The Importance of Comorbidities at Baseline and 5-Year Follow-Up in a Lung Cancer Biomarker Screening Trial
Abstract
1. Background
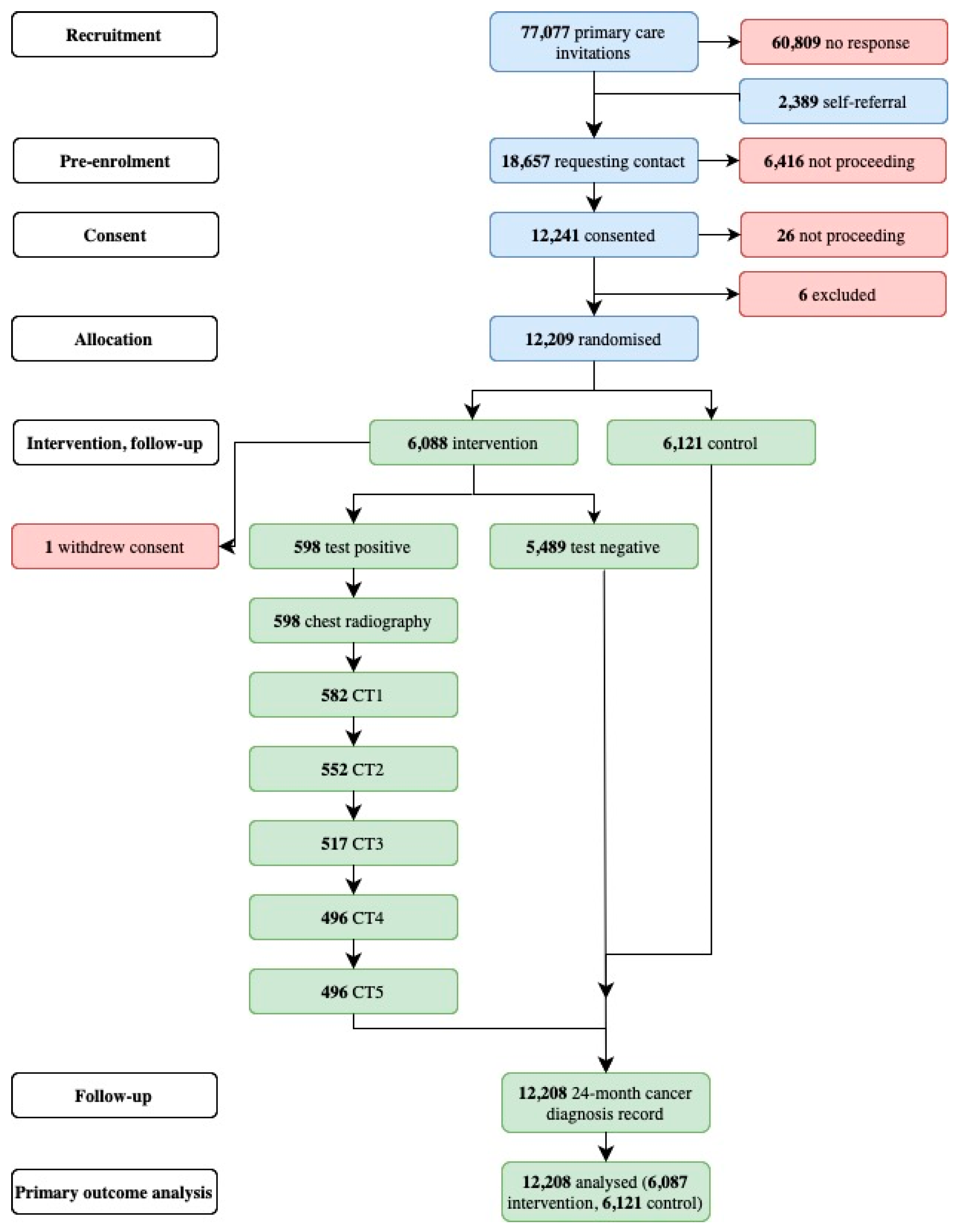
2. Materials and Methods
3. Results
3.1. Summary
3.2. Baseline Analysis
3.2.1. Overview Based on eDRIS Prescription and Comorbidity Data
3.2.2. Demographics
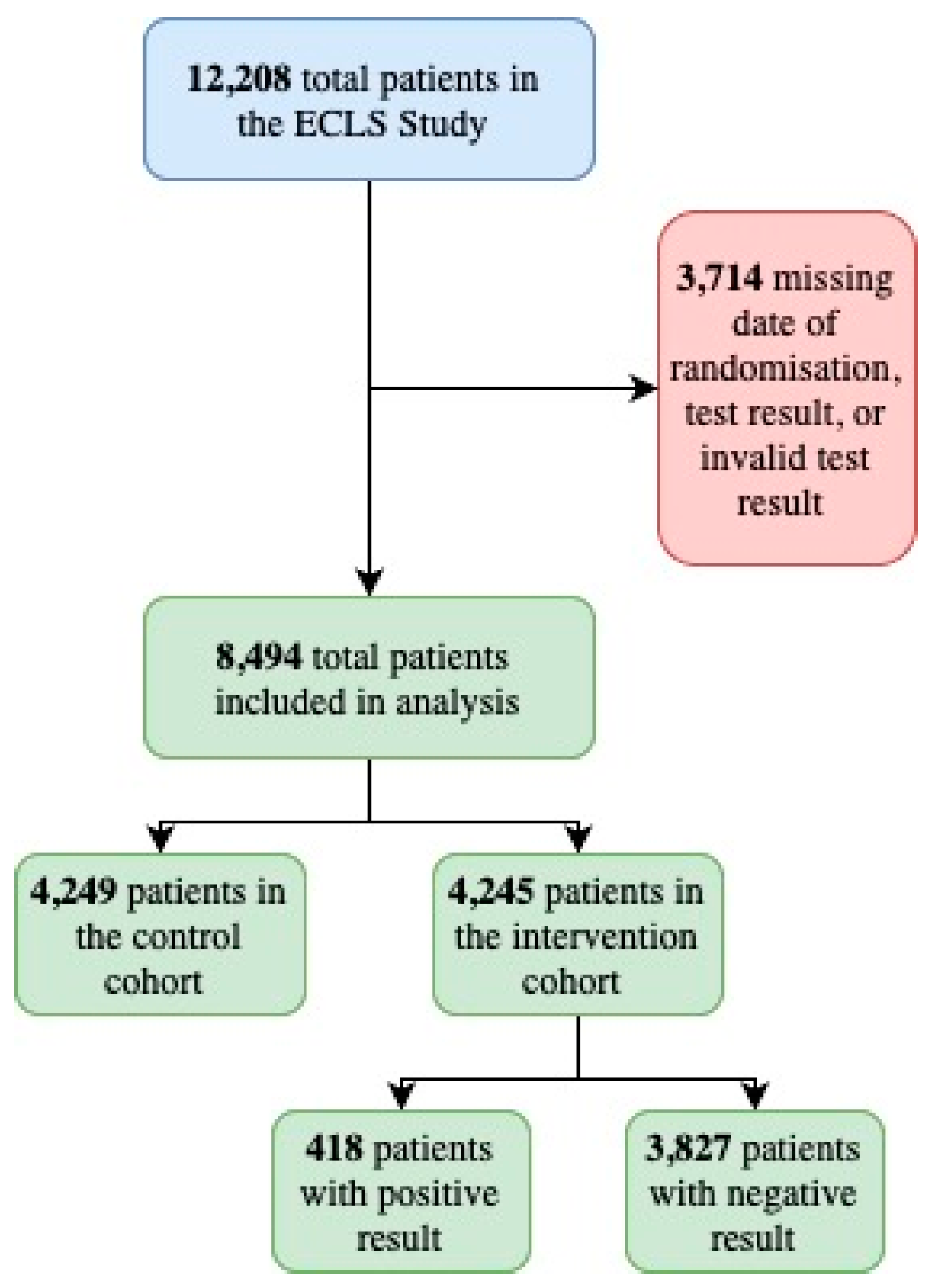
3.3. 5-Year Follow-Up
3.3.1. Baseline Overview Based on ICD10 Data
3.3.2. Chi-Square Analysis
Intervention and Control Cohorts
Positive Test and Negative Test Cohorts
4. Discussion
4.1. Summary of Findings
4.2. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| COPD | Chronic obstructive pulmonary disease |
| ECLS | Early Detection of Cancer of the Lung Scotland study |
| HIC | Health Informatics Centre |
| IFs | Incidental Findings |
| LCS | Lung cancer screening |
| LDCT | Low-dose CT |
| NLCS | National lung cancer screening |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, F.M.; Mair, F.S.; Anderson, W.; Armory, P.; Briggs, A.; Chew, C.; Dorward, A.; Haughney, J.; Hogarth, F.; Kendrick, D.; et al. Earlier diagnosis of lung cancer in a randomised trial of an autoantibody blood test followed by imaging. Eur. Respir. J. 2021, 57, 2000670. [Google Scholar] [CrossRef] [PubMed]
- Oudkerk, M.; Liu, S.; Heuvelmans, M.A.; Walter, J.E.; Field, J.K. Lung cancer LDCT screening and mortality reduction—Evidence, pitfalls and future perspectives. Nat. Rev. Clin. Oncol. 2021, 18, 135–151. [Google Scholar] [CrossRef] [PubMed]
- Aberle, D.R.; Adams, A.M.; Berg, C.D.; Black, W.C.; Clapp, J.D.; Fagerstrom, R.M.; Gareen, I.F.; Gatsonis, C.; Marcus, P.M.; Sicks, J.D. Reduced lung-cancer mortality with low-dose computed tomographic screening. N. Engl. J. Med. 2011, 365, 395–409. [Google Scholar] [CrossRef]
- de Koning, H.J.; van der Aalst, C.M.; de Jong, P.A.; Scholten, E.T.; Nackaerts, K.; Heuvelmans, M.A.; Lammers, J.J.; Weenink, C.; Yousaf-Khan, U.; Horeweg, N.; et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N. Engl. J. Med. 2020, 382, 503–513. [Google Scholar] [CrossRef]
- GOV.UK. UK National Screening Committee: Lung Cancer. 2022. Available online: https://view-health-screening-recommendations.service.gov.uk/lung-cancer/ (accessed on 8 September 2024).
- Kale, M.S.; Sigel, K.; Arora, A.; Ferket, B.S.; Wisnivesky, J.; Kong, C.Y. The Benefits and Harms of Lung Cancer Screening in Individuals With Comorbidities. JTO Clin. Res. Rep. 2024, 5, 100635. [Google Scholar] [CrossRef]
- Gareen, I.F.; Gutman, R.; Sicks, J.; Tailor, T.D.; Hoffman, R.M.; Trivedi, A.N.; Flores, E.; Underwood, E.; Cochancela, J.; Chiles, C. Significant Incidental Findings in the National Lung Screening Trial. JAMA Intern. Med. 2023, 183, 677–684. [Google Scholar] [CrossRef]
- Tsai, E.B.; Chiles, C.; Carter, B.W.; Godoy, M.C.B.; Shroff, G.S.; Munden, R.F.; Truong, M.T.; Wu, C.C. Incidental Findings on Lung Cancer Screening: Significance and Management. Semin. Ultrasound CT MR 2018, 39, 273–281. [Google Scholar] [CrossRef]
- Treadwell, J.; McCartney, M. Overdiagnosis and overtreatment: Generalists--it’s time for a grassroots revolution. Br. J. Gen. Pract. 2016, 66, 116–117. [Google Scholar] [CrossRef]
- Morgan, L.; Choi, H.; Reid, M.; Khawaja, A.; Mazzone, P.J. Frequency of Incidental Findings and Subsequent Evaluation in Low-Dose Computed Tomographic Scans for Lung Cancer Screening. Ann. Am. Thorac. Soc. 2017, 14, 1450–1456. [Google Scholar] [CrossRef]
- Toumazis, I.; Cao, P.; de Nijs, K.; Bastani, M.; Munshi, V.; Hemmati, M.; Ten Haaf, K.; Jeon, J.; Tammemagi, M.; Gazelle, G.S.; et al. Risk Model-Based Lung Cancer Screening: A Cost-Effectiveness Analysis. Ann. Intern. Med. 2023, 176, 320–332. [Google Scholar] [CrossRef] [PubMed]
- Tan, N.Q.P.; Nargund, R.S.; Douglas, E.E.; Lopez-Olivo, M.A.; Resong, P.J.; Ishizawa, S.; Nofal, S.; Krause, K.; Volk, R.J.; Toumazis, I. Acceptability and perceptions of personalised risk-based cancer screening among health-care professionals and the general public: A systematic review and meta-analysis. Lancet Public Health 2025, 10, e85–e96. [Google Scholar] [CrossRef]
- Sullivan, F.M.; van Beusekom, M. Early diagnosis of lung cancer in people most at risk. Br. J. Gen. Pract. 2020, 70, 572–573. [Google Scholar] [CrossRef]
- Voigt, W.; Prosch, H.; Silva, M. Clinical Scores, Biomarkers and IT Tools in Lung Cancer Screening-Can an Integrated Approach Overcome Current Challenges? Cancers 2023, 15, 1218. [Google Scholar] [CrossRef] [PubMed]
- Chapman, C.J.; Healey, G.F.; Murray, A.; Boyle, P.; Robertson, C.; Peek, L.J.; Allen, J.; Thorpe, A.J.; Hamilton-Fairley, G.; Parsy-Kowalska, C.B.; et al. EarlyCDT(R)-Lung test: Improved clinical utility through additional autoantibody assays. Tumour Biol. 2012, 33, 1319–1326. [Google Scholar] [CrossRef]
- Sullivan, F.M.; Mair, F.S.; Anderson, W.; Chew, C.; Dorward, A.; Haughney, J.; Hogarth, F.; Kendrick, D.; Littleford, R.; McConnachie, A.; et al. Five year mortality in an RCT of a lung cancer biomarker to select people for low dose CT screening. PLoS ONE 2025, 20, e0306163. [Google Scholar] [CrossRef]
- Robles-Zurita, J.A.; McMeekin, N.; Sullivan, F.; Mair, F.S.; Briggs, A. Health Economic Evaluation of Lung Cancer Screening Using a Diagnostic Blood Test: The Early Detection of Cancer of the Lung Scotland (ECLS). Curr. Oncol. 2024, 31, 3546–3562. [Google Scholar] [CrossRef]
- Sullivan, F.M.; Farmer, E.; Mair, F.S.; Treweek, S.; Kendrick, D.; Jackson, C.; Robertson, C.; Briggs, A.; McCowan, C.; Bedford, L.; et al. Detection in blood of autoantibodies to tumour antigens as a case-finding method in lung cancer using the EarlyCDT(R)-Lung Test (ECLS): Study protocol for a randomized controlled trial. BMC Cancer 2017, 17, 187. [Google Scholar] [CrossRef] [PubMed]
- Bennett Institute for Applied Data Science, U.o.O. 2024. Available online: https://openprescribing.net/ (accessed on 1 July 2024).
- Foundation, T.R. The R Foundation. 2024. Available online: https://www.r-project.org (accessed on 8 May 2024).
- Almatrafi, A.; Thomas, O.; Callister, M.; Gabe, R.; Beeken, R.J.; Neal, R. The prevalence of comorbidity in the lung cancer screening population: A systematic review and meta-analysis. J. Med. Screen. 2023, 30, 3–13. [Google Scholar] [CrossRef]
- Barnett, K.; Mercer, S.W.; Norbury, M.; Watt, G.; Wyke, S.; Guthrie, B. Epidemiology of multimorbidity and implications for health care, research, and medical education: A cross-sectional study. Lancet 2012, 380, 37–43. [Google Scholar] [CrossRef]
- PHE. Prescribed Medicines Review: Summary. 3 December 2020. Available online: https://www.gov.uk/government/publications/prescribed-medicines-review-report/prescribed-medicines-review-summary#fn:1 (accessed on 1 February 2025).
- Berkowitz, L.; Schultz, B.M.; Salazar, G.A.; Pardo-Roa, C.; Sebastian, V.P.; Alvarez-Lobos, M.M.; Bueno, S.M. Impact of Cigarette Smoking on the Gastrointestinal Tract Inflammation: Opposing Effects in Crohn’s Disease and Ulcerative Colitis. Front. Immunol. 2018, 9, 74. [Google Scholar] [CrossRef] [PubMed]
- Li, L.F.; Chan, R.L.; Lu, L.; Shen, J.; Zhang, L.; Wu, W.K.; Wang, L.; Hu, T.; Li, M.X.; Cho, C.H. Cigarette smoking and gastrointestinal diseases: The causal relationship and underlying molecular mechanisms (review). Int. J. Mol. Med. 2014, 34, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, F.M.; Mair, F.S.; Anderson, W.; Chew, C.; Dorward, A.; Haughney, J.; Hogarth, F.; Kendrick, D.; Littleford, R.; McConnachie, A.; et al. Improved five year mortality in an RCT of a lung cancer biomarker to select people for screening. medRxiv 2024. [Google Scholar] [CrossRef]
- Ali, N.; Lifford, K.J.; Carter, B.; McRonald, F.; Yadegarfar, G.; Baldwin, D.R.; Weller, D.; Hansell, D.M.; Duffy, S.W.; Field, J.K.; et al. Barriers to uptake among high-risk individuals declining participation in lung cancer screening: A mixed methods analysis of the UK Lung Cancer Screening (UKLS) trial. BMJ Open 2015, 5, e008254. [Google Scholar] [CrossRef] [PubMed]
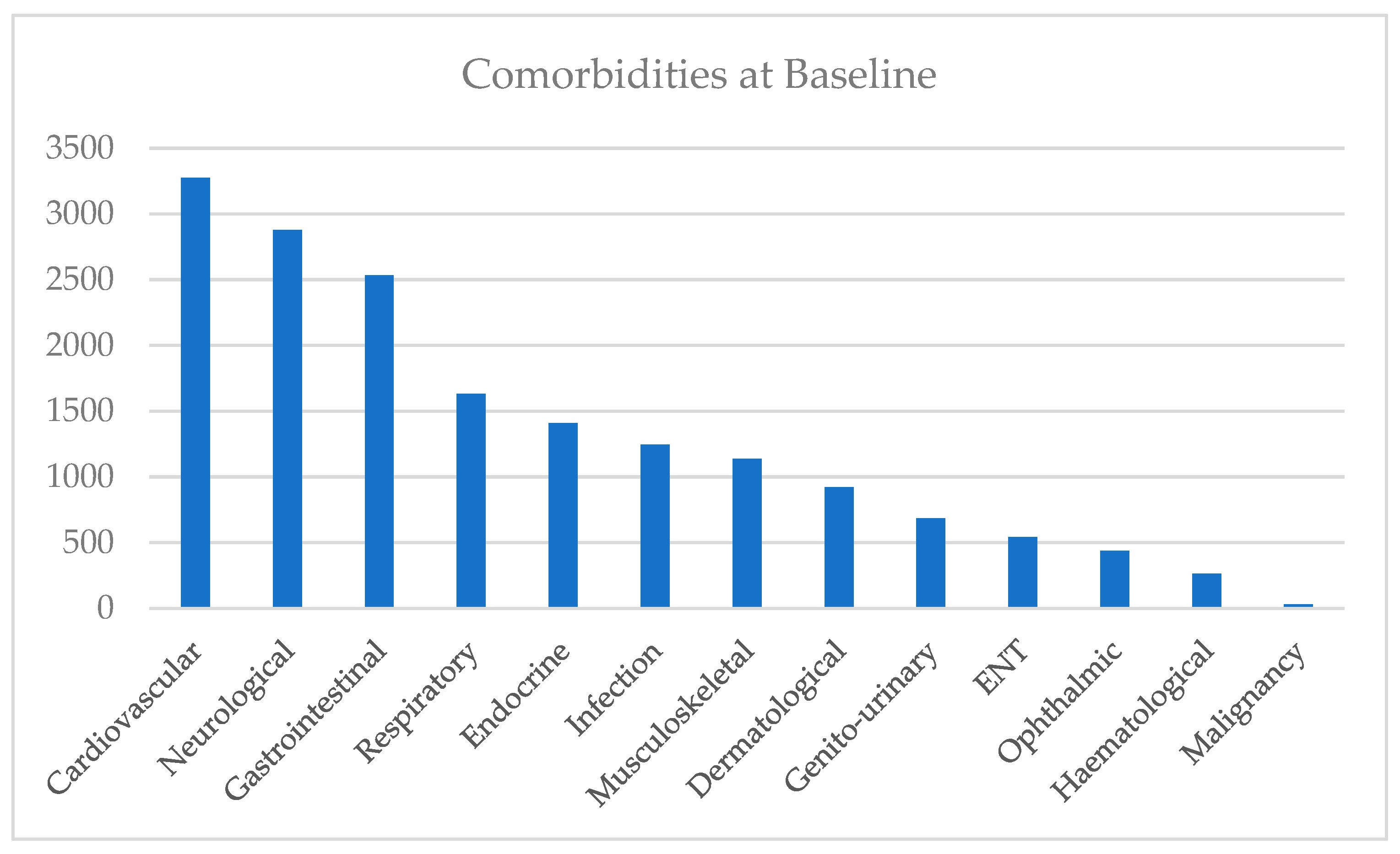
| Gender | Comorbidity | No Comorbidity | Total | % with Comorbidity |
| Female | 2454 | 1596 | 4050 | 60.6 |
| Male | 2539 | 1777 | 4316 | 58.8 |
| Pack Years | Comorbidity | No Comorbidity | Total | % with Comorbidity |
| 0–50 | 4000 | 2778 | 6778 | 59 |
| 51–100 | 927 | 554 | 1481 | 62.6 |
| 101–150 | 60 | 37 | 97 | 61.9 |
| 151–200 | 3 | 3 | 6 | 50 |
| 201–250 | 3 | 0 | 3 | 100 |
| >250 | 0 | 1 | 1 | 0 |
| Age | Comorbidity | No Comorbidity | Total | % with Comorbidity |
| 45–55 | 319 | 261 | 580 | 55 |
| 55–65 | 2972 | 2108 | 5080 | 58.5 |
| 66–75 | 1702 | 1004 | 2706 | 62.9 |
| >75 | 0 | 0 | 0 | / |
| Test Group | Malignancy | Cardiovascular Disease | COPD | Total Patients |
|---|---|---|---|---|
| Control | 257 | 477 | 426 | 856 |
| % of cohort | 6.05 | 11.23 | 10.03 | 20.15 |
| Intervention | 227 | 516 | 465 | 866 |
| % of cohort | 5.35 | 12.16 | 10.95 | 20.40 |
| Difference in % | 0.70 | −0.93 | −0.93 | −0.25 |
| Comorbidity | p Value | Chi-Square Statistic | Odds Ratio | Lower CI | Upper CI |
|---|---|---|---|---|---|
| Malignancy | 0.163 | 1.942 | 0.878 | 0.730 | 1.055 |
| Cardiovascular Disease | 0.183 | 1.776 | 1.094 | 0.958 | 1.249 |
| COPD | 0.163 | 1.948 | 1.104 | 0.961 | 1.268 |
| Total Patients | 0.770 | 0.085 | 1.016 | 0.914 | 1.129 |
| Comorbidity | p Value | Chi-Square Statistic | Odds Ratio | Lower CI | Upper CI |
|---|---|---|---|---|---|
| Malignancy | 0.590 | 0.290 | 0.879 | 0.549 | 1.407 |
| Cardiovascular Disease | 0.730 | 0.119 | 1.055 | 0.778 | 1.430 |
| COPD | 0.897 | 0.017 | 0.979 | 0.707 | 1.355 |
| Total Patients | 0.871 | 0.027 | 0.979 | 0.761 | 1.260 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romeikat, N.L.; Sullivan, F.; Daly, F.; Kong, W. The Importance of Comorbidities at Baseline and 5-Year Follow-Up in a Lung Cancer Biomarker Screening Trial. J. Clin. Med. 2025, 14, 2116. https://doi.org/10.3390/jcm14062116
Romeikat NL, Sullivan F, Daly F, Kong W. The Importance of Comorbidities at Baseline and 5-Year Follow-Up in a Lung Cancer Biomarker Screening Trial. Journal of Clinical Medicine. 2025; 14(6):2116. https://doi.org/10.3390/jcm14062116
Chicago/Turabian StyleRomeikat, Nimue Lilith, Frank Sullivan, Fergus Daly, and Wenyan Kong. 2025. "The Importance of Comorbidities at Baseline and 5-Year Follow-Up in a Lung Cancer Biomarker Screening Trial" Journal of Clinical Medicine 14, no. 6: 2116. https://doi.org/10.3390/jcm14062116
APA StyleRomeikat, N. L., Sullivan, F., Daly, F., & Kong, W. (2025). The Importance of Comorbidities at Baseline and 5-Year Follow-Up in a Lung Cancer Biomarker Screening Trial. Journal of Clinical Medicine, 14(6), 2116. https://doi.org/10.3390/jcm14062116






