | Yes | 3.235 (0.903) | −0.173 (−0.637–0.290) | 0.460 | 6.714 (1.271) | −0.295 (−0.932–0.341) | 0.359 | 12.667(1.723) | −0.797 (−1.915–0.320) | 0.159 |
| No | 3.062 (0.888) | 6.419 (1.303) | 11.870 (1.806) | |||||||
| Asymmetrical pain | Yes | 3.25 (0.888) | −0.181 (−0.720–0.357) | 0.506 | 6.273 (1.272) | 0.239 (−0.589–1.066) | 0.567 | 12.769(1.589) | −0.931 (−2.006–0.144) | 0.088 |
| No | 3.069 (0.893) | 6.512 (1.303) | 11.838(1.817) | |||||||
| Fibrosis | Yes | 3.295 (0.823) | −0.446 (−0.767–0.125) | 0.007 | 6.546 (1.284) | −0.114 (−0.646–0.417) | 0.671 | 11.939(1.463) | 0.124 (−0.698– 0.945) | 0.765 |
| No | 2.849 (0.907) | 6.431 (1.315) | 12.063(2.257) | |||||||
| Genital edema | Yes | 3.235 (0.903) | −0.173 (−0.637–0.290) | 0.460 | 6.667 (1.397) | −0.217 (−0.943–0.510) | 0.555 | 11.272(1.272) | 0.827 (−0.331–1.986) | 0.159 |
| No | 3.062(0.888) | 6.45 (1.282) | 12.1 (1.858) | |||||||
| (SD) | Mobility N | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre-treatment VAS | Post-treatment VAS | 90 Days Post-Treatment VAS | Mobility Day 3 | |||||||
| (SD) | 7.041 (0.917) | 3.976 (0.984) | 2.341 (0.947) | Independent | Somewhat Dependent | Dependent | Total | |||
| Pre-treatment VAS | Mean difference (95% CI) | 3.065 (2.911–3.219) | 4.700 (4.548–4.851) | Mobility day 1 | Independent | 0 | 0 | 0 | 0 | |
| p | 0.000 | 0.000 | ||||||||
| Post-treatment VAS | Mean difference (95% CI) | 1.635 (1.478–1.792) | Somewhat dependent | 10 | 0 | 0 | 10 | |||
| p | 0.000 | |||||||||
| 90 days post-treatment VAS | Mean difference (95% CI) | Dependent | 127 | 151 | 2 | 280 | ||||
| p | Total | 137 | 151 | 2 | 290 | |||||
| χ2 (2) = 11.567 | p = 0.003 | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Río-González, Á.; Delgado-Pérez, E.; García-García, E.; González-Fernández, L.; García-Isidoro, S.; Cerezo-Téllez, E. Physiotherapy Intervention in the Immediate Postoperative Phase of Lipedema Surgery—Observational Study. J. Clin. Med. 2025, 14, 2137. https://doi.org/10.3390/jcm14072137
Río-González Á, Delgado-Pérez E, García-García E, González-Fernández L, García-Isidoro S, Cerezo-Téllez E. Physiotherapy Intervention in the Immediate Postoperative Phase of Lipedema Surgery—Observational Study. Journal of Clinical Medicine. 2025; 14(7):2137. https://doi.org/10.3390/jcm14072137
Chicago/Turabian StyleRío-González, Ángela, Esther Delgado-Pérez, Elisa García-García, Laura González-Fernández, Sara García-Isidoro, and Ester Cerezo-Téllez. 2025. "Physiotherapy Intervention in the Immediate Postoperative Phase of Lipedema Surgery—Observational Study" Journal of Clinical Medicine 14, no. 7: 2137. https://doi.org/10.3390/jcm14072137
APA StyleRío-González, Á., Delgado-Pérez, E., García-García, E., González-Fernández, L., García-Isidoro, S., & Cerezo-Téllez, E. (2025). Physiotherapy Intervention in the Immediate Postoperative Phase of Lipedema Surgery—Observational Study. Journal of Clinical Medicine, 14(7), 2137. https://doi.org/10.3390/jcm14072137








