Clinically Evident Portal Hypertension Is an Independent Risk Factor of Hepatocellular Carcinoma Recurrence Following Liver Transplantation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Cohort
2.2. Definition and Assessment of CEPH
2.3. Transplant Procedure and Post-LT Surveillance
2.4. Outcome Variables
2.5. Statistical Analysis
3. Results
3.1. Clinicopathologic Characteristics
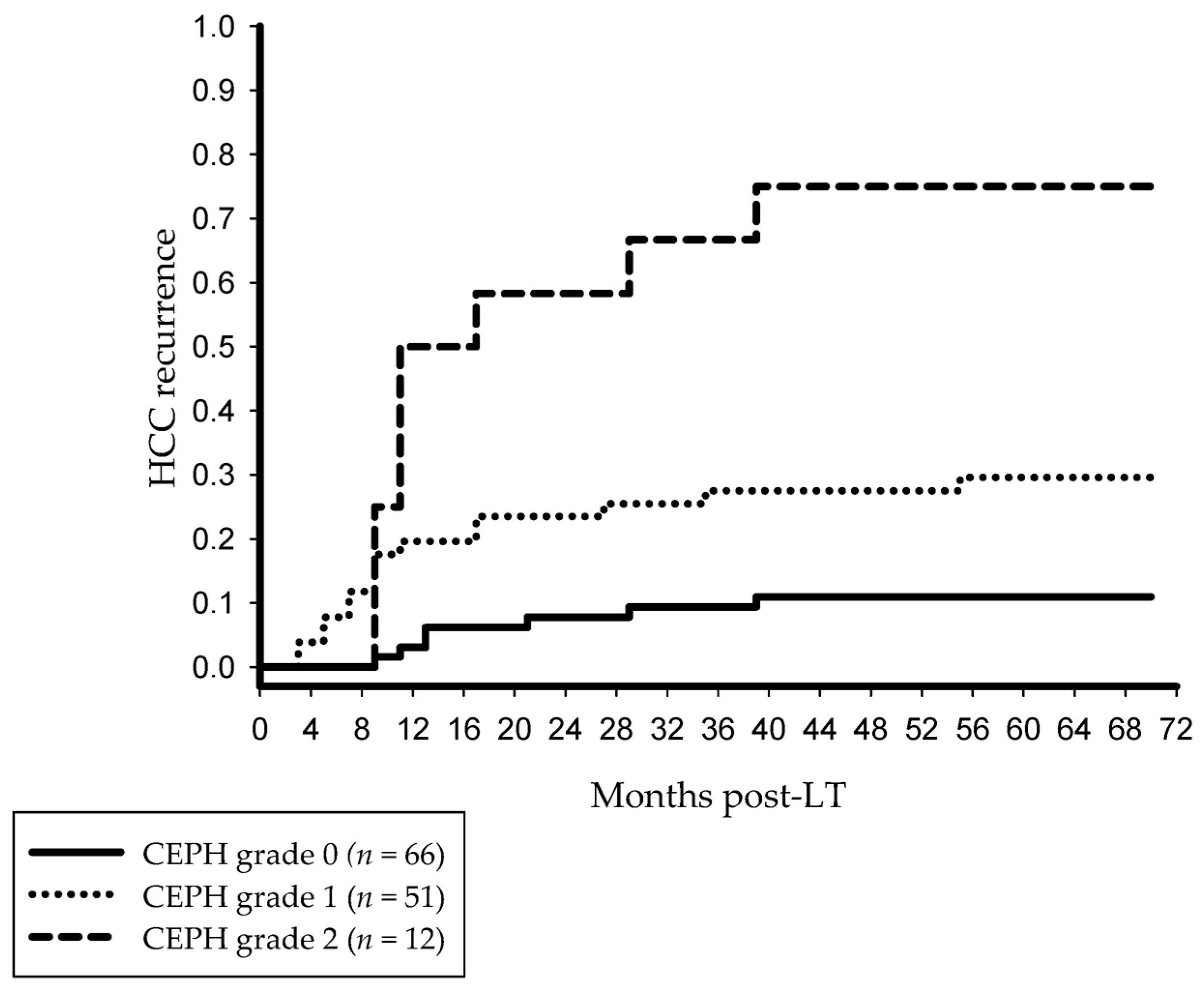
3.2. Post-LT Outcome and Risk Factors of HCC Recurrence
3.3. CEPH and TACE
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AFP | alfa-fetoprotein |
| CEPH | clinically evident portal hypertension |
| CNI | calcineurin inhibitor |
| CRP | C-reactive protein |
| CT | computed tomography |
| 18F-FDG | 18F-fluorodeoxy-glucose |
| HCC | hepatocellular carcinoma |
| HVPG | hepatic venous-portal gradient |
| IL-6 | interleukin-6 |
| LT | liver transplantation |
| MC | Milan criteria |
| MELD | model of end-stage liver disease |
| mTOR | mammalian target of rapamycin |
| MVI | microvascular invasion |
| OR | odds ratio |
| OS | overall survival |
| PET | positron emission tomography |
| PH | portal hypertension |
| PP | portal pressure |
| STD | standard deviation |
| TACE | transarterial chemoembolization |
| TME | tumor microenvironment |
| TSUVmax/LSUVmax | tumor-to-normal liver standardized uptake value |
| VEGF | vascular endothelial growth factor |
References
- Zhang, X.; Chen, C.; Wang, Y.; Xu, J. Recurrence risk prediction models for hepatocellular carcinoma after liver transplantation. J. Gastroenterol. Hepatol. 2024, 39, 2272–2280. [Google Scholar] [CrossRef] [PubMed]
- Goldman, M.L.; Zhou, K.; Dodge, J.L.; Yao, F.; Mehta, N. Lower Alpha-Fetoprotein Threshold of 500 ng/mL for Liver Transplantation May Improve Posttransplant Outcomes in Patients With Hepatocellular Carcinoma. Liver Transpl. 2022, 28, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Sung, P.S.; You, Y.K.; Kim, D.G.; Oh, J.S.; Chun, H.J.; Jang, J.W.; Bae, S.H.; Choi, J.Y.; Yoon, S.K. Pathologic complete response to chemoembolization improves survival outcomes after curative surgery for hepatocellular carcinoma: Predictive factors of response. HPB 2019, 21, 1718–1726. [Google Scholar] [CrossRef] [PubMed]
- Kornberg, A.; Küpper, B.; Tannapfel, A.; Büchler, P.; Krause, B.; Witt, U.; Gottschild, D.; Friess, H. Patients with non-[18 F]fludeoxyglucose-avid advanced hepatocellular carcinoma on clinical staging may achieve long-term recurrence-free survival after liver transplantation. Liver Transpl. 2012, 18, 53–61. [Google Scholar] [CrossRef]
- Grąt, K.; Pacho, R.; Grąt, M.; Krawczyk, M.; Zieniewicz, K.; Rowiński, O. Impact of Body Composition on the Risk of Hepatocellular Carcinoma Recurrence After Liver Transplantation. J. Clin. Med. 2019, 13, 1672. [Google Scholar] [CrossRef]
- Yasukawa, K.; Shimizu, A.; Kubota, K.; Notake, T.; Hosoda, K.; Hayashi, H.; Soejima, Y. Impact of Liver Fibrosis Severity on Oncological Prognosis in Hepatocellular Carcinoma. Liver Cancer 2023, 13, 150–160. [Google Scholar] [CrossRef]
- Allaire, M.; Rudler, M.; Thabut, D. Portal hypertension and hepatocellular carcinoma: Des liaisons dangereuses. Liver Int. 2021, 41, 1734–1743. [Google Scholar] [CrossRef]
- Müller, L.; Hahn, F.; Mähringer-Kunz, A.; Stoehr, F.; Gairing, S.J.; Foerster, F.; Weinmann, A.; Galle, P.R.; Mittler, J.; Pinto Dos Santos, D.; et al. Prevalence and clinical significance of clinically evident portal hypertension in patients with hepatocellular carcinoma undergoing transarterial chemoembolization. United Eur. Gastroenterol. J. 2022, 10, 41–53. [Google Scholar] [CrossRef]
- Ripoll, C.; Groszmann, R.J.; Garcia-Tsao, G.; Bosch, J.; Grace, N.; Burroughs, A.; Planas, R.; Escorsell, A.; Garcia-Pagan, J.C.; Makuch, R.; et al. Hepatic venous pressure gradient predicts development of hepatocellular carcinoma independently of severity of cirrhosis. J. Hepatol. 2009, 50, 923–928. [Google Scholar] [CrossRef]
- Xia, F.; Huang, Z.; Zhang, Q.; Ndhlovu, E.; Chen, X.; Zhang, B.; Zhu, P. Clinically significant portal hypertension (CSPH) on early-stage HCC following hepatectomy: What’s the impact? Eur. J. Surg. Oncol. 2023, 49, 771–779. [Google Scholar] [CrossRef]
- Dioguardi Burgio, M.; Garzelli, L.; Cannella, R.; Ronot, M.; Vilgrain, V. Hepatocellular Carcinoma: Optimal Radiological Evaluation before Liver Transplantation. Life 2023, 13, 2267. [Google Scholar] [CrossRef] [PubMed]
- Ling, L.L.; Hsu, C.C.; Yong, C.C.; Elsarawy, A.M.; Chan, Y.C.; Wang, C.C.; Li, W.F.; Lin, T.L.; Kuo, F.Y.; Cheng, Y.F.; et al. FDG-PET predicted unfavorable tumor histology in living donor liver transplant recipients; a retrospective cohort study. Int. J. Surg. 2019, 69, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.; Simbrunner, B.; Jachs, M.; Hartl, L.; Bauer, D.; Paternostro, R.; Schwabl, P.; Scheiner, B.; Stättermayer, A.F.; Pinter, M.; et al. Systemic inflammation increases across distinct stages of advanced chronic liver disease and correlates with decompensation and mortality. J. Hepatol. 2021, 74, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Kornberg, A.; Witt, U.; Matevossian, E.; Küpper, B.; Assfalg, V.; Drzezga, A.; Hüser, N.; Wildgruber, M.; Friess, H. Extended postinterventional tumor necrosis-implication for outcome in liver transplant patients with advanced HCC. PLoS ONE 2013, 8, e53960. [Google Scholar] [CrossRef]
- Choi, J.W.; Chung, J.W.; Lee, D.H.; Kim, H.C.; Hur, S.; Lee, M.; Jae, H.J. Portal hypertension is associated with poor outcome of transarterial chemoembolization in patients with hepatocellular carcinoma. Eur. Radiol. 2018, 28, 2184–2193. [Google Scholar] [CrossRef]
- Sapisochin, G.; Bruix, J. Liver transplantation for hepatocellular carcinoma: Outcomes and novel surgical approaches. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 203–217. [Google Scholar] [CrossRef]
- Kornberg, A.; Witt, U.; Schernhammer, M.; Kornberg, J.; Müller, K.; Friess, H.; Thrum, K. The role of preoperative albumin-bilirubin grade for oncological risk stratification in liver transplant patients with hepatocellular carcinoma. J. Surg. Oncol. 2019, 120, 1126–1136. [Google Scholar] [CrossRef]
- Mehta, N.; Dodge, J.L.; Roberts, J.P.; Yao, F.Y. A novel waitlist dropout score for hepatocellular carcinoma—Identifying a threshold that predicts worse post-transplant survival. J. Hepatol. 2021, 74, 829–837. [Google Scholar] [CrossRef]
- Faitot, F.; Allard, M.A.; Pittau, G.; Ciacio, O.; Adam, R.; Castaing, D.; Cunha, A.S.; Pelletier, G.; Cherqui, D.; Samuel, D.; et al. Impact of clinically evident portal hypertension on the course of hepatocellular carcinoma in patients listed for liver transplantation. Hepatology 2015, 62, 179–187. [Google Scholar] [CrossRef]
- Suk, K.T.; Kim, E.J.; Kim, D.J.; Kim, H.S.; Bang, C.S.; Park, T.Y.; Baik, G.H.; Kim, S.E.; Park, J.W.; Park, S.H.; et al. Prognostic Significance of Hemodynamic and Clinical Stages in the Prediction of Hepatocellular Carcinoma. J. Clin. Gastroenterol. 2017, 51, 285–293. [Google Scholar] [CrossRef]
- Kim, M.Y.; Baik, S.K.; Yea, C.J.; Lee, I.Y.; Kim, H.J.; Park, K.W.; Kim, H.K.; Suk, K.T.; Kim, J.W.; Kim, H.S.; et al. Hepatic venous pressure gradient can predict the development of hepatocellular carcinoma and hyponatremia in decompensated alcoholic cirrhosis. Eur. J. Gastroenterol. Hepatol. 2009, 21, 1241–1246. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Civelek, A.C.; Li, X.F. The application of (18)F-FDG PET/CT imaging for human hepatocellular carcinoma: A narrative review. Quant. Imaging Med. Surg. 2023, 13, 6268–6279. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Chen, J.; Gao, H.; Kong, S.N.; Deivasigamani, A.; Shi, M.; Xie, T.; Hui, K.M. Hypoxia-induced modulation of glucose transporter expression impacts (18)F-fluorodeoxyglucose PET-CT imaging in hepatocellular carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Tan, M.; Gao, Z.; Wang, S.; Lyu, L.; Ding, H. Role of Hydrogen Sulfide and Hypoxia in Hepatic Angiogenesis of Portal Hypertension. J. Clin. Transl. Hepatol. 2023, 28, 675–681. [Google Scholar] [CrossRef]
- Oura, K.; Morishita, A.; Tadokoro, T.; Fujita, K.; Tani, J.; Kobara, H. Immune Microenvironment and the Effect of Vascular Endothelial Growth Factor Inhibition in Hepatocellular Carcinoma. Int. J. Mol. Sci. 2024, 25, 13590. [Google Scholar] [CrossRef]
- Zhou, X.; Hu, Y.; Sun, H.; Chen, R.; Huang, G.; Liu, J. SUVmax on 18F-FDG PET and PD-L1 expression between in hepatocellular carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 3107–3115. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Huang, Z.; Yu, X.; Zheng, L.; Xu, J. C-Reactive Protein Is an Indicator of the Immunosuppressive Microenvironment Fostered by Myeloid Cells in Hepatocellular Carcinoma. Front. Oncol. 2022, 11, 774823. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, S.; Yang, Z.; Hu, J.; Hu, W.; Sun, P.; Wu, L.; Han, B. Association between the expression levels of IL-6 and IL-6R in the hepatocellular carcinoma microenvironment and postoperative recurrence. Oncol. Lett. 2018, 16, 7158–7165. [Google Scholar] [CrossRef]
- Minici, R.; Siciliano, M.A.; Ammendola, M.; Santoro, R.C.; Barbieri, V.; Ranieri, G.; Laganà, D. Prognostic Role of Neutrophil-to-Lymphocyte Ratio (NLR), Lymphocyte-to-Monocyte Ratio (LMR), Platelet-to-Lymphocyte Ratio (PLR) and Lymphocyte-to-C Reactive Protein Ratio (LCR) in Patients with Hepatocellular Carcinoma (HCC) undergoing Chemoembolizations (TACE) of the Liver: The Unexplored Corner Linking Tumor Microenvironment, Biomarkers and Interventional Radiology. Cancers 2022, 15, 257. [Google Scholar] [CrossRef]
- He, Q.; Yang, J.; Jin, Y. Development and Validation of TACE Refractoriness-Related Diagnostic and Prognostic Scores and Characterization of Tumor Microenvironment Infiltration in Hepatocellular Carcinoma. Front. Immunol. 2022, 13, 869993. [Google Scholar] [CrossRef]
- Patidar, Y.; Chandel, K.; Condati, N.K.; Srinivasan, S.V.; Mukund, A.; Sarin, S.K. Transarterial Chemoembolization (TACE) Combined With Sorafenib versus TACE in Patients With BCLC Stage C Hepatocellular Carcinoma—A Retrospective Study. J. Clin. Exp. Hepatol. 2022, 12, 745–754. [Google Scholar] [CrossRef] [PubMed]
- D’Avola, D.; Granito, A.; Torre-Aláez, M.; Piscaglia, F. The importance of liver functional reserve in the non-surgical treatment of hepatocellular carcinoma. J. Hepatol. 2022, 76, 1185–1198. [Google Scholar] [CrossRef] [PubMed]
- El Hajji, S.; Lacotte, S.; Moeckli, B.; Cauchy, F.; Compagnon, P.; Toso, C. Transjugular Intrahepatic Portosystemic Shunt Is Associated With Better Waitlist Management of Liver Transplant Candidates With Hepatocellular Carcinoma. Transpl. Int. 2024, 37, 12781. [Google Scholar] [CrossRef] [PubMed]
- Allaire, M.; Garcia, H.; Meyblum, L.; Mouri, S.; Spitzer, E.; Goumard, C.; Lucidarme, O.; Rudler, M.; Scatton, O.; Roux, C.; et al. Non selective beta-blockers prevent PHT-related complications occurrence in HCC patients with esophageal varices treated by TACE. Clin. Res. Hepatol. Gastroenterol. 2025, 49, 102496. [Google Scholar] [CrossRef]
- Wei, G.; Zhao, Y.; Feng, S.; Yuan, J.; Xu, G.; Lv, T.; Yang, J.; Kong, L.; Yang, J. Does depressurization of the portal vein before liver transplantation affect the recurrence of HCC? A nested case-control study. BMC Cancer 2024, 24, 558. [Google Scholar] [CrossRef]
- Li, M.; Bhoori, S.; Mehta, N.; Mazzaferro, V. Immunotherapy for hepatocellular carcinoma: The next evolution in expanding access to liver transplantation. J. Hepatol. 2024, 81, 743–755. [Google Scholar] [CrossRef]
- Rodríguez-Perálvarez, M.; Tsochatzis, E.; Naveas, M.C.; Pieri, G.; García-Caparrós, C.; O’Beirne, J.; Poyato-González, A.; Ferrín-Sánchez, G.; Montero-Álvarez, J.L.; Patch, D.; et al. Reduced exposure to calcineurin inhibitors early after liver transplantation prevents recurrence of hepatocellular carcinoma. J. Hepatol. 2013, 59, 1193–1199. [Google Scholar] [CrossRef]
- Lei, H.W.; Cai, J.; Li, C.M.; Yang, F.; Shi, W.Q.; Shi, W.Q.; Wang, L.P.; Feng, Y.Y. Rapamycin Combi with TAE on the Growth, Metastasis, and Prognosis of Hepatocellular Carcinoma in Rat Models. Ann. Hepatol. 2018, 17, 645–654. [Google Scholar] [CrossRef]
- Aliseda, D.; Zozaya, G.; Martí-Cruchaga, P.; Herrero, I.; Iñarrairaegui, M.; Argemí, J.; Martínez De La Cuesta, A.; Blanco, N.; Sabatella, L.; Sangro, B.; et al. The Impact of Portal Hypertension Assessment Method on the Outcomes of Hepatocellular Carcinoma Resection: A Meta-Analysis of Matched Cohort and Prospective Studies. Ann. Surg. 2024, 280, 46–55. [Google Scholar] [CrossRef]
- Jachs, M.; Hartl, L.; Simbrunner, B.; Semmler, G.; Balcar, L.; Hofer, B.S.; Schwarz, M.; Bauer, D.; Stättermayer, A.F.; Pinter, M.; et al. Prognostic performance of non-invasive tests for portal hypertension is comparable to that of hepatic venous pressure gradient. J. Hepatol. 2024, 80, 744–752. [Google Scholar] [CrossRef]


| Prognostic Factors | All Patients (n = 129) | Without CEPH (n = 66) | With CEPH (n = 63) | p Value |
|---|---|---|---|---|
| Median age in years (range) | 58.3 (38–71) | 60.5 (38–71) | 58.0 (41–70) | 0.441 |
| Sex, n (%) Female Male | 53 (41.1) 76 (58.9) | 28 (42.4) 38 (57.6) | 25 (39.7) 38 (60.3) | 0.752 |
| Alcohol-related cirrhosis, n (%) No Yes | 59 (45.7) 70 (54.3) | 29 (43.9) 37 (56.1) | 30 (47.6) 33 (52.4) | 0.675 |
| Viral cirrhosis, n (%) No Yes | 87 (67.4) 42 (32.6) | 49 (74.2) 17 (25.8) | 38 (60.3) 25 (39.7) | 0.092 |
| Child–Pugh, n (%) A B or C | 53 (41.1) 76 (58.9) | 46 (69.7) 20 (30.3) | 7 (11.1) 56 (88.9) | < 0.001 |
| MELD score, n (%) ≤20 >20 | 72 (55.8) 57 (44.2) | 56 (84.8) 10 (15.2) | 16 (25.4) 47 (74.6) | < 0.001 |
| Ascites, n (%) Absent Present | 87 (67.4) 42 (32.6) | 66 (100) 0 (0) | 19 (30.2) 44 (69.8) | < 0.001 |
| Esophago-gastric varices, n (%) No Yes | 73 (56.6) 56 (43.4) | 66 (100) 0 (0) | 7 (11.1) 56 (88.9) | < 0.001 |
| Splenomegaly, n (%) No Yes | 76 (58.9) 53 (41.1) | 47 (71.2) 19 (28.8) | 29 (46.0) 34 (54.0) | 0.004 |
| Thrombocytopenia, n (%) No Yes | 101 (78.3) 28 (21.7) | 57 (86.4) 9 (13.6) | 44 (69.8) 19 (30.2) | 0.023 |
| Clinical features of CEPH, n (%) 0 1 2 3 | 66 (51.2) 24 (18.6) 27 (20.9) 12 (9.3) | 66 (100) 0 (0) 0 (0) 0 (0) | 0 (0) 24 (38.1) 27 (42.9) 12 (19.0) | <0.001 |
| Mean number of tumor nodules, (±STD) | 2.1 ± 1.4 | 2.0 ± 1.5 | 2.3 ± 1.2 | 0.286 |
| Number of tumor nodules, n (%) ≤3 >3 | 109 (84.5%) 20 (15.5%) | 54 (81.8%) 12 (18.2%) | 55 (87.3%) 8 (12.7%) | 0.390 |
| Tumor manifestation, n (%) Solitary Multifocal | 58 (45.0) 71 (55.0) | 35 (53.0) 31 (47.0) | 23 (36.5) 40 (63.5) | 0.059 |
| Mean size largest nodule, cm, (±STD) | 4.2 ± 2.3 | 4.1 ± 2.6 | 4.3 ± 2.0 | 0.665 |
| Size largest nodule, n (%) ≤5 cm >5 cm | 107 (82.9%) 22 (17.1%) | 54 (81.8%) 12 (18.2%) | 53 (84.1%) 10 (15.9%) | 0.727 |
| Mean total tumor diameter, cm, (±STD) | 6.9 ± 3.8 | 6.5 ± 4.0 | 7.4 ± 3.6 | 0.180 |
| Total tumor diameter, n (%) ≤10 cm >10 cm | 111 (86.0) 18 (14.0) | 56 (84.8) 10 (15.5) | 55 (87.3) 8 (12.7) | 0.688 |
| Milan status, n (%) In Out | 71 (55.0) 58 (45.0) | 38 (57.6) 28 (42.4) | 33 (52.4) 30 (47.6) | 0.553 |
| TACE prior LT, n (%) Yes No | 94 (72.9) 35 (27.1) | 49 (74.2) 17 (25.8) | 45 (71.4) 18 (28.6) | 0.719 |
| Median AFP level, mg/dL, (range) | 70 (2.7–46,926) | 56 (2.7–5580) | 100 (3.2–46,926) | 0.151 |
| Median CRP level, mg/dL, (range) | 1 (0.1–9.5) | 0.7 (0.1–4) | 1 (0.2–9.5) | 0.008 |
| Median IL-6 level, ng/dL, (range) | 17 (7–35) | 15 (7–35) | 17 (6–70) | 0.001 |
| Mean TSUVmax/LSUVmax, (±STD) | 1.53 (0.79) | 1.25 (0.45) | 1.82 (0.94) | <0.001 |
| PET status, n (%) Negative Positive | 80 (62) 49 (38) | 47 (71.2) 19 (28.8) | 33 (52.4) 30 (47.6) | 0.028 |
| Grading, n (%) Well/moderate Poor | 105 (81.4) 24 (18.6) | 58 (87.9) 8 (12.1) | 47 (74.6) 16 (25.4) | 0.053 |
| MVI, n (%) No Yes | 79 (61.2) 50 (38.8) | 46 (69.7) 20 (30.3) | 33 (52.4) 30 (47.6) | 0.044 |
| Post-TACE tumor necrosis, n (%) >75% ≤75% | 33 (35.1) 61 (64.9) | 26 (53.1) 23 (46.9) | 7 (15.6) 38 (84.4) | 0.001 |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Prognostic Factors | OR (95% CI) | p Value | OR (95% CI) | p Value |
| Male sex | 1.14 (0.498–2.603) | 0.758 | ||
| Age recipients’ > 60 years | 1.48 (0.659–3.336) | 0.341 | ||
| Virus-related liver cirrhosis | 1.53 (0.617–3.778) | 0.359 | ||
| Child–Pugh cirrhosis B or C | 3.03 (1.196–7.693) | 0.019 | ||
| MELD score > 20 | 3.00 (1.292–6.945) | 0.011 | ||
| Ascites | 4.62 (1.969–10.856) | 0.001 | ||
| Esophago-gastric varices | 4.60 (1.908–11.094) | 0.001 | ||
| Splenomegaly | 2.09 (0.923–4.738) | 0.077 | ||
| Thrombocytopenia | 2.17 (0.852–5.259) | 0.106 | ||
| CEPH | 5.19 (2.038–13.199) | 0.001 | 6.88 (1.663–28.484) | 0.008 |
| Number of tumor nodules > 3 | 4.19 (1.546–11.362) | 0.005 | ||
| Multifocal tumor manifestation | 2.99 (1.222–7.342) | 0.017 | ||
| Size of largest nodule > 5 cm | 1.61 (0.590–4.412) | 0.096 | ||
| Total tumor diameter > 10 cm | 4.05 (1.437–11.390) | 0.008 | ||
| Milan-out | 4.21 (1.750–10.125) | 0.001 | 3.90 (1.019–14.923) | 0.047 |
| No TACE prior LT | 0.55 (0.240–1.279) | 0.167 | ||
| AFP level > 100 mg/dL | 8.87 (3.508–22.398) | <0.001 | 8.24 (2.122–31.962) | 0.002 |
| CRP level > 1 mg/dL | 6.85 (2.82–16.612) | <0.001 | ||
| IL-6 level > 17 ng/dL | 3.95 (1.672–9.344) | 0.002 | 7.53 (1.863–30.394) | 0.005 |
| PET+ status | 23.31 (7.366–73.813) | <0.001 | 26.38 (5.884–118.276) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kornberg, A.; Seyfried, N.; Friess, H. Clinically Evident Portal Hypertension Is an Independent Risk Factor of Hepatocellular Carcinoma Recurrence Following Liver Transplantation. J. Clin. Med. 2025, 14, 2032. https://doi.org/10.3390/jcm14062032
Kornberg A, Seyfried N, Friess H. Clinically Evident Portal Hypertension Is an Independent Risk Factor of Hepatocellular Carcinoma Recurrence Following Liver Transplantation. Journal of Clinical Medicine. 2025; 14(6):2032. https://doi.org/10.3390/jcm14062032
Chicago/Turabian StyleKornberg, Arno, Nick Seyfried, and Helmut Friess. 2025. "Clinically Evident Portal Hypertension Is an Independent Risk Factor of Hepatocellular Carcinoma Recurrence Following Liver Transplantation" Journal of Clinical Medicine 14, no. 6: 2032. https://doi.org/10.3390/jcm14062032
APA StyleKornberg, A., Seyfried, N., & Friess, H. (2025). Clinically Evident Portal Hypertension Is an Independent Risk Factor of Hepatocellular Carcinoma Recurrence Following Liver Transplantation. Journal of Clinical Medicine, 14(6), 2032. https://doi.org/10.3390/jcm14062032






