The Present and Future of Robotic Surgery in Breast Cancer and Breast Reconstruction
Abstract
1. Introduction
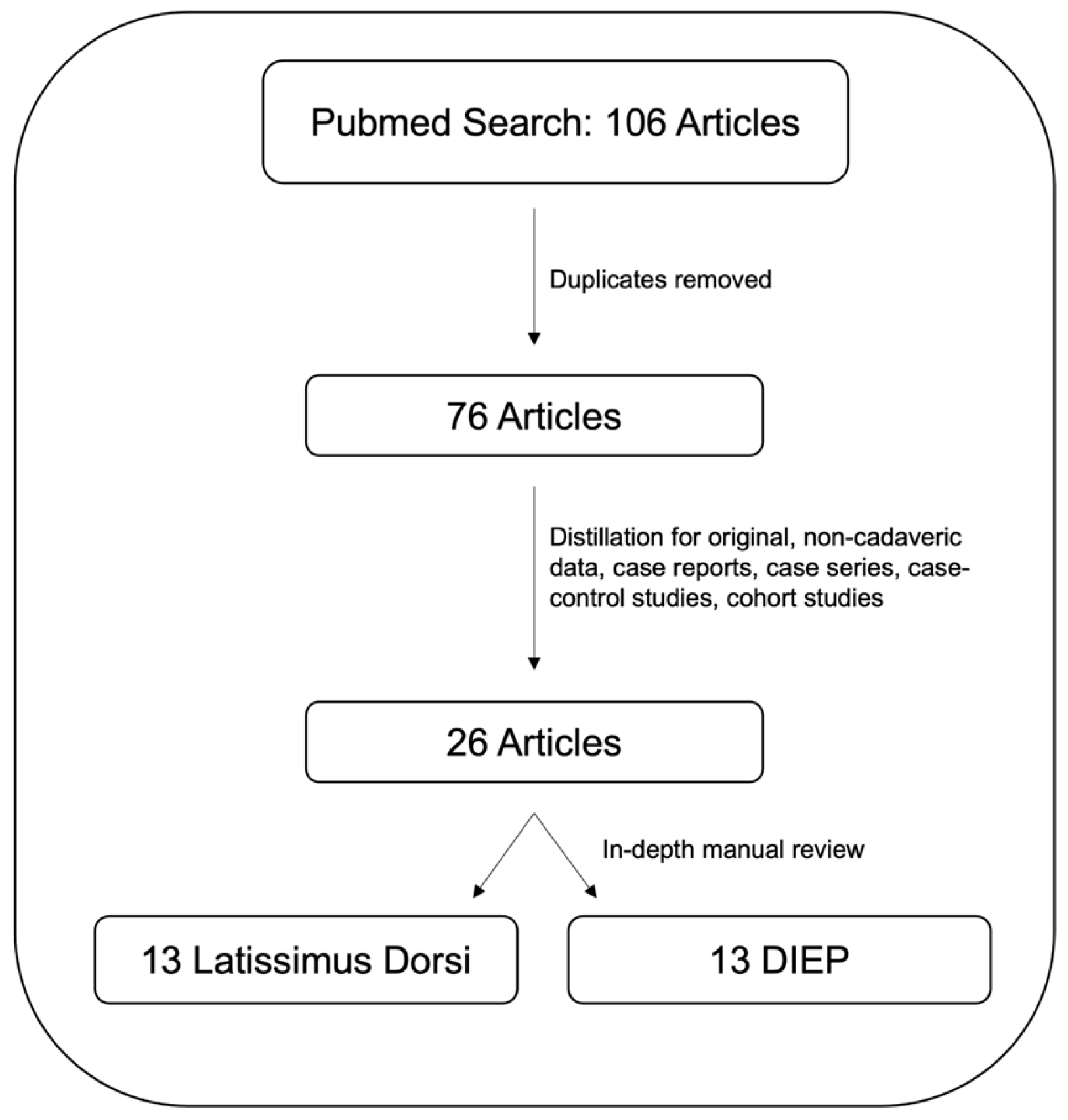
2. Materials and Methods
3. Results
3.1. Robotic-Assisted Latissimus Dorsi (LD) Flap Breast Reconstruction
3.2. Robotic-Assisted Deep Inferior Epigastric Perforator (DIEP) Flap Breast Reconstruction
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LD | latissimus dorsi |
| DIEP | deep inferior epigastric perforator |
| TAP/TAPP | trans-abdominal pre-peritoneal |
| TEP | total extra-peritoneal |
| TRAM | transverse rectus abdominal muscle |
| UNLV | University of Nevada Las Vegas |
| CT | computed tomography |
| SP | single port |
References
- Caswell-Jin, J.L.; Sun, L.P.; Munoz, D.; Lu, Y.; Li, Y.; Huang, H.; Hampton, J.M.; Song, J.; Jayasekera, J.; Schechter, C.; et al. Analysis of Breast Cancer Mortality in the US-1975 to 2019. JAMA 2024, 331, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Kohler, B.A.; Sherman, R.L.; Howlader, N.; Jemal, A.; Ryerson, A.B.; Henry, K.A.; Boscoe, F.P.; Cronin, K.A.; Lake, A.; Noone, A.M.; et al. Annual Report to the Nation on the Status of Cancer, 1975-2011, Featuring Incidence of Breast Cancer Subtypes by Race/Ethnicity, Poverty, and State. J. Natl. Cancer Inst. 2015, 107, djv048. [Google Scholar] [CrossRef] [PubMed]
- Lakhtakia, R. A Brief History of Breast Cancer: Part I: Surgical domination reinvented. Sultan Qaboos Univ. Med. J. 2014, 14, e166–e169. [Google Scholar] [PubMed]
- Wilkins, E.G.; Alderman, A.K. Breast reconstruction practices in north america: Current trends and future priorities. Semin. Plast. Surg. 2004, 18, 149–155. [Google Scholar] [CrossRef]
- Kronowitz, S.J.; Robb, G.L. Radiation therapy and breast reconstruction: A critical review of the literature. Plast. Reconstr. Surg. 2009, 124, 395–408. [Google Scholar] [CrossRef]
- Lynch, J.B.; Madden, J.J.; Franklin, J.D. Breast reconstruction following mastectomy for cancer. Ann. Surg. 1978, 187, 490–501. [Google Scholar] [CrossRef]
- Uyulmaz, S.; Fontein, D.; Sarvan, M.; Grünherz, L.; Giovanoli, P.; Lindenblatt, N. Management of Delayed Vascular Occlusion in Free Flap Breast Reconstruction: A Systematic Review of Literature and Case Report. Ann. Plast. Surg. 2024, 93, 713–721. [Google Scholar] [CrossRef]
- Maxwell, G.P. Iginio Tansini and the origin of the latissimus dorsi musculocutaneous flap. Plast. Reconstr. Surg. 1980, 65, 686–692. [Google Scholar] [CrossRef]
- Sood, R.; Easow, J.M.; Konopka, G.; Panthaki, Z.J. Latissimus Dorsi Flap in Breast Reconstruction: Recent Innovations in the Workhorse Flap. Cancer Control 2018, 25, 1073274817744638. [Google Scholar] [CrossRef]
- Koshima, I.; Soeda, S. Inferior epigastric artery skin flaps without rectus abdominis muscle. Br. J. Plast. Surg. 1989, 42, 645–648. [Google Scholar] [CrossRef]
- Allen, R.J.; Treece, P. Deep inferior epigastric perforator flap for breast reconstruction. Ann. Plast. Surg. 1994, 32, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Blondeel, P.N. One hundred free DIEP flap breast reconstructions: A personal experience. Br. J. Plast. Surg. 1999, 52, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Healy, C.; Allen, R.J. The evolution of perforator flap breast reconstruction: Twenty years after the first DIEP flap. J. Reconstr. Microsurg. 2014, 30, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Wei, F.C.; Levin, L.S.; Chen, M.C. Donor-site morbidity comparison between endoscopically assisted and traditional harvest of free latissimus dorsi muscle flap. Plast. Reconstr. Surg. 1999, 104, 1070–1077; quiz 1078. [Google Scholar] [CrossRef]
- Pomel, C.; Missana, M.C.; Lasser, P. Endoscopic harvesting of the latissimus dorsi flap in breast reconstructive surgery. Feasibility study and review of the literature. Ann. Chir. 2002, 127, 337–342. [Google Scholar] [CrossRef]
- Eo, P.S.; Kim, H.; Lee, J.S.; Lee, J.; Park, H.Y.; Yang, J.D. Robot-Assisted Latissimus Dorsi Flap Harvest for Partial Breast Reconstruction: Comparison With Endoscopic and Conventional Approaches. Aesthet. Surg. J. 2023, 44, 38–46. [Google Scholar] [CrossRef]
- Selber, J.C.; Baumann, D.P.; Holsinger, C.F. Robotic harvest of the latissimus dorsi muscle: Laboratory and clinical experience. J. Reconstr. Microsurg. 2012, 28, 457–464. [Google Scholar] [CrossRef]
- Parekattil, S.J.; Moran, M.E. Robotic instrumentation: Evolution and microsurgical applications. Indian J. Urol. 2010, 26, 395–403. [Google Scholar] [CrossRef]
- Coker, A.; Sebastian, R.; Tatum, J.; Cornejo, J.; Zevallos, A.; Li, C.; Schweitzer, M.; Adrales, G. Do advances in technology translate to improved outcomes? Comparing robotic bariatric surgery outcomes over two-time intervals utilizing the MBSAQIP database. Surg. Endosc. 2023, 37, 7970–7979. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, B.; Weng, B.; Liu, X.; Hou, S. Comparison of robotic-assisted and laparoscopic partial nephrectomy based on the PADUA score and the predictive value of the PADUA score and the Mayo Adhesive Probability score for postoperative complications: A single-center retrospective study. J. Cancer Res. Clin. Oncol. 2024, 151, 1. [Google Scholar] [CrossRef]
- Mohammed, H.; Gabra, I.M.; Halawa, N.; Naeem, S.; Ogah, C.O.; Nath, T.S. Comparative Systematic Review and Meta-Analysis Between Robotic and Laparoscopic Abdominoperineal Resection for Rectal Cancer: Oncological and Short-Term Outcomes. Cureus 2024, 16, e72877. [Google Scholar] [CrossRef] [PubMed]
- Morrell, A.L.G.; Morrell-Junior, A.C.; Morrell, A.G.; Mendes, J.M.F.; Tustumi, F.; DE-Oliveira-E-Silva, L.G.; Morrell, A. The history of robotic surgery and its evolution: When illusion becomes reality. Rev. Col. Bras. Cir. 2021, 48, e20202798. [Google Scholar] [CrossRef] [PubMed]
- Boyd, B.; Umansky, J.; Samson, M.; Boyd, D.; Stahl, K. Robotic harvest of internal mammary vessels in breast reconstruction. J. Reconstr. Microsurg. 2006, 22, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Gundlapalli, V.S.; Ogunleye, A.A.; Scott, K.; Wenzinger, E.; Ulm, J.P.; Tavana, L.; Pullatt, R.C.; Delaney, K.O. Robotic-assisted deep inferior epigastric artery perforator flap abdominal harvest for breast reconstruction: A case report. Microsurgery 2018, 38, 702–705. [Google Scholar] [CrossRef]
- Selber, J.C. The Robotic DIEP Flap. Plast. Reconstr. Surg. 2020, 145, 340–343. [Google Scholar] [CrossRef]
- Toesca, A.; Peradze, N.; Manconi, A.; Galimberti, V.; Intra, M.; Colleoni, M.; Bonanni, B.; Curigliano, G.; Rietjens, M.; Viale, G.; et al. Robotic nipple-sparing mastectomy for the treatment of breast cancer: Feasibility and safety study. Breast 2017, 31, 51–56. [Google Scholar] [CrossRef]
- Joo, O.Y.; Song, S.Y.; Lew, D.H.; Park, H.S.; Lee, D.W. Robotic harvest of a latissimus dorsi flap using a single-port surgical robotic system in breast reconstruction. Arch. Plast. Surg. 2021, 48, 577–582. [Google Scholar] [CrossRef]
- Chung, J.H.; You, H.J.; Kim, H.S.; Lee, B.I.; Park, S.H.; Yoon, E.S. A novel technique for robot assisted latissimus dorsi flap harvest. J. Plast. Reconstr. Aesthet. Surg. 2015, 68, 966–972. [Google Scholar] [CrossRef]
- Thomsen, J.B.; Rindom, M.B.; Rancati, A.; Angrigiani, C. Thoracodorsal artery flaps for breast reconstruction-the variants and its approach. Arch. Plast. Surg. 2021, 48, 15–25. [Google Scholar] [CrossRef]
- Selber, J.C.; Baumann, D.P.; Holsinger, F.C. Robotic latissimus dorsi muscle harvest: A case series. Plast. Reconstr. Surg. 2012, 129, 1305–1312. [Google Scholar] [CrossRef]
- Clemens, M.W.; Kronowitz, S.; Selber, J.C. Robotic-assisted latissimus dorsi harvest in delayed-immediate breast reconstruction. Semin. Plast. Surg. 2014, 28, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.W.; Chen, S.T.; Lin, S.L.; Lin, Y.L.; Wu, H.K.; Pai, S.H.; Chen, D.R.; Kuo, S.J. Technique for single axillary incision robotic assisted quadrantectomy and immediate partial breast reconstruction with robotic latissimus dorsi flap harvest for breast cancer: A case report. Medicine 2018, 97, e11373. [Google Scholar] [CrossRef] [PubMed]
- Houvenaeghel, G.; Bannier, M.; Rua, S.; Barrou, J.; Heinemann, M.; Knight, S.; Lambaudie, E.; Cohen, M. Robotic breast and reconstructive surgery: 100 procedures in 2-years for 80 patients. Surg. Oncol. 2019, 31, 38–45. [Google Scholar] [CrossRef]
- Houvenaeghel, G.; Bannier, M.; Rua, S.; Barrou, J.; Heinemann, M.; Lambaudie, E.; Cohen, M. Skin sparing mastectomy and robotic latissimus dorsi-flap reconstruction through a single incision. World J. Surg. Oncol. 2019, 17, 176. [Google Scholar] [CrossRef] [PubMed]
- Houvenaeghel, G.; Bannier, M.; Rua, S.; Barrou, J.; Heinemann, M.; Van Troy, A.; Lambaudie, E.; Cohen, M. Breast cancer robotic nipple sparing mastectomy: Evaluation of several surgical procedures and learning curve. World J. Surg. Oncol. 2019, 17, 27. [Google Scholar] [CrossRef]
- Houvenaeghel, G.; Cohen, M.; Ribeiro, S.R.; Barrou, J.; Heinemann, M.; Frayret, C.; Lambaudie, E.; Bannier, M. Robotic Nipple-Sparing Mastectomy and Immediate Breast Reconstruction with Robotic Latissimus Dorsi Flap Harvest: Technique and Results. Surg. Innov. 2020, 27, 481–491. [Google Scholar] [CrossRef]
- Houvenaeghel, G.; El Hajj, H.; Schmitt, A.; Cohen, M.; Rua, S.; Barrou, J.; Lambaudie, E.; Bannier, M. Robotic-assisted skin sparing mastectomy and immediate reconstruction using latissimus dorsi flap a new effective and safe technique: A comparative study. Surg. Oncol. 2020, 35, 406–411. [Google Scholar] [CrossRef]
- Moon, K.C.; Yeo, H.D.; Yoon, E.S.; Lee, B.I.; Park, S.H.; Chung, J.H.; Lee, H.C. Robotic-assisted latissimus dorsi muscle flap for autologous chest reconstruction in poland syndrome. J. Plast. Reconstr. Aesthet. Surg. 2020, 73, 1506–1513. [Google Scholar] [CrossRef]
- Winocour, S.; Tarassoli, S.; Chu, C.K.; Liu, J.; Clemens, M.W.; Selber, J.C. Comparing Outcomes of Robotically Assisted Latissimus Dorsi Harvest to the Traditional Open Approach in Breast Reconstruction. Plast. Reconstr. Surg. 2020, 146, 1221–1225. [Google Scholar] [CrossRef]
- Cheon, J.H.; Kim, H.E.; Park, S.H.; Yoon, E.S. Ten-year experience of robotic latissimus muscle flap reconstructive surgery at a single institution. J. Plast. Reconstr. Aesthet. Surg. 2022, 75, 3664–3672. [Google Scholar] [CrossRef]
- Hwang, Y.J.; Chung, J.H.; Lee, H.C.; Park, S.H.; Yoon, E.S. Single-Port Transaxillary Robot-Assisted Latissimus Dorsi Muscle Flap Reconstruction for Poland Syndrome: Concomitant Application of Robotic System to Contralateral Augmentation Mammoplasty. Arch. Plast. Surg. 2022, 49, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Kurlander, D.E.; Le-Petross, H.T.; Shuck, J.W.; Butler, C.E.; Selber, J.C. Robotic DIEP Patient Selection: Analysis of CT Angiography. Plast. Reconstr. Surg. Glob. Open 2021, 9, e3970. [Google Scholar] [CrossRef] [PubMed]
- Rezania, N.; Harmon, K.A.; Frauchiger-Ankers, R.; La-Anyane, O.; Idrizi, K.; To, J.; Ritz, E.M.; Kurlander, D.E.; Shenaq, D.; Kokosis, G. A DIEP Dive into Patient Risk Factors for Hernia and Bulge Development: A Meta-regression. J. Reconstr. Microsurg. 2024, 41, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Selber, J.C. (Ed.) Robotics in Plastic and Reconstructive Surgery; Springer Nature Switzerland AG: Cham, Switzerland, 2021; Volume 1, p. 150. [Google Scholar]
- Choi, J.H.; Song, S.Y.; Park, H.S.; Kim, C.H.; Kim, J.Y.; Lew, D.H.; Roh, T.S.; Lee, D.W. Robotic DIEP Flap Harvest through a Totally Extraperitoneal Approach Using a Single-Port Surgical Robotic System. Plast. Reconstr. Surg. 2021, 148, 304–307. [Google Scholar] [CrossRef]
- Piper, M.; Ligh, C.A.; Shakir, S.; Messa, C.; Soriano, I.; Kanchwala, S. Minimally invasive robotic-assisted harvest of the deep inferior epigastric perforator flap for autologous breast reconstruction. J. Plast. Reconstr. Aesthet. Surg. 2021, 74, 890–930. [Google Scholar] [CrossRef]
- Shakir, S.; Spencer, A.B.; Piper, M.; Kozak, G.M.; Soriano, I.S.; Kanchwala, S.K. Laparoscopy allows the harvest of the DIEP flap with shorter fascial incisions as compared to endoscopic harvest: A single surgeon retrospective cohort study. J. Plast. Reconstr. Aesthet. Surg. 2021, 74, 1203–1212. [Google Scholar] [CrossRef]
- Wittesaele, W.; Vandevoort, M. Implementing the Robotic deep inferior epigastric perforator Flap in daily practice: A series of 10 cases. J. Plast. Reconstr. Aesthet. Surg. 2022, 75, 2577–2583. [Google Scholar] [CrossRef]
- Lee, M.J.; Won, J.; Song, S.Y.; Park, H.S.; Kim, J.Y.; Shin, H.J.; Kwon, Y.I.; Lee, D.W.; Kim, N.Y. Clinical outcomes following robotic versus conventional DIEP flap in breast reconstruction: A retrospective matched study. Front. Oncol. 2022, 12, 989231. [Google Scholar] [CrossRef]
- Jung, J.H.; Jeon, Y.R.; Lee, D.W.; Park, H.S.; Lew, D.H.; Roh, T.S.; Song, S.Y. Initial report of extraperitoneal pedicle dissection in deep inferior epigastric perforator flap breast reconstruction using the da Vinci SP. Arch. Plast. Surg. 2022, 49, 34–38. [Google Scholar] [CrossRef]
- Bishop, S.N.; Asaad, M.; Liu, J.; Chu, C.K.; Clemens, M.W.; Kapur, S.S.; Largo, R.D.; Selber, J.C. Robotic Harvest of the Deep Inferior Epigastric Perforator Flap for Breast Reconstruction: A Case Series. Plast. Reconstr. Surg. 2022, 149, 1073–1077. [Google Scholar] [CrossRef]
- Tsai, C.Y.; Kim, B.S.; Kuo, W.L.; Liu, K.H.; Chang, T.N.; Cheong, D.C.; Huang, J.J. Novel Port Placement in Robot-Assisted DIEP Flap Harvest Improves Visibility and Bilateral DIEP Access: Early Controlled Cohort Study. Plast. Reconstr. Surg. 2023, 152, 590e–595e. [Google Scholar] [CrossRef] [PubMed]
- Murariu, D.; Chen, B.; Bailey, E.; Nelson, W.; Fortunato, R.; Nosik, S.; Moreira, A. Transabdominal Robotic Harvest of Bilateral DIEP Pedicles in Breast Reconstruction: Technique and Interdisciplinary Approach. J. Reconstr. Microsurg. 2024. [Google Scholar] [CrossRef] [PubMed]
- Nelson, W.; Murariu, D.; Moreira, A.A. Indocyanine Green-Guided Near-Infrared Fluorescence Enhances Vascular Anatomy in Robot-Assisted DIEP Flap Harvest. Plast. Reconstr. Surg. 2024, 153, 796–798. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.; Chen, B.; Bailey, E.; Nelson, W.; Murariu, D. Learning Curve Analysis for Robotic-assisted Harvest of Deep Inferior Epigastric Perforator Flap. Plast. Reconstr. Surg. Glob. Open 2024, 12, e6242. [Google Scholar] [CrossRef]
- Moreira, A.; Bailey, E.A.; Chen, B.; Nelson, W.; Li, J.; Fortunato, R.; Nosik, S.; Murariu, D. A New Era in Perforator Flap Surgery for Breast Reconstruction: A Comparative Study of Robotic versus Standard Harvest of Bilateral Deep Inferior Epigastric Artery Perforator Flaps. J. Reconstr. Microsurg. 2024. [Google Scholar] [CrossRef]
- Ibrahim, A.E.; Sarhane, K.A.; Selber, J.C. New Frontiers in Robotic-Assisted Microsurgical Reconstruction. Clin. Plast. Surg. 2017, 44, 415–423. [Google Scholar] [CrossRef]
- Vollbach, F.H.; Bigdeli, A.K.; Struebing, F.; Weigel, J.L.; Gazyakan, E.; Kneser, U. Using a Microsurgical Robotic Platform for In-flap Anastomosis in Autologous Bipedicular Breast Reconstruction. Plast. Reconstr. Surg. Glob. Open 2024, 12, e5511. [Google Scholar] [CrossRef]
- Tanna, N.; Sugiyama, G.; Smith, M.L.; Sanchez, S.B.; Minasian, R.A.; Robinson, E.; Silverman, J.; Shuck, J.W.; Selber, J. The Full Continuum of Robotic Breast Surgery: Robotic-assisted Mastectomy, Robotic DIEP Flap, and Robotic Supermicrosurgery. Plast. Reconstr. Surg. Glob. Open 2023, 11, e5491. [Google Scholar] [CrossRef]
- Kueckelhaus, M. Minimally Invasive Robotic-assisted Perforator-to-Perforator DIEP Flap Breast Reconstruction. Plast. Reconstr. Surg. Glob. Open 2024, 12, e5800. [Google Scholar] [CrossRef]
- Dastagir, N.; Obed, D.; Tamulevicius, M.; Dastagir, K.; Vogt, P.M. The Use of the Symani Surgical System® in Emergency Hand Trauma Care. Surg. Innov. 2024, 31, 460–465. [Google Scholar] [CrossRef]
- Van Mulken, T.J.M.; Wolfs, J.A.G.N.; Qiu, S.S.; Scharmga, A.M.J.; Schols, R.M.; Spiekerman van Weezelenburg, M.A.; Cau, R.; van der Hulst, R.R.W.J.; Group, M.R.R. One-Year Outcomes of the First Human Trial on Robot-Assisted Lymphaticovenous Anastomosis for Breast Cancer-Related Lymphedema. Plast. Reconstr. Surg. 2022, 149, 151–161. [Google Scholar] [CrossRef]
| Source | Number of Robotic Flaps | Number of Open Flaps | Incisional Length (Robotic) (Avg) | Console Time (Avg) | Surgery Duration (Robotic) (Avg) | Surgery Duration (Open) (Avg) | Hospital Stay (Robot) (Avg Days) | Hospital Stay (Open) (Avg Days) | Complication Rate (Robot) | Complication Rate (Open) | Patient Satisfaction Analysis |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Selber 2012 Case series [30] | 8 | 0 | 5 cm | 111 min | NR | NR | NR | NR | 1: Transient peripheral neuropathy | NR | NR |
| Clemens 2014 Retrospective cohort study [31] | 12 | 64 | 5 cm | NR | NR | NR | 2.7 Days | 3.4 Days | 16.7% Total (p = 0.31), Seroma 8.3%, Infection 14.1%, Re-operation 8.3% | Seroma 8.9%, Delayed healing 7.8%, Infection 8.3%, re-operation 12.5%, Capsular contracture 4.7% | NR |
| Chung 2015 Retrospective case series [28] | 12 | 0 | 5.5 cm | 85.8 min | NR | NR | NR | NR | One patient dissatisfied with upper inner quadrant appearance, resolved with 3x outpatient fat grafting | NR | 9.2 for breast symmetry, 9.9 for scar and 9.6 for general aesthetic result out of 10 |
| Lai 2018 Case report [32] | 1 | 0 | 4 cm | 97 min | NR | NR | NR | NR | 1× seroma; 100% | NR | NR |
| Houvenaeghel 2019 Prospective [33,34,35,36] | 80 | 0 | 5.5 cm | NR | 310 min | NR | 4 Days | NR | 41.10%. Grade 1: 29 seromas. Grade 3: 1 = bleeding requiring re-operation | NR | NR |
| Houvenaeghel 2020 Prospective case–control [37] | 46 | 59 | 5. 5 cm | NR | 290.5 min | 259.7 min open p = 0.016 | 4.3 Days | 3.8 Days p = 0.089 | 45.70% Total. 12 Seroma, 1 bleeding, 2 dorsal infection, 2 re-operation | 62.70% Total. 23 seroma, 2 bleeding, 1 re-operation | NR |
| Moon 2020 Prospective case series [38] | 21 | 0 | 5.5 cm | 58 min | 295 min | NR | 7 Days | NR | 4x Seromas all resolved w/aspiration, 1× axillary wound complication | NR | improvement in chest deformity, satisfaction with overall outcomes, chest symmetry, and scars were 4.80, 4.72, 4.18, and 4.87 out of 5 |
| Wincour 2020 Retrospective cohort study [39] | 25 | 27 | 5.5 cm | NR | 388 min | 311 min | 2 Days | 3 Days | 16% Seroma | 3% Hematoma | NR |
| Joo 2021 Case report SP [27] | 1 | 0 | 4.5 cm | 100 min | 328 min | NR | 6 Days | NR | 0% | NR | Breast-Q 67/100. Avg test score is 55 |
| Cheon 2022 Retrospective cohort series SP [40] | 41 Total: 13S, 18Xi 10Sp | 0 | 4.5 cm | Si 85 min, Xi 64 min, SP 49 min, Total 70 min | Si 399 min, Xi 451 min, SP 496 min, total 439 min | NR | Si 12.6, Xi 9.7, Sp 7, Total 9.0 | NR | Si: 1 infection (7.7%), 5 seroma (38.5%), 2 donor site morbidity (15.4%), 8 wound problems (61.5%). Xi: 2 infections (11.1%), 6 seroma (33.3%), 1 flap loss (5.6%), 4 donor site morbidities (22.2%), 10 wound-related problems (55.6%). Sp: 1 hematoma (10%), 2 seroma (20%), 2 donor site morbidities (20%), 2 wound-related problems (20%) | NR | Harris breast scale, no significant difference among groups but Sp patients did have higher scores |
| Hwang 2022 Retrospective case series SP [41] | 3 | 0 | 5 cm | NR | 449 min | NR | NR | NR | 0% | NR | NR |
| Eo 2023 Prospective Cohort study (Also included 17 endoscopically harvested LD) [16] | 20 | 20 | 4 cm | 75.7 min, Endoscopic 34.5 min p < 0.001 | 394.4 min, 316.6 min endoscopic | 279 min | 10.2, 10.8 endoscopic | 9.75 | 15% robotic, 11.8% endoscopic; 3 seroma (robotic), 2 seroma (endoscopic), 1 wound dehiscence each | 20% Total; 3 Seroma (15%), 1 wound dehischence (5%). | Modified BREAST-Q: Significantly lower satisfaction with scar on the back and overall between open vs. robotic/endoscopic. No significant difference between endoscopic and robotic. |
| Source | Number of Robotic Flaps | Number of Open Flaps | Fascial Incisional Length (Avg) | Surgery Duration (Avg) | Follow-Up Interval |
|---|---|---|---|---|---|
| Gundlapalli 2018 Case report TAP [24] | 1 | NR | 1.5 cm | 531 min | 9 Mos |
| Selber 2021 Technique TAP [44] | NR | NR | 1.5–3.0 cm | NR | NR |
| Choi 2021 Retrospective case series TEP [45] | 17 | 4 | 1.5 cm | 487 min | NR |
| Kurlander 2021 Retrospective case review TAP [42] | 13 | 49 | 3.5 cm | NR | NR |
| Piper 2021 Case series TAP [46] | 8 | NR | NR | NR | NR |
| Shakir 2021 Retrospective cohort study TAP/TEP [47] | 3 | 94 endoscopic, 38 TEP laparoscopic | 2.0 TEPLAP, 4.5 endoscopic, NR robotic | 249 min unilateral endoscopic, 535 min b/l endo, 335 min unilateral TEPLAP, 453 min b/l TEPLAP, 535 min TAPRAP | 8 Mos |
| Wittesaele 2021 Case series TAP [48] | 10 | NR | 3 cm | 479 min | 2 weeks and 6 weeks |
| Lee 2022 Retrospective cohort study TEP SP [49] | 21 | 186 | 4.3 | 509 min robotic, 438 open | NR |
| Jung 2022 Case report TEP SP [50] | 1 | 0 | 5 cm | NR | 7 Mos |
| Bishop 2022 Case series TAP [51] | 21 | 0 | 3.6 cm | 425 min unilateral, 511 bilateral min | 5 Mos |
| Tsai 2023 Retrospective cohort study TAP [52] | 13 (11 unilateral 2 bilateral) | 86 (62 unilateral 24 bilateral) | 2.7 cm vs. 8.1 open (p < 0.0001) | ~100 additional minutes robotic | 3 days ICU, 2, 4, 12 weeks |
| Murariu 2024 Retrospective cohort study TAP [53,54] | 46 (All bilateral robot) | NR | 4.1 cm | 739 min | 90-day follow-up |
| Moreira 2024 Retrospective case series TAP [55,56] | 46 | 48 (total flaps including bilateral) | 4.1 cm robot vs. 11.7 cm open (p < 0.001) | 739 min robotic, 683 min open | 90 days, 284.6 days robot, 357.6 days open |
| Source | Hospital Stay (Robot) (Avg) | Hospital Stay (Open) (Avg) | Complication Rate (Robot) | Complication Rate (Open) | Patient Satisfaction | Cost Analysis |
|---|---|---|---|---|---|---|
| Gundlapalli 2018 Case report TAP [24] | NR | NR | 0% | NR | NR | USD 16,300 robotic, USD 14,800 open |
| Selber 2021 Technique TAP [44] | NR | NR | NR | NR | NR | NR |
| Choi 2021 Retrospective case series TEP [45] | NR | NR | NR | NR | NR | NR |
| Piper 2021 Case series TAP [46] | 4 Days | NR | NR | NR | NR | NR |
| Shakir 2021 Retrospective cohort study TAP/TEP [47] | 4.7 Days | 2.8 endoscopic 2.5 TEPLAP | NR | Pedicle injury flap loss ×1, 1× abdominal bulge endoscopic | NR | ∼USD 234 per case of disposable cost, ∼USD 495 for TEP-LAP harvest, and ∼USD 1487 for RAP harvest. |
| Wittesaele 2021 Case series TAP [48] | NR | NR | 1× Chest hematoma requiring drainage | NR | NR | NR |
| Lee 2022 Retrospective cohort study TEP SP [49] | 7.92 Days | 8.77 | 5.3% (1) Flap loss, 5.3% (1) Fat necrosis | 2.2% Flap loss (4), 1.1% (2) fat necrosis, 1.1% (2) Seroma, 6.5% (12) Donor site wound problem | Breast-Q: 16 Robot, 59 Open. Robotic group had significantly higher scores for post-operative psychosocial well-being (p = 0.007), physical well-being of the chest (p = 0.028), and physical well-being of the abdomen (p = 0.02) | NR |
| Jung 2022 Case report TEP SP [50] | 7 Days | NR | 0% | NR | NR | NR |
| Bishop 2022 Case series TAP [51] | 3.8 Days | NR | 31.3% (5) Surgical site occurrence, 1× wound healing complication | NR | Five patients had bilateral flaps, one harvested robotically and the other open and patient was blinded. Four in five patients reported less pain on the robotic side. | NR |
| Tsai 2023 Retrospective cohort study TAP [52] | 3 Days ICU | 3 Days ICU | 1/13 Wound healing minor complication | 2/86: Minor wound complications | No significant difference in post-operative pain | ~USD 3500 increase in robot instruments and disposables |
| Murariu 2024 Retrospective cohort study TAP [53,54] | 3.9 | NR | 1× Partial flap necrosis, 1× abdominal wound complication | NR | NR | NR |
| Moreira 2024 Retrospective case series TAP [55,56] | 3.9 | 4.3 | 1 Partial flap loss, 1 return to OR, 7 wound-related issues, 2 readmission for any reason | 2 Abdominal bulges requiring intervention at revision surgery. 2 partial flap loss/return to OR, 3 readmissions any reason, 1 readmission for flap, 8 wound healing issues | NR | NR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allen, B.; Knutson, A.; Iftekhar, N.; Giles, C.; Patterson, J.; MacDavid, J.; Baynosa, R. The Present and Future of Robotic Surgery in Breast Cancer and Breast Reconstruction. J. Clin. Med. 2025, 14, 2100. https://doi.org/10.3390/jcm14062100
Allen B, Knutson A, Iftekhar N, Giles C, Patterson J, MacDavid J, Baynosa R. The Present and Future of Robotic Surgery in Breast Cancer and Breast Reconstruction. Journal of Clinical Medicine. 2025; 14(6):2100. https://doi.org/10.3390/jcm14062100
Chicago/Turabian StyleAllen, Brett, Alexis Knutson, Noama Iftekhar, Casey Giles, Jarrell Patterson, Joshua MacDavid, and Richard Baynosa. 2025. "The Present and Future of Robotic Surgery in Breast Cancer and Breast Reconstruction" Journal of Clinical Medicine 14, no. 6: 2100. https://doi.org/10.3390/jcm14062100
APA StyleAllen, B., Knutson, A., Iftekhar, N., Giles, C., Patterson, J., MacDavid, J., & Baynosa, R. (2025). The Present and Future of Robotic Surgery in Breast Cancer and Breast Reconstruction. Journal of Clinical Medicine, 14(6), 2100. https://doi.org/10.3390/jcm14062100






