A Retrospective Review of the Deep Parasternal Intercostal Plane Block in Patients Undergoing Cardiac Surgery with Median Sternotomy
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Intraoperative Management
2.3. Block Technique
2.4. Postoperative Period
2.5. Neurocognitive Function
2.6. Data
2.7. Statistical Analysis
3. Results
4. Discussion
4.1. Previous Literature
4.2. PSB Technique
4.3. Adjuvants
4.4. Timing: Pre vs. Post Sternotomy
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ERACS | Enhanced Recovery After Cardiac Surgery |
| Deep-PIP | Deep parasternal-intercostal plane |
| TTMPB | Transversus thoracic muscle plane block |
| PSB | Parasternal block |
| CABG | Coronary artery bypass grafting |
| BMI | Body mass index |
| CTICU | Cardiothoracic intensive care unit |
| EMR | Electronic medical record |
| RASS | Richmond agitation sedation scale |
| CPOT | Critical care pain observation tool |
| VAS | Visual analogue scale |
| FEV1 | Forced expiratory volume in 1 s |
| OSA | Obstructive sleep apnea |
| LVEF | Left ventricular ejection fraction |
| PASP | Pulmonary artery systolic pressure |
| CPB | Cardiopulmonary bypass |
| ACC | Aortic cross clamp |
| MME | Morphine milligram equivalents |
| AUC24 | Area under the curve (24 h) |
References
- Mondal, S.; Bergbower, E.A.S.; Cheung, E.; Grewal, A.S.; Ghoreishi, M.; Hollander, K.N.; Anders, M.G.; Taylor, B.S.; Tanaka, K.A. Role of Cardiac Anesthesiologists in Intraoperative Enhanced Recovery After Cardiac Surgery (ERACS) Protocol: A Retrospective Single-Center Study Analyzing Preliminary Results of a Yearlong ERACS Protocol Implementation. J. Cardiothorac. Vasc. Anesth. 2023, 37, 2450–2460. [Google Scholar] [CrossRef] [PubMed]
- Grant, M.C.; Crisafi, C.; Alvarez, A.; Arora, R.C.; Brindle, M.E.; Chatterjee, S.; Ender, J.; Fletcher, N.; Gregory, A.J.; Gunaydin, S.; et al. Perioperative Care in Cardiac Surgery: A Joint Consensus Statement by the Enhanced Recovery After Surgery (ERAS) Cardiac Society, ERAS International Society, and The Society of Thoracic Surgeons (STS). Ann. Thorac. Surg. 2024, 117, 669–689. [Google Scholar] [CrossRef] [PubMed]
- Mertes, P.-M.; Kindo, M.; Amour, J.; Baufreton, C.; Camilleri, L.; Caus, T.; Chatel, D.; Cholley, B.; Curtil, A.; Grimaud, J.-P.; et al. Guidelines on Enhanced Recovery after Cardiac Surgery under Cardiopulmonary Bypass or Off-Pump. Anaesth. Crit. Care Pain Med. 2022, 41, 101059. [Google Scholar] [CrossRef] [PubMed]
- Ball, M.; Falkson, S.; Fakoya, A. Anatomy, Angle of Louis. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK459336/ (accessed on 2 October 2024).
- El-Boghdadly, K.; Wolmarans, M.; Stengel, A.D.; Albrecht, E.; Chin, K.J.; Elsharkawy, H.; Kopp, S.; Mariano, E.R.; Xu, J.L.; Adhikary, S.; et al. Standardizing Nomenclature in Regional Anesthesia: An ASRA-ESRA Delphi Consensus Study of Abdominal Wall, Paraspinal, and Chest Wall Blocks. Reg. Anesth. Pain Med. 2021, 46, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.J.; Versyck, B.; Elsharkawy, H.; Rojas Gomez, M.F.; Sala-Blanch, X.; Reina, M.A. Anatomical Basis of Fascial Plane Blocks. Reg. Anesth. Pain Med. 2021, 46, 581–599. [Google Scholar] [CrossRef]
- Chin, K.J.; Lirk, P.; Hollmann, M.W.; Schwarz, S.K.W. Mechanisms of Action of Fascial Plane Blocks: A Narrative Review. Reg. Anesth. Pain Med. 2021, 46, 618–628. [Google Scholar] [CrossRef]
- Ritter, M.J.; Christensen, J.M.; Yalamuri, S.M. Regional Anesthesia for Cardiac Surgery. Adv. Anesth. 2021, 39, 215–240. [Google Scholar] [CrossRef]
- Hong, B.; Oh, C.; Jo, Y.; Lee, S.; Park, S.; Kim, Y.-H. Current Evidence of Ultrasound Guided Fascial Plane Blocks for Cardiac Surgery: A Narrative Literature Review. Korean J. Anesthesiol. 2022, 75, 460–472. [Google Scholar] [CrossRef]
- Fujii, S.; Roche, M.; Jones, P.M.; Vissa, D.; Bainbridge, D.; Zhou, J.R. Transversus Thoracis Muscle Plane Block in Cardiac Surgery: A Pilot Feasibility Study. Reg. Anesth. Pain Med. 2019, 44, 556–560. [Google Scholar] [CrossRef]
- Sepolvere, G.; Tognù, A.; Tedesco, M.; Coppolino, F.; Cristiano, L. Avoiding the Internal Mammary Artery During Parasternal Blocks: Ultrasound Identification and Technique Considerations. J. Cardiothorac. Vasc. Anesth. 2021, 35, 1594–1602. [Google Scholar] [CrossRef]
- Aydin, M.E.; Ahiskalioglu, A.; Ates, I.; Tor, I.H.; Borulu, F.; Erguney, O.D.; Celik, M.; Dogan, N. Efficacy of Ultrasound-Guided Transversus Thoracic Muscle Plane Block on Postoperative Opioid Consumption After Cardiac Surgery: A Prospective, Randomized, Double-Blind Study. J. Cardiothorac. Vasc. Anesth. 2020, 34, 2996–3003. [Google Scholar] [CrossRef] [PubMed]
- Murata, H.; Hida, K.; Hara, T. Transverse Thoracic Muscle Plane Block: Tricks and Tips to Accomplish the Block. Reg. Anesth. Pain Med. 2016, 41, 411–412. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, E.; Vorobeichik, L.; Jacot-Guillarmod, A.; Fournier, N.; Abdallah, F.W. Dexamethasone Is Superior to Dexmedetomidine as a Perineural Adjunct for Supraclavicular Brachial Plexus Block: Systematic Review and Indirect Meta-analysis. Anesth. Analg. 2019, 128, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Ammar, A.; Mahmoud, K. Effect of adding dexamethasone to bupivacaine on transversus abdominis plane block for abdominal hysterectomy: A prospective randomized controlled trial. Saudi J. Anaesth. 2012, 6, 229. [Google Scholar] [CrossRef]
- Gao, Z.; Xiao, Y.; Wang, Q.; Li, Y. Comparison of dexmedetomidine and dexamethasone as adjuvant for ropivacaine in ultrasound-guided erector spinae plane block for video-assisted thoracoscopic lobectomy surgery: A randomized, double-blind, placebo-controlled trial. Ann. Transl. Med. 2019, 7, 668. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, Z.; Feng, C.; Jin, Y.; Zhao, X. Dexmedetomidine as an Adjuvant in Peripheral Nerve Block. Drug Des. Dev. Ther. 2023, 17, 1463–1484. [Google Scholar] [CrossRef]
- Tan, S.; Lee, E.; Lee, S.; Sakaria, S.; Roh, J. Morphine Equianalgesic Dose Chart in the Emergency Department. J. Educ. Teach. Emerg. Med. 2022, 7, L1–L20. [Google Scholar] [CrossRef]
- McPherson, M.L. (Ed.) Demystifying Opioid Conversion Calculations: A Guide for Effective Dosing; American Society of Health-System Pharmacists: Bethesda, MD, USA, 2018; ISBN 978-1-58528-430-6. [Google Scholar]
- Nielsen, S.; Degenhardt, L.; Hoban, B.; Gisev, N. A Synthesis of Oral Morphine Equivalents (OME) for Opioid Utilisation Studies. Pharmacoepidemiol. Drug Saf. 2016, 25, 733–737. [Google Scholar] [CrossRef]
- Barr, J.; Zomorodi, K.; Bertaccini, E.J.; Shafer, S.L.; Geller, E. A Double-Blind, Randomized Comparison of IV Lorazepam versus Midazolam for Sedation of ICU Patients via a Pharmacologic Model. Anesthesiology 2001, 95, 286–298. [Google Scholar] [CrossRef]
- Loria, C.M.; Zborek, K.; Millward, J.B.; Anderson, M.P.; Richardson, C.M.; Namburi, N.; Faiza, Z.; Timsina, L.R.; Lee, L.S. Enhanced Recovery after Cardiac Surgery Protocol Reduces Perioperative Opioid Use. JTCVS Open 2022, 12, 280–296. [Google Scholar] [CrossRef]
- Li, J.; Lin, L.; Peng, J.; He, S.; Wen, Y.; Zhang, M. Efficacy of Ultrasound-Guided Parasternal Block in Adult Cardiac Surgery: A Meta-Analysis of Randomized Controlled Trials. Minerva Anestesiol. 2022, 88, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.J.; Versyck, B.; Pawa, A. Ultrasound-guided Fascial Plane Blocks of the Chest Wall: A State-of-the-art Review. Anaesthesia 2021, 76, 110–126. [Google Scholar] [CrossRef] [PubMed]
- Amour, J.; Cholley, B.; Ouattara, A.; Longrois, D.; Leprince, P.; Fellahi, J.-L.; Riou, B.; Hariri, S.; Latrémouille, C.; Rémy, A.; et al. The Effect of Local Anesthetic Continuous Wound Infusion for the Prevention of Postoperative Pneumonia after On-Pump Cardiac Surgery with Sternotomy: The STERNOCAT Randomized Clinical Trial. Intensive Care Med. 2019, 45, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Song, W.; Wang, W.; Peng, Y.; Zhai, C.; Yao, L.; Xia, Z. Ultrasound-Guided Parasternal Intercostal Nerve Block for Postoperative Analgesia in Mediastinal Mass Resection by Median Sternotomy: A Randomized, Double-Blind, Placebo-Controlled Trial. BMC Anesthesiol. 2021, 21, 98. [Google Scholar] [CrossRef]
- Nasr, D.; Abdelhamid, H.; Mohsen, M.; Aly, A. The Analgesic Efficacy of Continuous Presternal Bupivacaine Infusion through a Single Catheter after Cardiac Surgery. Ann. Card. Anaesth. 2015, 18, 15. [Google Scholar] [CrossRef]
- Bloc, S.; Perot, B.P.; Gibert, H.; Law Koune, J.-D.; Burg, Y.; Leclerc, D.; Vuitton, A.-S.; De La Jonquière, C.; Luka, M.; Waldmann, T.; et al. Efficacy of Parasternal Block to Decrease Intraoperative Opioid Use in Coronary Artery Bypass Surgery via Sternotomy: A Randomized Controlled Trial. Reg. Anesth. Pain Med. 2021, 46, 671–678. [Google Scholar] [CrossRef]
- Khera, T.; Murugappan, K.R.; Leibowitz, A.; Bareli, N.; Shankar, P.; Gilleland, S.; Wilson, K.; Oren-Grinberg, A.; Novack, V.; Venkatachalam, S.; et al. Ultrasound-Guided Pecto-Intercostal Fascial Block for Postoperative Pain Management in Cardiac Surgery: A Prospective, Randomized, Placebo-Controlled Trial. J. Cardiothorac. Vasc. Anesth. 2021, 35, 896–903. [Google Scholar] [CrossRef]
- Kumar, A.K.; Chauhan, S.; Bhoi, D.; Kaushal, B. Pectointercostal Fascial Block (PIFB) as a Novel Technique for Postoperative Pain Management in Patients Undergoing Cardiac Surgery. J. Cardiothorac. Vasc. Anesth. 2021, 35, 116–122. [Google Scholar] [CrossRef]
- Vilvanathan, S.; Saravanababu, M.; Sreedhar, R.; Gadhinglajkar, S.; Dash, P.; Sukesan, S. Ultrasound-Guided Modified Parasternal Intercostal Nerve Block: Role of Preemptive Analgesic Adjunct for Mitigating Poststernotomy Pain. Anesth. Essays Res. 2020, 14, 300. [Google Scholar] [CrossRef]
- Mansour, M.A.; Mahmoud, H.E.; Fakhry, D.M.; Kassim, D.Y. Comparison of the Effects of Transversus Thoracic Muscle Plane Block and Pecto-Intercostal Fascial Block on Postoperative Opioid Consumption in Patients Undergoing Open Cardiac Surgery: A Prospective Randomized Study. BMC Anesthesiol. 2024, 24, 63. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Chen, S. Bilateral Transversus Thoracis Muscle Plane Block Provides Effective Analgesia and Enhances Recovery after Open Cardiac Surgery. J. Card. Surg. 2021, 36, 2818–2823. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.M.K.; Chen, P.Y.; Tang, G.C.C.; Chiu, S.L.C.; Mok, L.Y.H.; Au, S.S.W.; Wong, R.H.L. Deep Parasternal Intercostal Plane Block for Intraoperative Pain Control in Cardiac Surgical Patients for Sternotomy: A Prospective Randomized Controlled Trial. J. Cardiothorac. Vasc. Anesth. 2024, 38, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Abdelbaser, I.I.; Mageed, N.A. Analgesic Efficacy of Ultrasound Guided Bilateral Transversus Thoracis Muscle Plane Block in Pediatric Cardiac Surgery: A Randomized, Double-Blind, Controlled Study. J. Clin. Anesth. 2020, 67, 110002. [Google Scholar] [CrossRef]
- Barr, A.M.; Tutungi, E.; Almeida, A.A. Parasternal Intercostal Block with Ropivacaine for Pain Management After Cardiac Surgery: A Double-Blind, Randomized, Controlled Trial. J. Cardiothorac. Vasc. Anesth. 2007, 21, 547–553. [Google Scholar] [CrossRef]
- King, M.; Stambulic, T.; Hassan, S.M.A.; Norman, P.A.; Derry, K.; Payne, D.M.; El Diasty, M. Median Sternotomy Pain after Cardiac Surgery: To Block, or Not? A Systematic Review and Meta-analysis. J. Card. Surg. 2022, 37, 3729–3742. [Google Scholar] [CrossRef]
- Dost, B.; De Cassai, A.; Balzani, E.; Tulgar, S.; Ahiskalioglu, A. Effects of Ultrasound-Guided Regional Anesthesia in Cardiac Surgery: A Systematic Review and Network Meta-Analysis. BMC Anesthesiol. 2022, 22, 409. [Google Scholar] [CrossRef]
- Samerchua, A.; Leurcharusmee, P.; Supphapipat, K.; Unchiti, K.; Lapisatepun, P.; Maikong, N.; Kantakam, P.; Navic, P.; Mahakkanukrauh, P. Optimal Techniques of Ultrasound-Guided Superficial and Deep Parasternal Intercostal Plane Blocks: A Cadaveric Study. Reg. Anesth. Pain Med. 2024, 49, 320–325. [Google Scholar] [CrossRef]
- Fujii, S.; Vissa, D.; Ganapathy, S.; Johnson, M.; Zhou, J. Transversus Thoracic Muscle Plane Block on a Cadaver with History of Coronary Artery Bypass Grafting. Reg. Anesth. Pain Med. 2017, 42, 535–537. [Google Scholar] [CrossRef]
- Pehora, C.; Pearson, A.M.; Kaushal, A.; Crawford, M.W.; Johnston, B. Dexamethasone as an Adjuvant to Peripheral Nerve Block. Cochrane Database Syst. Rev. 2017, 11, CD011770. [Google Scholar] [CrossRef]
- Zhao, Y.; He, J.; Yu, N.; Jia, C.; Wang, S. Mechanisms of Dexmedetomidine in Neuropathic Pain. Front. Neurosci. 2020, 14, 330. [Google Scholar] [CrossRef]
- Chapman, B.C.; Shepherd, B.; Moore, R.; Stanley, D.J.; Nelson, E.C. Dual Adjunct Therapy with Dexamethasone and Dexmedetomidine in Transversus Abdominis Plane Blocks Reduces Postoperative Opioid Use in Colorectal Surgery. Am. J. Surg. 2021, 222, 198–202. [Google Scholar] [CrossRef]

| IV Dose | Oral Dose | Equivalent IV Morphine | Equivalent Oral Morphine | |
|---|---|---|---|---|
| Morphine | 10 mg | 30 mg | 10 mg | 30 mg |
| Hydromorphone | 1.5 mg | 7.5 mg | 10 mg | 30 mg |
| Fentanyl | 0.1 mg (100 ug) | NA | 10 mg | 30 mg |
| Oxycodone | NA | 20 mg | NA | 30 mg |
| NOBlock n = 351 | BLOCK n = 232 | Standardized Difference | |
|---|---|---|---|
| Sex | |||
| Female | 25.6% | 22.0% | 0.0860 |
| Male | 74.4% | 78.0% | −0.0860 |
| Age (years) Mean (Median) ± SD | 67.0 (68.0) ± 10.2 | 66.3 (66.0) ± 9.8 | 0.0732 |
| BMI (kg/m2) Mean (Median) ± SD | 30.0 (29.4) ± 5.9 | 29.7 (28.7) ± 5.2 | 0.0588 |
| Organ Function | |||
| Lung Disease | 17.38% | 13.36% | 0.1115 |
| OSA | 18.52% | 15.52% | 0.0799 |
| Home O2 | 0.28% | 0.00% | 0.0756 |
| Dialysis | 1.99% | 0.86% | 0.0955 |
| Liver Disease | 2.85% | 1.72% | 0.0753 |
| Creatinine (mg/dL) Mean (Median) ± SD | 1.1 (0.9) ± 0.8 | 1.0 (0.9) ± 0.7 | 0.1124 |
| LVEF (%) Mean (Median) ± SD | 55.5 (60.0) ± 11.5 | 56.6 (59.0) ± 10.6 | −0.0979 |
| PASP (mmHg) Mean (Median) ± SD | 34.1 (30.0) ± 13.5 | 33.2 (30.0) ± 12.9 | 0.0692 |
| Procedure Type | |||
| CABG | 58.97% | 60.34% | −0.0279 |
| CABG+ | 8.26% | 6.90% | 0.0516 |
| Non-CABG | 32.76% | 32.76% | 0.0001 |
| CPB Time (mins) Mean (Median) ± SD | 113.9 (106.0) ± 41.8 | 113.2 (105.0) ± 43.7 | 0.0168 |
| ACC Time (mins) Mean (Median) ± SD | 87.7 (82.0) ± 33.6 | 90.2 (84.0) ± 36.0 | −0.0727 |
| Urgency | |||
| Elective | 54.70% | 56.47% | −0.0355 |
| Urgent | 45.30% | 43.53% | 0.0355 |
| Adjusted for Matching | |||||
|---|---|---|---|---|---|
| No Block (n = 351) | Block (n = 232) | Difference (95% CI) | Odds Ratio (95% CI) | p-Value | |
| Intraoperative Medications | |||||
| Ketamine (%) mean (median) ± sd (mg) | 21.1% 12.3 (0.0) ± 25.5 | 16.4% 8.1 (0.0) ± 18.4 | 4.7% [−1.7%,11.1%] 4.2 [0.6, 7.7] | 0.72 (0.47, 1.13) | 0.157 0.032 |
| Intraop MME (mg) Mean (Median) ± SD | 99.9 (80.0) ± 76.9 | 96.3 (75.0) ± 83.6 | 3.6 [−9.8, 17.1] | 1.00 (0.99, 1.00) | 0.684 |
| Intraop BenzoEquiv (mg) Mean (Median) ± SD | 2.8 (2.0) ± 1.9 | 2.5 (2.0) ± 1.8 | 0.3 [−0.01, 0.6] | 0.92 (0.84,1.01) | 0.066 |
| Postoperative Cticu Medications | |||||
| Postop MME (mg) Mean (Median) ± SD | 68.8 (60.0) ± 43.2 | 78.3 (69.0) ± 49.7 | −9.5 [−17.3, −1.7] | 1.00 (1.00, 1.00) | 0.021 |
| Postop BenzoEquiv (mg) Mean (Median) ± SD | 1.8 (4.0) ± 4.2 | 1.4 (4.0) ± 4.1 | 0.42 [−0.17, 1.21] | 0.97 (0.93, 1.01) | 0.204 |
| Acetaminophen (%) | 96.6% | 98.7% | −2.1% [−4.5%, 0.3%] | 2.60 (0.73, 9.33) | 0.142 |
| Ketorolac (%) | 26.2% | 20.7% | 5.5% [−1.4%, 12.5%] | 0.65 (0.43, 0.99) | 0.047 |
| Antipsychotic (%) | 0.6% | 1.7% | −1.2% [−3.0%, 0.7%] | 3.61 (0.65, 19.91) | 0.141 |
| Total medications | |||||
| Total MME (mg) Mean (Median) ± SD | 168.8 (155.0) ± 89.7 | 174.6 (153.0) ± 104.6 | −5.9 [−22.3, 10.5] | 1.00 (0.99, 1.00) | 0.435 |
| Unadjusted | Adjusted for Matching | ||||
|---|---|---|---|---|---|
| No Block (n = 351) | Block (n = 232) | Difference (95% CI) | Odds Ratio (95% CI) | p-Value | |
| Pain Score AUC24 Mean (Median) ± SD | 96.7 (98.0) ± 31.0 | 82.8 (82.5) ± 34.0 | 14.0 [8.5, 19.5] | 0.99 (0.98, 0.99) | <0.001 |
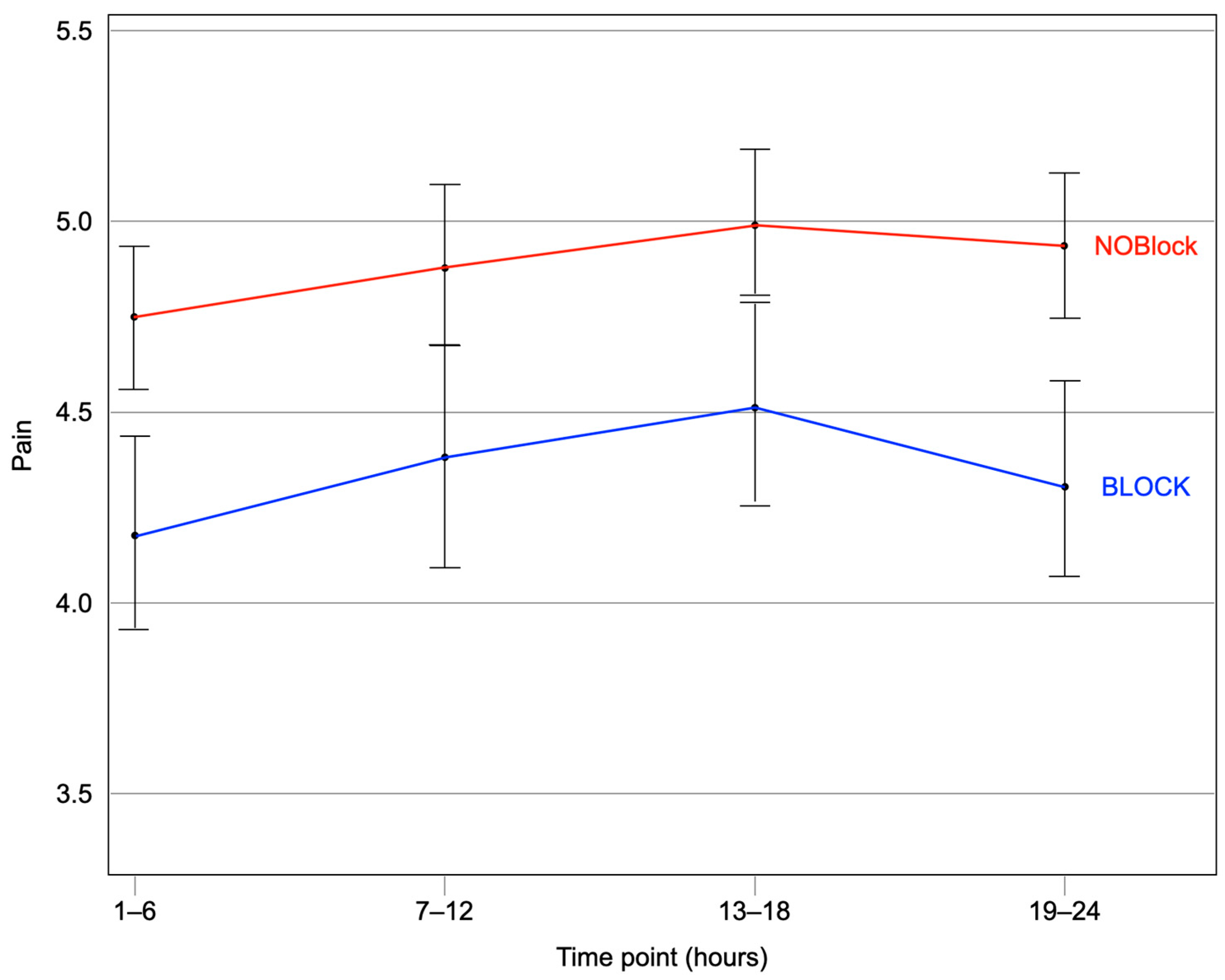
| Pain Score, 1–6 h Mean (Median) ± SD | 4.72 (4.75) ±1.56 | 4.18 (4.0) ±1.69 | 0.54 [0.23, 0.86] | 0.84 (0.72, 0.97) | 0.015 |
| Pain Score, 7–12 h Mean (Median) ± SD | 4.88 (5.0) ± 1.70 | 4.39 (4.5) ± 1.96 | 0.48 [0.14, 0.82] | 0.88 (0.78, 0.98) | 0.022 |
| Pain Score, 13–18 h Mean (Median) ± SD | 4.99 (5.0) ± 1.71 | 4.52 (4.55) ± 1.77 | 0.46 [0.15, 0.78] | 0.85 (0.76, 0.96) | 0.007 |
| Pain Score, 19–24 h Mean (Median) ± SD | 4.92 (5.0) ± 1.58 | 4.33 (4.0) ± 1.76 | 0.59 [0.29, 0.89] | 0.82 (0.73, 0.92) | <0.001 |
| Time to Extubation (mins) Mean (Median) ± SD | 794.9 (349.5) ± 3183.3 | 366.8 (259) ± 439.4 | 428.1 [89.8, 766.4] | 1.00 (0.99, 1.0) | <0.001 |
| ICU Time (hours) Mean (Median) ± SD | 98.7 (74.0) ± 95.5 | 91.1 (74.4) ± 66.9 | 7.55 [−5.64, 20.74] | 1.00 (0.99, 1.00) | 0.434 |
| Discharge Time (days) Mean (Median) ± SD | 8.3 (7.0) ± 4.9 | 6.6 (6.0) ± 3.3 | 1.71 [1.04, 2.37] | 0.89 (0.84, 0.94) | <0.001 |
| 30 Day Mortality (%) | 1.42% | 0.00% | 1.42% [0.18, 2.66] | 0.25 (0.00, 1.40) | 0.0988 |
| Unadjusted | Adjusted for Matching | ||||
|---|---|---|---|---|---|
| Single-PIP | Multi-PIP | Difference (95% CI) | Odds Ratio (95% CI) | p-Value | |
| Intraoperative Medications | |||||
| Intraop BenzoEquiv (mg) Mean ± SD | 2.6 ± 1.6 | 3.0 ± 2.7 | −0.36 [−1.25, 0.53] | 1.086 (0.904, 1.304) | 0.380 |
| Intraop Ketamine (mg) Mean ± SD | 10.5 ± 24.6 | 8.14 ± 18.68 | 2.35 [−5.4, 10.1] | 0.994 (0.976, 1.012) | 0.531 |
| Intraop MME (mg) Mean ± SD | 105.8 ± 94.9 | 36.4 ± 34.7 | 69.4 [46.3, 92.5] | 0.977 (0.963, 0.990) | <0.001 |
| Block Bupivacaine 0.25% (mL) Mean ± SD | 51.0 ± 10.3 | 72.8 ± 12.6 | −21.8 [−26.2, −17.4] | 1.343 (1.108, 1.628) | 0.003 |
| Block adjuvants (%) | 44.4% | 93.0% | −48.6% [−61.8%, −35.4%] | 31.484 (4.235, 234.076) | <0.001 |
| Block dexamethasone (%) | 44.4% | 93.0% | −48.6% [−61.8%, −35.4%] | 31.484 (4.235, 234.076) | <0.001 |
| Block dexmedetomidine (%) | 37.5% | 93.0% | −56.0% [−69.0%, −43.0%] | 37.909 (5.128, 280.225) | <0.001 |
| Postoperative CTIC | |||||
| Postop BenzoEquiv (mg) Mean ± SD (N) | 2.5 ± 5.0 | 1.2 ± 2.2 | 1.37 [0.10, 2.65} | 0.913 (0.811, 1.029) | 0.136 |
| Postop MME (mg) Mean ± SD | 78.1 ± 46.8 | 60.3 ± 44.7 | 17.8 [1.0, 34.6] | 0.989 (0.979, 0.999) | 0.031 |
| Total MME (mg) Mean ± SD | 183.9 ± 105.1 | 96.7 ± 57.9 | 87.19 [58.5, 115.9] | 0.985 (0.977, 0.992) | <0.001 |
| Pain Score AUC24 Mean ± SD | 85.3 ± 32.5 | 79.4 ± 34.2 | 5.97 [−6.6, 18.5] | 0.994 (0.982, 1.006) | 0.325 |
| Pain Score 1–6 h Mean ± SD | 4.0 ± 1.7 | 4.1 ± 1.6 | −0.05 [−0.77, 0.67] | 1.023 (0.768, 1.363) | 0.875 |
| Pain Score 7–12 h Mean ± SD | 4.6 ± 1.9 | 4.7 ± 2.0 | −0.07 [−0.92, 0.79] | 1.094 (0.862, 1.389) | 0.460 |
| Pain Score 13–18 h Mean ± SD | 4.6 ± 1.7 | 4.1 ± 2.0 | 0.54 [−0.25, 1.34] | 0.870 (0.663, 1.140) | 0.311 |
| Pain Score 19–24 h Mean ± SD | 4.7 ± 1.6 | 4.3 ± 1.9 | 0.40 [−0.31, 1.11] | 0.862 (0.657, 1.131) | 0.285 |
| Pain Score 1–12 h Mean ± SD | 4.3 ± 1.6 | 4.2 ± 1.7 | 0.11 [−0.56, 0.77] | 0.9827 (0.7817, 1.2353) | 0.881 |
| Pain Score 13–24 h Mean ± SD | 4.6 ± 1.3 | 4.1 ± 1.6 | 0.54 [−0.05, 1.12] | 0.7465 (0.5539, 1.0060) | 0.055 |
| Time to Extubation (mins) Mean (Median) ± SD | 449.8 (249) ± 687.6 | 389.6 (308) ± 243.5 | 60.17 [−107.17, 227.51] | 0.9998 (0.9990, 1.0006) | 0.648 |
| ICU Time (hours) Mean (Median) ± SD | 96.9 (75.1) ± 75.9 | 95.9 (92.5) ± 76.4 | 0.97 [−27.23, 29.16] | 0.9995 (0.9947, 1.0043) | 0.832 |
| Discharge Time (days) Mean ± SD | 7.1 (6) ± 5.7 | 6.81 (6) ± 4.03 | 0.30 [−1.43, 2.02] | 0.9836 (0.9107, 1.0623) | 0.674 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.; Vargas Galvan, L.A.; Walsh, K.L.; Winegarner, A.; Apruzzese, P.; Asher, S.; Maslow, A. A Retrospective Review of the Deep Parasternal Intercostal Plane Block in Patients Undergoing Cardiac Surgery with Median Sternotomy. J. Clin. Med. 2025, 14, 2074. https://doi.org/10.3390/jcm14062074
Chen T, Vargas Galvan LA, Walsh KL, Winegarner A, Apruzzese P, Asher S, Maslow A. A Retrospective Review of the Deep Parasternal Intercostal Plane Block in Patients Undergoing Cardiac Surgery with Median Sternotomy. Journal of Clinical Medicine. 2025; 14(6):2074. https://doi.org/10.3390/jcm14062074
Chicago/Turabian StyleChen, Tzonghuei, Leslie Annette Vargas Galvan, Kendra L. Walsh, Andrew Winegarner, Patricia Apruzzese, Shyamal Asher, and Andrew Maslow. 2025. "A Retrospective Review of the Deep Parasternal Intercostal Plane Block in Patients Undergoing Cardiac Surgery with Median Sternotomy" Journal of Clinical Medicine 14, no. 6: 2074. https://doi.org/10.3390/jcm14062074
APA StyleChen, T., Vargas Galvan, L. A., Walsh, K. L., Winegarner, A., Apruzzese, P., Asher, S., & Maslow, A. (2025). A Retrospective Review of the Deep Parasternal Intercostal Plane Block in Patients Undergoing Cardiac Surgery with Median Sternotomy. Journal of Clinical Medicine, 14(6), 2074. https://doi.org/10.3390/jcm14062074






