Orbital Implant Surgery with Costal Cartilage Graft Is Associated with Better Symmetry and Improved Cosmetic Appearance
Abstract
1. Introduction
- The group that underwent cartilage grafting shows significantly less upper eyelid asymmetry;
- In cases involving orbital fractures, additional surgery is often required due to enophthalmos or blepharoptosis resulting from increased orbital volume caused by insufficient orbital fracture reduction.
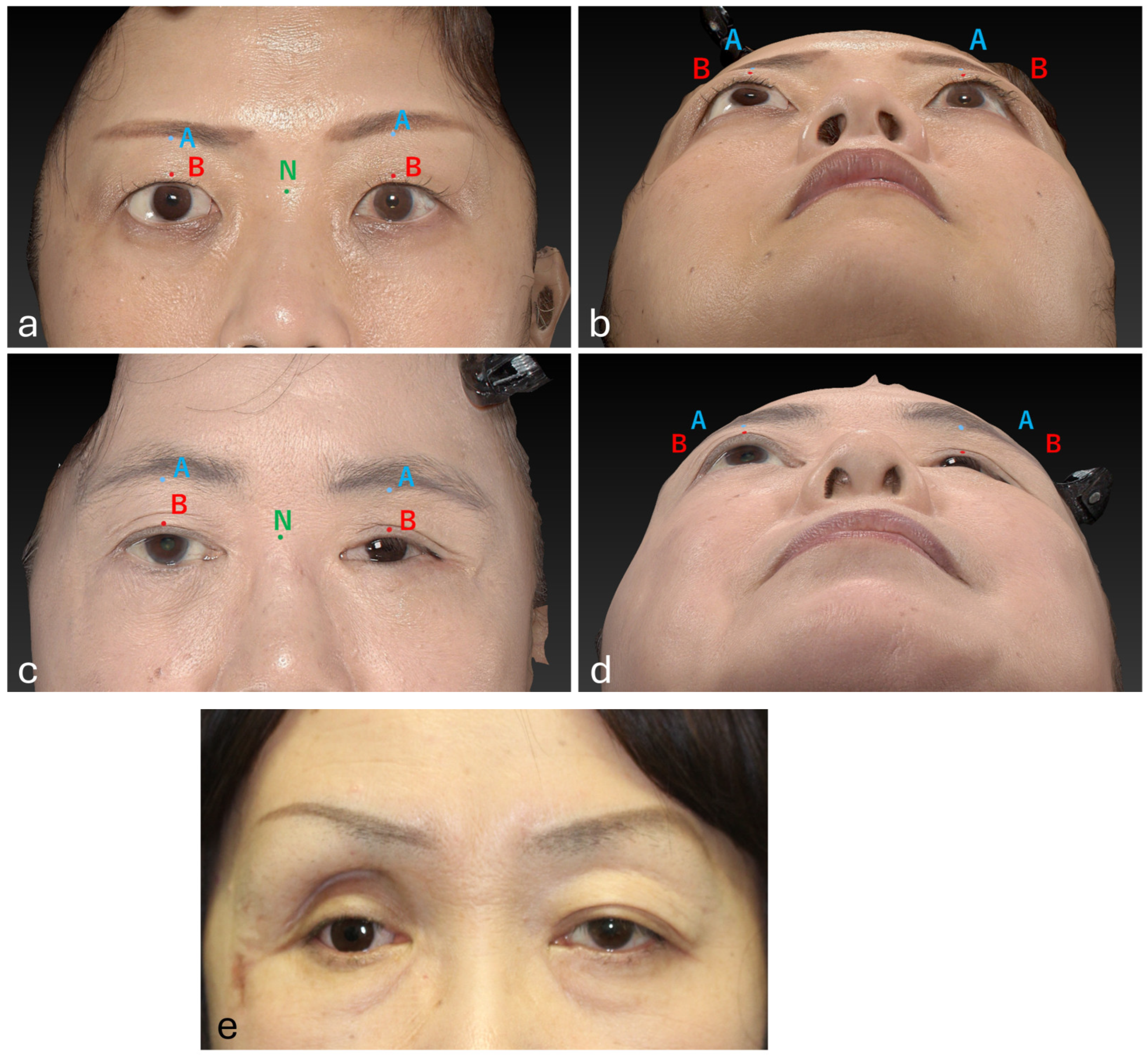
2. Materials and Methods
2.1. Study Design/Sample
2.2. Surgical Technique
2.2.1. Evisceration Procedure
2.2.2. Orbital Implant Surgery
2.3. Postoperative Follow-Up
2.4. Variables
2.5. Data Collection Methods
2.6. Standardization of 3D Imaging
2.7. Data Analysis
3. Results
3.1. Characteristics of the Study Population
3.2. Upper Eyelid Measurement Differences Between Groups
3.3. Complications and Additional Surgeries in Group 1
3.4. Complications in Group 2
4. Discussion
4.1. Current Status of Orbital Implant Surgery
4.2. Influence of Age and Gender on Surgical Selection
4.3. Measurement Methods and Evaluation
4.4. Postoperative Complications
4.4.1. Enophthalmos
4.4.2. Blepharoptosis
4.4.3. Lower Eyelid Malposition and Laxity
4.4.4. Narrow Conjunctival Sac
4.5. Relationship Between Orbital Fractures and the Need for Additional Surgery
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Park, H.Y.; Kim, T.H.; Yoon, J.S.; Ko, J. Quantitative assessment of increase in orbital volume after orbital floor fracture reconstruction using a bioabsorbable implant. Graefe’s Arch. Clin. Exp. Ophthalmol. 2022, 260, 3027–3036. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.Y.; Fujioka, J.K.; Daigle, P.; Tran, S.D. The Use of Functional Biomaterials in Aesthetic and Functional Restoration in Orbital Surgery. J. Funct. Biomater. 2024, 15, 33. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Grob, S.; Yonkers, M.; Tao, J. Orbital Fracture Repair. Semin. Plast. Surg. 2017, 31, 31–39. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Carraway, J.H.; Mellow, C.G.; Mustarde, J.C. Use of cartilage graft for an orbital socket implant. Ann. Plast. Surg. 1990, 24, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Morton, A.D. Enucleation and Evisceration. In Ophthalmic Care of the Combat Casualty; Albert, D.M., Gragoudas, E.S., Eds.; Office of the Surgeon General, Department of the Army: Washington, DC, USA, 2003; pp. 405–420. [Google Scholar]
- Motomura, H.; Deguchi, A.; Ataka, S.; Fujii, N.; Hatano, T.; Fujikawa, H.; Maeda, S.; Haraoka, G. A Dynamic Costal Cartilage Platform Promotes Ocular Prosthetic Excursion: Preliminary Report. Plast. Reconstr. Surg. Glob. Open 2021, 9, e3352. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Taylor, H.O.; Morrison, C.S.; Linden, O.; Phillips, B.; Chang, J.; Byrne, M.E.; Sullivan, S.R.; Forrest, C.R. Quantitative facial asymmetry: Using three-dimensional photogrammetry to measure baseline facial surface symmetry. J. Craniofac. Surg. 2014, 25, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Lane, C.; Harrell, W., Jr. Completing the 3-dimensional picture. Am. J. Orthod. Dentofac. Orthop. 2008, 133, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Christmas, N.J.; Gordon, C.D.; Murray, T.G.; Tse, D.; Johnson, T.; Garonzik, S.; O’Brien, J.M. Intraorbital implants after enucleation and their complications: A 10-year review. Arch. Ophthalmol. 1998, 116, 1199–1203. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.J.; Choung, H.K.; Khwarg, S.I. Free orbital fat graft to prevent porous polyethylene orbital implant exposure in patients with retinoblastoma. Ophthalmic Plast. Reconstr. Surg. 2005, 21, 253–258. [Google Scholar] [CrossRef]
- Custer, P.L.; Trinkaus, K.M. Porous implant exposure: Incidence, management, and morbidity. Ophthalmic Plast. Reconstr. Surg. 2007, 23, 1–7. [Google Scholar] [CrossRef]
- Su, G.W.; Yen, M.T. Current trends in managing the anophthalmic socket after primary enucleation and evisceration. Ophthalmic Plast. Reconstr. Surg. 2004, 20, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, K.; Hirose, T.; Furuta, S. Semiquantitative correction of posttraumatic enophthalmos with sliced cartilage grafts. Plast. Reconstr. Surg. 1989, 83, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Park, I.H.; Hong, S.H.; Eun, S.C. Sliced Costochondral Chip Grafts in Posttraumatic Enophthalmos Correction. J. Craniofac. Surg. 2017, 28, 418–421. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhen, J.; Li, H.; Sun, S.; Wu, H.; Shen, P.; Chen, Z.; Yang, C. Characteristics of Chinese Costal Cartilage and Costa Calcification Using Dual-Energy Computed Tomography Imaging. Sci. Rep. 2017, 7, 2923. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Andrades, P.; Hernandez, D.; Falguera, M.I.; Millan, J.M.; Heredero, S.; Gutierrez, R.; Sánchez-Aniceto, G. Degrees of tolerance in post-traumatic orbital volume correction: The role of prefabricated mesh. J. Oral Maxillofac. Surg. 2009, 67, 2404–2411. [Google Scholar] [CrossRef] [PubMed]
- Rokohl, A.C.; Kopecky, A.; Trester, M.; Wawer Matos, P.A.; Pine, K.R.; Heindl, L.M. Post-enucleation socket syndrome-a novel pathophysiological definition. Graefe’s Arch. Clin. Exp. Ophthalmol. 2022, 260, 2427–2431. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rubin, P.A.; Shore, J.W.; Yaremchuk, M.J. Complex orbital fracture repair using rigid fixation of the internal orbital skeleton. Ophthalmology 1992, 99, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Kaltreider, S.A.; Shields, M.D.; Hippeard, S.C.; Patrie, J. Anophthalmic ptosis: Investigation of the mechanisms and statistical analysis. Ophthalmic Plast. Reconstr. Surg. 2003, 19, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Kaltreider, S.A. The ideal ocular prosthesis: Analysis of prosthetic volume. Ophthalmic Plast. Reconstr. Surg. 2000, 16, 388–392. [Google Scholar] [CrossRef] [PubMed]
- van der Meulen, J.C. Reconstruction of the socket. In Color Atlas and Text of Ocular Plastic Surgery, 1st ed.; Van der Meulen, J.C., Gruss, J.S., Eds.; Mosby-Wolfe: London, UK, 1996; pp. 275–297. [Google Scholar]
- Vistnes, L.M. Mechanism of upper lid ptosis in the anophthalmic orbit. Plast. Reconstr. Surg. 1976, 58, 539–545. [Google Scholar] [CrossRef]
- Birgfeld, C.; Gruss, J. The importance of accurate, early bony reconstruction in orbital injuries with globe loss. Craniomaxillofacial Trauma Reconstr. 2011, 4, 121–128. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ataullah, S.; Whitehouse, R.W.; Stelmach, M.; Shah, S.; Leatherbarrow, B. Missed orbital wall blow-out fracture as a cause of post-enucleation socket syndrome. Eye 1999, 13 Pt 4, 541–544. [Google Scholar] [CrossRef] [PubMed]


| Causes of Blindness | Number | Percentage | |
|---|---|---|---|
| Group 1 | Congenital | 2 | 15.4% |
| Diabetic retinopathy | 2 | 15.4% | |
| Injury | 7 | 53.8% | |
| Keratitis | 2 | 15.4% | |
| Group 2 | Cytomegalovirus | 1 | 10% |
| Injury | 7 | 70% | |
| Septic endophthalmitis | 1 | 10% | |
| Thyroid eye disease | 1 | 10% | |
| Variables | Group 1 Median (IQR) (mm) | Group 2 Median (IQR) (mm) | p Value |
|---|---|---|---|
| Point A normal side | −3.84 (−5.84 to −2.92) | −4.02 (−7.50 to −2.67) | 0.410 a |
| Point A affected side | −4.23 (−6.28 to −2.59) | −6.47 (−15.08 to −4.96) | 0.042 * a |
| Point A difference in measurements | 0.75 (−0.26 to 2.44) | 3.23 (2.42 to 5.11) | 0.003 * a |
| Point B normal side | −0.37 (−3.17 to 0.45) | 0.10 (−2.31 to 2.08) | 0.208 a |
| Point B affected side | −2.07 (−3.92 to −0.13) | −3.45 (−7.68 to −1.36) | 0.284 a |
| Point B difference in measurements | 0.86 (−0.53 to 1.78) | 4.20 (2.29 to 5.80) | 0.001 * a |
| Complications | Number | Percentage | |
|---|---|---|---|
| Preoperative complications | Bulbar atrophy | 1 | 7.7% |
| Multiple facial fractures | 2 | 15.4% | |
| Orbital fracture | 4 | 30.8% | |
| Orbital fracture with levator palpebrae muscle injury | 1 | 7.7% | |
| Absent | 6 | 46.2% | |
| Postoperative complications | Blepharoptosis | 3 | 23.1% |
| Enophthalmos and narrow conjunctival sac | 1 | 7.7% | |
| Enophthalmos, narrow conjunctival sac, and traumatic blepharoptosis | 1 | 7.7% | |
| Narrow conjunctival sac | 2 | 15.4% | |
| Narrow conjunctival sac and blepharoptosis | 1 | 7.7% | |
| Absent | 5 | 38.5% | |
| Additional Surgeries | Number | Percentage |
|---|---|---|
| Blepharoptosis surgery | 3 | 23.1% |
| Conjunctival sacroplasty | 2 | 15.4% |
| Conjunctival sacroplasty and blepharoptosis surgery | 1 | 7.7% |
| Orbital fracture reduction and conjunctival sacroplasty | 1 | 7.7% |
| Orbital fracture reduction, conjunctival sacroplasty, and blepharoptosis surgery | 1 | 7.7% |
| Absent | 5 | 38.5% |
| Orbital Fracture | Additional Surgery | p Value | |
|---|---|---|---|
| Not Performed | Performed | ||
| Number (%) | Number (%) | ||
| Absent | 5 (62.5) | 3 (37.5) | 0.075 b |
| Present | 0 (0) | 5 (100) | |
| Postoperative Complications | Number | Percentage |
|---|---|---|
| Blepharoptosis | 5 | 50.0% |
| Enophthalmos | 6 | 60.0% |
| Narrow conjunctival sac | 6 | 60.0% |
| Lower Eyelid Malposition and Laxity | 5 | 50.0% |
| Ulceration | 2 | 20.0% |
| Absent | 0 | 0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanai, U.; Tsunoda, Y.; Nemoto, H.; Nakagawa, Y.; Suzuki, T.; Akamatsu, T. Orbital Implant Surgery with Costal Cartilage Graft Is Associated with Better Symmetry and Improved Cosmetic Appearance. J. Clin. Med. 2025, 14, 2052. https://doi.org/10.3390/jcm14062052
Hanai U, Tsunoda Y, Nemoto H, Nakagawa Y, Suzuki T, Akamatsu T. Orbital Implant Surgery with Costal Cartilage Graft Is Associated with Better Symmetry and Improved Cosmetic Appearance. Journal of Clinical Medicine. 2025; 14(6):2052. https://doi.org/10.3390/jcm14062052
Chicago/Turabian StyleHanai, Ushio, Yotaro Tsunoda, Hitoshi Nemoto, Yoshihiro Nakagawa, Takahiro Suzuki, and Tadashi Akamatsu. 2025. "Orbital Implant Surgery with Costal Cartilage Graft Is Associated with Better Symmetry and Improved Cosmetic Appearance" Journal of Clinical Medicine 14, no. 6: 2052. https://doi.org/10.3390/jcm14062052
APA StyleHanai, U., Tsunoda, Y., Nemoto, H., Nakagawa, Y., Suzuki, T., & Akamatsu, T. (2025). Orbital Implant Surgery with Costal Cartilage Graft Is Associated with Better Symmetry and Improved Cosmetic Appearance. Journal of Clinical Medicine, 14(6), 2052. https://doi.org/10.3390/jcm14062052






