Cancer Is a Major Determinant of Postoperative Atrial Fibrillation After Cardiac Surgery
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Sample and Data Collection
2.2. Statistical Analysis
2.3. Ethical Considerations
3. Results
4. Discussion
5. Clinical Implications
6. Strengths and Limitations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dobrev, D.; Aguilar, M.; Heijman, J.; Guichard, J.-B.; Nattel, S. Postoperative atrial fibrillation: Mechanisms, manifestations and management. Nat. Rev. Cardiol. 2019, 16, 417–436. [Google Scholar] [CrossRef] [PubMed]
- Almassi, G.H.; Wagner, T.H.; Carr, B.; Hattler, B.; Collins, J.F.; Quin, J.A.; Ebrahimi, R.; Grover, F.L.; Bishawi, M.; Shroyer, A.L.W. Postoperative atrial fibrillation impacts on costs and one-year clinical outcomes: The veterans affairs randomized on/off bypass trial. Ann. Thorac. Surg. 2015, 99, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Al-Khatib, S.M.; Hafley, G.; Harrington, R.A.; Mack, M.J.; Ferguson, T.B.; Peterson, E.D.; Califf, R.M.; Kouchoukos, N.T.; Alexander, J.H. Patterns of management of atrial fibrillation complicating coronary artery bypass grafting: Results from the project of ex-vivo vein graft engineering via transfection iv (prevent-iv) trial. Am. Heart J. 2009, 158, 792–798. [Google Scholar] [CrossRef]
- Lin, M.-H.; Kamel, H.; Singer, D.E.; Wu, Y.-L.; Lee, M.; Ovbiagele, B. Perioperative/postoperative atrial fibrillation and risk of subsequent stroke and/or mortality. Stroke 2019, 50, 1364–1371. [Google Scholar] [CrossRef] [PubMed]
- Eikelboom, R.; Sanjanwala, R.; Le, M.-L.; Yamashita, M.H.; Arora, R.C. Postoperative atrial fibrillation after cardiac surgery: A systematic review and meta-analysis. Ann. Thorac. Surg. 2021, 111, 544–554. [Google Scholar] [CrossRef]
- Bessissow, A.; Khan, J.; Devereaux, P.J.; Alvarez-Garcia, J.; Alonso-Coello, P. Postoperative atrial fibrillation in non-cardiac and cardiac surgery: An overview. J. Thromb. Haemost. 2015, 13 (Suppl. S1), S304–S312. [Google Scholar] [CrossRef]
- Yuan, M.; Zhang, Z.; Tse, G.; Feng, X.; Korantzopoulos, P.; Letsas, K.P.; Yan, B.P.; Wu, W.K.K.; Zhang, H.; Li, G.; et al. Association of cancer and the risk of developing atrial fibrillation: A systematic review and meta-analysis. Cardiol. Res. Pract. 2019, 2019, 8985273. [Google Scholar] [CrossRef]
- Conen, D.; Wong, J.A.; Sandhu, R.K.; Cook, N.R.; Lee, I.-M.; Buring, J.E.; Albert, C.M. Risk of malignant cancer among women with new-onset atrial fibrillation. JAMA Cardiol. 2016, 1, 389–396. [Google Scholar] [CrossRef]
- Farmakis, D.; Parissis, J.; Filippatos, G. Insights into onco-cardiology: Atrial fibrillation in cancer. J. Am. Coll. Cardiol. 2014, 63, 945–953. [Google Scholar] [CrossRef]
- Mennander, A.A.; Nielsen, S.J.; Huhtala, H.; Dellgren, G.; Hansson, E.C.; Jeppsson, A. History of cancer and survival after coronary artery bypass grafting: Experiences from the swedeheart registry. J. Thorac. Cardiovasc. Surg. 2022, 164, 107–114.e1. [Google Scholar] [CrossRef]
- Pandey, A.; Okaj, I.; Ichhpuniani, S.; Tao, B.; Kaur, H.; Spence, J.D.; Young, J.; Healey, J.S.; Devereaux, P.; Um, K.J.; et al. Risk scores for prediction of postoperative atrial fibrillation after cardiac surgery: A systematic review and meta-analysis. Am. J. Cardiol. 2023, 209, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the american society of echocardiography and the european association of cardiovascular imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Wang, Z.; Cao, F.; Song, J.; Fan, S.; Qiu, J.; Fan, X.; Yu, C. New-onset postoperative atrial fibrillation after total arch repair is associated with increased in-hospital mortality. J. Am. Heart Assoc. 2021, 10, e021980. [Google Scholar] [CrossRef] [PubMed]
- Akintoye, E.; Sellke, F.; Marchioli, R.; Tavazzi, L.; Mozaffarian, D. Factors associated with postoperative atrial fibrillation and other adverse events after cardiac surgery. J. Thorac. Cardiovasc. Surg. 2018, 155, 242–251.e10. [Google Scholar] [CrossRef]
- Greenberg, J.W.; Lancaster, T.S.; Schuessler, R.B.; Melby, S.J. Postoperative atrial fibrillation following cardiac surgery: A persistent complication. Eur. J. Cardio-Thoracic Surg. 2017, 52, 665–672. [Google Scholar] [CrossRef]
- Ishii, Y.; Schuessler, R.B.; Gaynor, S.L.; Yamada, K.; Fu, A.S.; Boineau, J.P.; Damiano, R.J., Jr. Inflammation of atrium after cardiac surgery is associated with inhomogeneity of atrial conduction and atrial fibrillation. Circulation 2005, 111, 2881–2888. [Google Scholar] [CrossRef]
- Ryu, K.; Li, L.; Khrestian, C.M.; Matsumoto, N.; Sahadevan, J.; Ruehr, M.L.; Van Wagoner, D.R.; Efimov, I.R.; Waldo, A.L. Effects of sterile pericarditis on connexins 40 and 43 in the atria: Correlation with abnormal conduction and atrial arrhythmias. Am. J. Physiol. Circ. Physiol. 2007, 293, H1231–H1241. [Google Scholar] [CrossRef]
- Wong, C.X.; Ganesan, A.N.; Selvanayagam, J.B. Epicardial fat and atrial fibrillation: Current evidence, potential mechanisms, clinical implications, and future directions. Eur. Heart J. 2016, 38, 1294–1302. [Google Scholar] [CrossRef]
- Amar, D.; Zhang, H.; Miodownik, S.; Kadish, A.H. Competing autonomic mechanisms precede the onset of postoperative atrial fibrillation. J. Am. Coll. Cardiol. 2003, 42, 1262–1268. [Google Scholar] [CrossRef]
- Kalman, J.M.; Munawar, M.; Howes, L.G.; Louis, W.J.; Buxton, B.F.; Gutteridge, G.; Tonkin, A.M. Atrial fibrillation after coronary artery bypass grafting is associated with sympathetic activation. Ann. Thorac. Surg. 1995, 60, 1709–1715. [Google Scholar] [CrossRef]
- Ovčariček, P.P.; Verburg, F.A.; Hoffmann, M.; Iakovou, I.; Mihailovic, J.; Vrachimis, A.; Luster, M.; Giovanella, L. Higher thyroid hormone levels and cancer. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 808–821. [Google Scholar] [CrossRef]
- Chiari, P.; Fellahi, J.-L. Myocardial protection in cardiac surgery: A comprehensive review of current therapies and future cardioprotective strategies. Front. Med. 2024, 11, 1424188. [Google Scholar] [CrossRef]
- Gaudino, M.; Di Franco, A.; Rong, L.Q.; Piccini, J.; Mack, M. Postoperative atrial fibrillation: From mechanisms to treatment. Eur. Heart J. 2023, 44, 1020–1039. [Google Scholar] [CrossRef] [PubMed]
- Taha, A.; Hjärpe, A.; Martinsson, A.; Nielsen, S.J.; Barbu, M.; Pivodic, A.; Lannemyr, L.; Bergfeldt, L.; Jeppsson, A. Cardiopulmonary bypass management and risk of new-onset atrial fibrillation after cardiac surgery. Interdiscip. Cardiovasc. Thorac. Surg. 2023, 37, ivad153. [Google Scholar] [CrossRef] [PubMed]
- Dieberg, G.; Smart, N.A.; King, N. On- vs. Off-pump coronary artery bypass grafting: A systematic review and meta-analysis. Int. J. Cardiol. 2016, 223, 201–211. [Google Scholar] [CrossRef]
- Aboumsallem, J.P.; Moslehi, J.; de Boer, R.A. Reverse cardio-oncology: Cancer development in patients with cardiovascular disease. J. Am. Heart Assoc. 2020, 9, e013754. [Google Scholar] [CrossRef]
- Moslehi, J.J. Cardiovascular toxic effects of targeted cancer therapies. N. Engl. J. Med. 2016, 375, 1457–1467. [Google Scholar] [CrossRef]
- Herrmann, J. Adverse cardiac effects of cancer therapies: Cardiotoxicity and arrhythmia. Nat. Rev. Cardiol. 2020, 17, 474–502. [Google Scholar] [CrossRef]
- Guha, A.; Fradley, M.G.; Dent, S.F.; Weintraub, N.L.; Lustberg, M.B.; Alonso, A.; Addison, D. Incidence, risk factors, and mortality of atrial fibrillation in breast cancer: A seer-medicare analysis. Eur. Heart J. 2021, 43, 300–312. [Google Scholar] [CrossRef]
- Parahuleva, M.S.; Kreutz, J.; Euler, G.; Terzieva, D.; Mardini, A.; Uchikova, E.; Parahuleva, N. Incidence of atrial fibrillation in postmenopausal women with endometrial cancer. J. Clin. Med. 2021, 10, 266. [Google Scholar] [CrossRef]
- Chen, D.-Y.; Liu, J.-R.; Tseng, C.-N.; Hsieh, M.-J.; Chuang, C.-K.; Pang, S.-T.; Chen, S.-W.; Hsieh, I.-C.; Chu, P.-H.; Chen, J.-S.; et al. Major adverse cardiovascular events in patients with renal cell carcinoma treated with targeted therapies. JACC CardioOncology 2022, 4, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Zoltek, M.; Andersson, T.M.; Hedman, C.; Ihre-Lundgren, C.; Nordenvall, C. Cardiovascular incidence in 6900 patients with differentiated thyroid cancer: A swedish nationwide study. World J. Surg. 2019, 44, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Sorigue, M.; Gual-Capllonch, F.; Garcia, O.; Sarrate, E.; Franch-Sarto, M.; Ibarra, G.; Grau, J.; Orna, E.; Ribera, J.-M.; Sancho, J.-M. Incidence, predictive factors, management, and survival impact of atrial fibrillation in non-hodgkin lymphoma. Ann. Hematol. 2018, 97, 1633–1640. [Google Scholar] [CrossRef]
- Shah, N.; Rochlani, Y.; Pothineni, N.V.; Paydak, H. Burden of arrhythmias in patients with multiple myeloma. Int. J. Cardiol. 2015, 203, 305–306. [Google Scholar] [CrossRef]
- Abdel-Qadir, H.; Sabrie, N.; Leong, D.; Pang, A.; Austin, P.C.; Prica, A.; Nanthakumar, K.; Calvillo-Argüelles, O.; Lee, D.S.; Thavendiranathan, P. Cardiovascular risk associated with ibrutinib use in chronic lymphocytic leukemia: A population-based cohort study. J. Clin. Oncol. 2021, 39, 3453–3462. [Google Scholar] [CrossRef]
- Leong, D.P.; Caron, F.; Hillis, C.; Duan, A.; Healey, J.S.; Fraser, G.; Siegal, D. The risk of atrial fibrillation with ibrutinib use: A systematic review and meta-analysis. Blood 2016, 128, 138–140. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, J.; Boismoreau, L.; Morice, P.-M.; Sassier, M.; Da-Silva, A.; Plane, A.-F.; Font, J.; Milliez, P.; Legallois, D.; Dolladille, C. Atrial fibrillation incidence associated with exposure to anticancer drugs used as monotherapy in clinical trials. JACC CardioOncology 2023, 5, 216–226. [Google Scholar] [CrossRef]
- Shaaban, A.; Scott, S.S.; Greenlee, A.N.; Binda, N.; Noor, A.; Webb, A.; Guo, S.; Purdy, N.; Pennza, N.; Habib, A.; et al. Atrial fibrillation in cancer, anticancer therapies, and underlying mechanisms. J. Mol. Cell. Cardiol. 2024, 194, 118–132. [Google Scholar] [CrossRef]
- Voigt, N.; Li, N.; Wang, Q.; Wang, W.; Trafford, A.W.; Abu-Taha, I.; Sun, Q.; Wieland, T.; Ravens, U.; Nattel, S.; et al. Enhanced sarcoplasmic reticulum Ca2+ leak and increased Na+-Ca 2+ exchanger function underlie delayed afterdepolarizations in patients with chronic atrial fibrillation. Circulation 2012, 125, 2059–2070. [Google Scholar] [CrossRef]
- Tan, R.; Cong, T.; Xu, G.; Hao, Z.; Liao, J.; Xie, Y.; Lin, Y.; Yang, X.; Li, Q.; Liu, Y.; et al. Anthracycline-induced atrial structural and electrical remodeling characterizes early cardiotoxicity and contributes to atrial conductive instability and dysfunction. Antioxid. Redox Signal. 2022, 37, 19–39. [Google Scholar] [CrossRef]
- Chen, Q.; van Rein, N.; van der Hulle, T.; Heemelaar, J.C.; Trines, S.A.; Versteeg, H.H.; Klok, F.A.; Cannegieter, S.C. Coexisting atrial fibrillation and cancer: Time trends and associations with mortality in a nationwide dutch study. Eur. Heart J. 2024, 45, 2201–2213. [Google Scholar] [CrossRef]
- Mauro, E.; Lucà, F.; Tetta, C.; Parise, O.; Parrini, I.; Parise, G.; Rao, C.M.; Matteucci, F.; Micali, L.R.; Gulizia, M.M.; et al. Breast cancer and atrial fibrillation. J. Clin. Med. 2022, 11, 1417. [Google Scholar] [CrossRef]
- Alexandre, J.; Moslehi, J.J.; Bersell, K.R.; Funck-Brentano, C.; Roden, D.M.; Salem, J.-E. Anticancer drug-induced cardiac rhythm disorders: Current knowledge and basic underlying mechanisms. Pharmacol. Ther. 2018, 189, 89–103. [Google Scholar] [CrossRef]
- Mariani, M.V.; Pierucci, N.; Trivigno, S.; Cipollone, P.; Piro, A.; Chimenti, C.; Della Rocca, D.G.; Miraldi, F.; Vizza, C.D.; Lavalle, C. Probability score to predict spontaneous conversion to sinus rhythm in patients with symptomatic atrial fibrillation when less could be more? J. Clin. Med. 2024, 13, 1470. [Google Scholar] [CrossRef] [PubMed]
- Egan, S.; Collins-Smyth, C.; Chitnis, S.; Head, J.; Chiu, A.; Bhatti, G.; McLean, S.R. Prevention of postoperative atrial fibrillation in cardiac surgery: A quality improvement project. Can. J. Anaesth. 2023, 70, 1880–1891. [Google Scholar] [CrossRef] [PubMed]
- Malektojari, A.; Javidfar, Z.; Ghazizadeh, S.; Lahuti, S.; Shokraei, R.; Zeinaee, M.; Badele, A.; Mirzadeh, R.; Ashrafi, M.; Afra, F.; et al. Effectiveness of anti-inflammatory agents to prevent atrial fibrillation after cardiac surgery: A systematic review and network meta-analysis. CJC Open 2025, 7, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Perezgrovas-Olaria, R.; Chadow, D.; Lau, C.; Rahouma, M.; Soletti, G.J.; Cancelli, G.; Harik, L.; Dimagli, A.; Rong, L.Q.; Gillinov, M.; et al. Characteristics of postoperative atrial fibrillation and the effect of posterior pericardiotomy. Ann. Thorac. Surg. 2022, 116, 615–622. [Google Scholar] [CrossRef]
- Ruan, Y.; Robinson, N.B.; Naik, A.; Silva, M.; Hameed, I.; Rahouma, M.; Oakley, C.; Di Franco, A.; Zamvar, V.; Girardi, L.N.; et al. Effect of atrial pacing on post-operative atrial fibrillation following coronary artery bypass grafting: Pairwise and network meta-analyses. Int. J. Cardiol. 2020, 302, 103–107. [Google Scholar] [CrossRef]
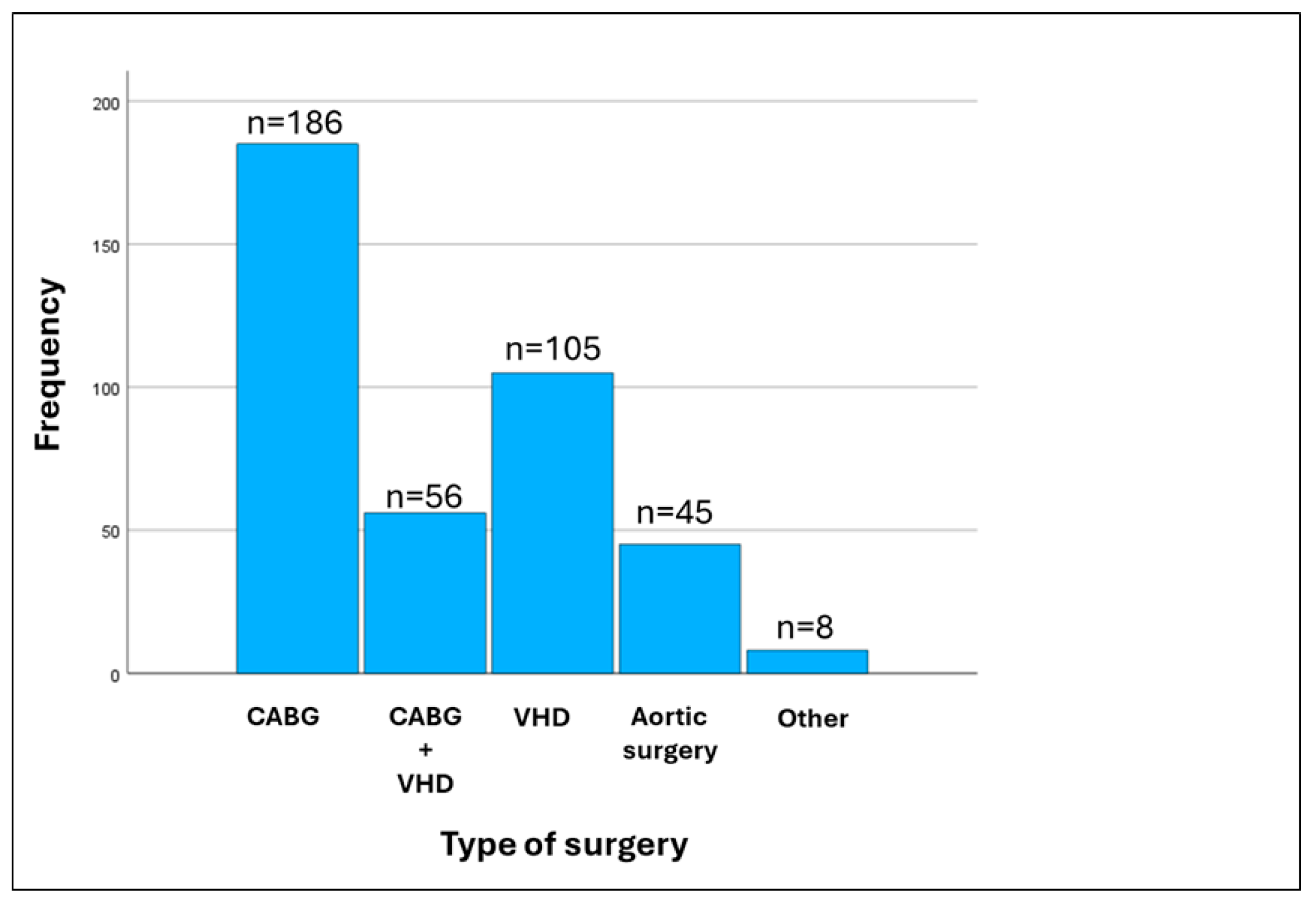
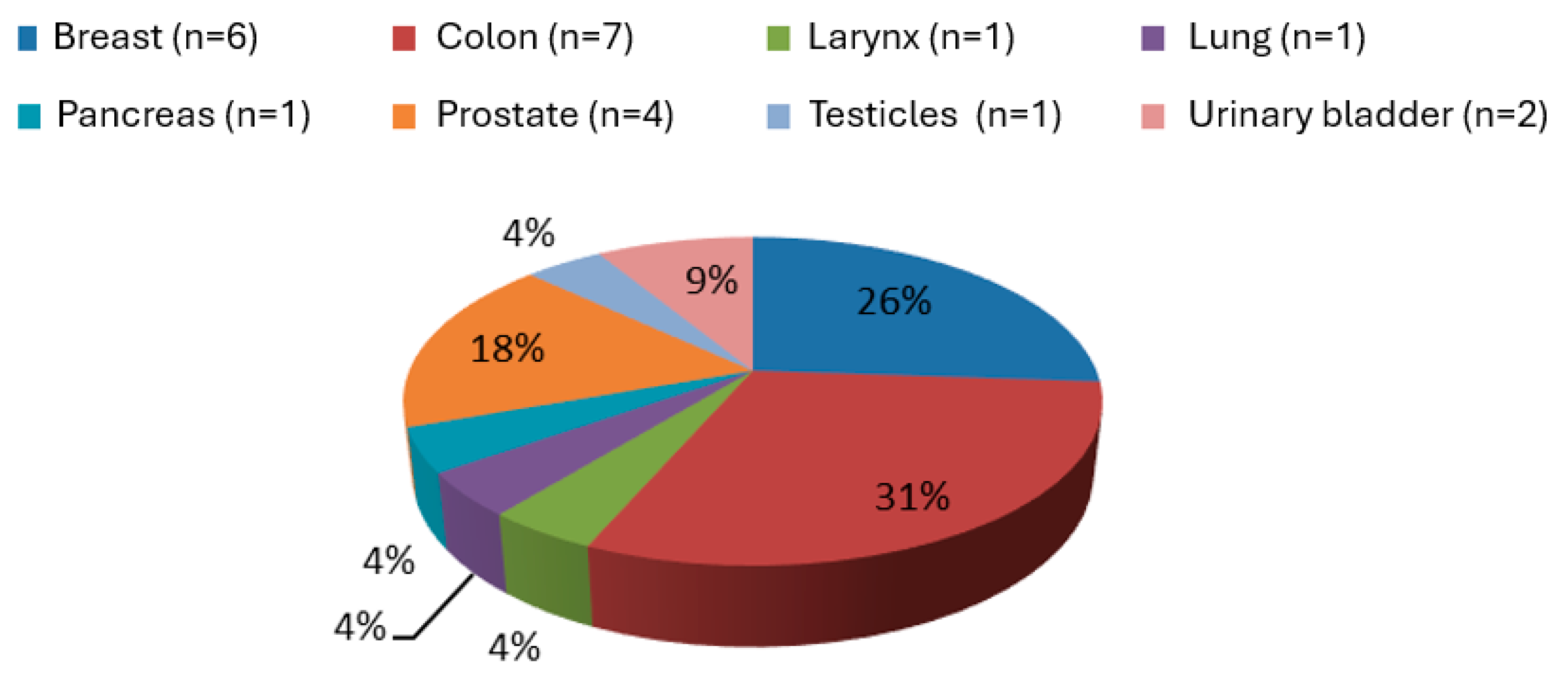
| Variables | No Atrial Fibrillation (n = 334) | Atrial Fibrillation (n = 66) | p |
|---|---|---|---|
| Demographic | |||
| Age (years) | 67 [59–73] | 71 [66–75] | 0.200 |
| Females (%) | 48 (14.4) | 16 (24.2) | 0.929 |
| Clinical | |||
| Systolic blood pressure (mmHg) | 130 [115–144] | 130 [115–150] | 0.088 |
| Diastolic blood pressure (mmHg) | 74 [65–81] | 63 [71, 72] | 0.698 |
| Heart rate (bpm) | 73 [65–80] | 70 [65–80] | 0.318 |
| Left ventricular ejection fraction (%) | 58 [55–63] | 60 [55–65] | 0.009 |
| Laboratory | |||
| Hemoglobin (mg/dL) | 13 [11.8–14.4] | 12.95 [11.7–14.9] | 0.561 |
| Hematocrit (%) | 39.9 [37.2–42.3] | 39.2 [36.1–42.6] | 0.495 |
| Creatinine (mg/dL) | 0.93 [0.80–1.14] | 0.92 [0.74–1.13] | 0.978 |
| Sodium (meq/L) | 140 [139–141] | 140 [139–141] | 0.989 |
| Glucose (mg/dL) | 112 [101–136] | 118 [101–138] | 0.493 |
| Potassium (meq/L) | 3.72 [3.57–3.90] | 3.79 [3.59–3.90] | 0.840 |
| Cardiopulmonary bypass time (min) | 126 [97–164] | 149 [118–189] | 0.002 |
| Cross clamp time (min) | 74 [55–117] | 92 [62–129] | 0.160 |
| Coexisting morbidities | |||
| Valvular heart disease (%) | 155 (46) | 41 (62) | 0.009 |
| Heart failure (%) | 23 (7) | 3 (5) | 0.518 |
| Coronary artery disease (%) | 214 (64) | 35 (53) | 0.164 |
| Hypertension (%) | 130 (39) | 18 (27) | 0.105 |
| Cancer (%) | 13 (4) | 10 (15) | <0.001 |
| Chronic obstructive pulmonary disease (%) | 111 (33) | 20 (30) | 0.769 |
| Dyslipidemia (%) | 207 (62) | 30 (45) | 0.026 |
| Hyperthyroidism (%) | 4 (1) | 0 (0) | 0.380 |
| Chronic kidney disease (%) | 35 (10) | 7 (11) | 0.907 |
| Diabetes mellitus (%) | 60 (18) | 8 (12) | 0.292 |
| Obesity (%) | 52 (16) | 8 (12) | 0.535 |
| Independent Variables | Wald | Odds Ratio | Lower 95% CI | Upper 95% CI | p Value |
|---|---|---|---|---|---|
| LVEF | 3.483 | 40.458 | 0.830 | 1971.388 | 0.062 |
| CBP | 6.582 | 1.001 | 1.000 | 1.001 | 0.010 |
| VHD | 6.628 | 2.070 | 1.190 | 3.602 | 0.062 |
| Cancer | 11.666 | 4.587 | 1.915 | 10.9.85 | <0.001 |
| Dyslipidemia | 4.869 | 0.546 | 0.319 | 0.935 | 0.027 |
| Independent Variable | Wald | Odds Ratio | Lower 95% CI | Upper 95% CI | p Value |
|---|---|---|---|---|---|
| Surgery type 1 | 3.134 | 0.173 | 0.025 | 1.207 | 0.077 |
| Surgery type 2 | 1.966 | 0.245 | 0.034 | 1.750 | 0.161 |
| Surgery type 3 | 2.357 | 0.223 | 0.033 | 1.515 | 0.125 |
| Surgery type 4 | <0.001 | <0.001 | <0.001 | . | 0.999 |
| LVEF | 3.654 | 47.976 | 0.907 | 2538.745 | 0.056 |
| CBP | 4.671 | 1.001 | 1.000 | 1.001 | 0.031 |
| VHD | 2.584 | 4.064 | 0.735 | 22.463 | 0.108 |
| Cancer | 8.257 | 3.852 | 1.535 | 9.664 | 0.004 |
| Dyslipidemia | 0.840 | 0.646 | 0.254 | 1.645 | 0.360 |
| Patient | Sex | Age | Cancer Type |
|---|---|---|---|
| POAF (−) | |||
| 1 | Female | 79 | Breast |
| 2 | Female | 76 | Colon |
| 3 | Male | 70 | Pancreatic |
| 4 | Male | 66 | Urinary Bladder |
| 5 | Male | 82 | Prostate |
| 6 | Female | 63 | Colon |
| 7 | Male | 79 | Colon |
| 8 | Male | 74 | Prostate |
| 9 | Male | 56 | Colon |
| 10 | Male | 60 | Urinary Bladder |
| 11 | Female | 62 | Breast |
| 12 | Male | 64 | Testicular |
| 13 | Female | 59 | Breast |
| POAF (+) | |||
| 1 | Male | 71 | Prostate |
| 2 | Male | 76 | Colon |
| 3 | Male | 71 | Colon |
| 4 | Female | 76 | Breast |
| 5 | Male | 75 | Laryngeal |
| 6 | Female | 66 | Breast |
| 7 | Male | 73 | Lung |
| 8 | Male | 74 | Prostate |
| 9 | Male | 73 | Breast |
| 10 | Female | 72 | Colon |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Georghiou, G.P.; Xanthopoulos, A.; Kanellopoulos, G.; Georghiou, P.; Georgiou, A.; Skoularigis, J.; Giamouzis, G.; Lampropoulos, K.; Patrikios, I.; Triposkiadis, F. Cancer Is a Major Determinant of Postoperative Atrial Fibrillation After Cardiac Surgery. J. Clin. Med. 2025, 14, 2117. https://doi.org/10.3390/jcm14062117
Georghiou GP, Xanthopoulos A, Kanellopoulos G, Georghiou P, Georgiou A, Skoularigis J, Giamouzis G, Lampropoulos K, Patrikios I, Triposkiadis F. Cancer Is a Major Determinant of Postoperative Atrial Fibrillation After Cardiac Surgery. Journal of Clinical Medicine. 2025; 14(6):2117. https://doi.org/10.3390/jcm14062117
Chicago/Turabian StyleGeorghiou, Georgios P., Andrew Xanthopoulos, George Kanellopoulos, Panos Georghiou, Amalia Georgiou, John Skoularigis, Grigorios Giamouzis, Konstantinos Lampropoulos, Ioannis Patrikios, and Filippos Triposkiadis. 2025. "Cancer Is a Major Determinant of Postoperative Atrial Fibrillation After Cardiac Surgery" Journal of Clinical Medicine 14, no. 6: 2117. https://doi.org/10.3390/jcm14062117
APA StyleGeorghiou, G. P., Xanthopoulos, A., Kanellopoulos, G., Georghiou, P., Georgiou, A., Skoularigis, J., Giamouzis, G., Lampropoulos, K., Patrikios, I., & Triposkiadis, F. (2025). Cancer Is a Major Determinant of Postoperative Atrial Fibrillation After Cardiac Surgery. Journal of Clinical Medicine, 14(6), 2117. https://doi.org/10.3390/jcm14062117








