Effect of Oral Microbiota Composition on Metabolic Dysfunction-Associated Steatotic Liver Disease in the General Population
Abstract
1. Introduction
2. Materials and Methods
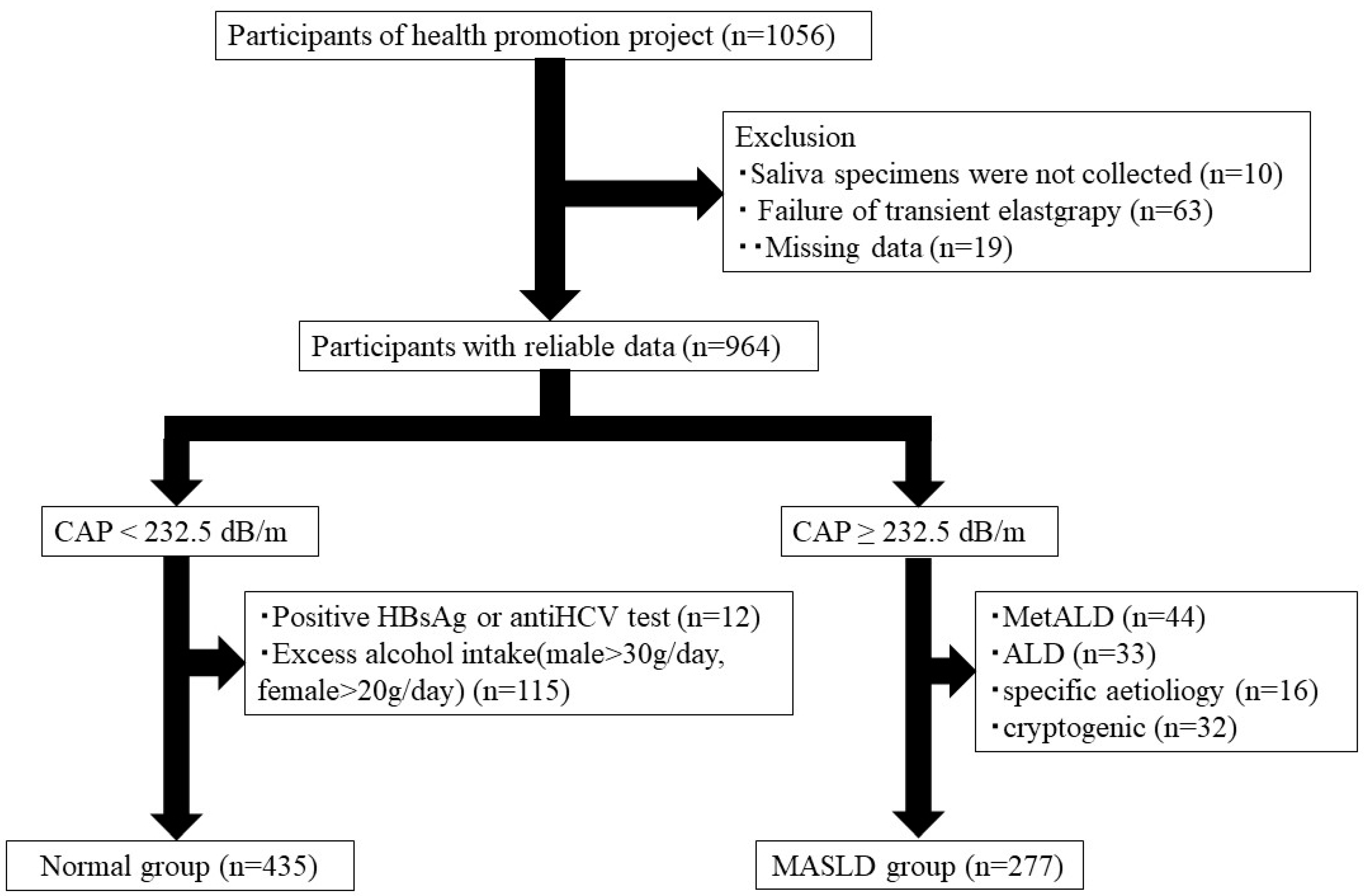
2.1. Study Participants
2.2. Transient Elastography
2.3. Clinical Parameters
2.4. MASLD Diagnosis
2.5. Measurements of Oral Microbiota
2.6. Oral Microbiota Pattern Analysis
2.7. Statistical Analysis
2.8. Ethics Statement
3. Results
3.1. Participant Characteristics
3.2. Risk Factors for Liver Fibrosis in Patients with MASLD
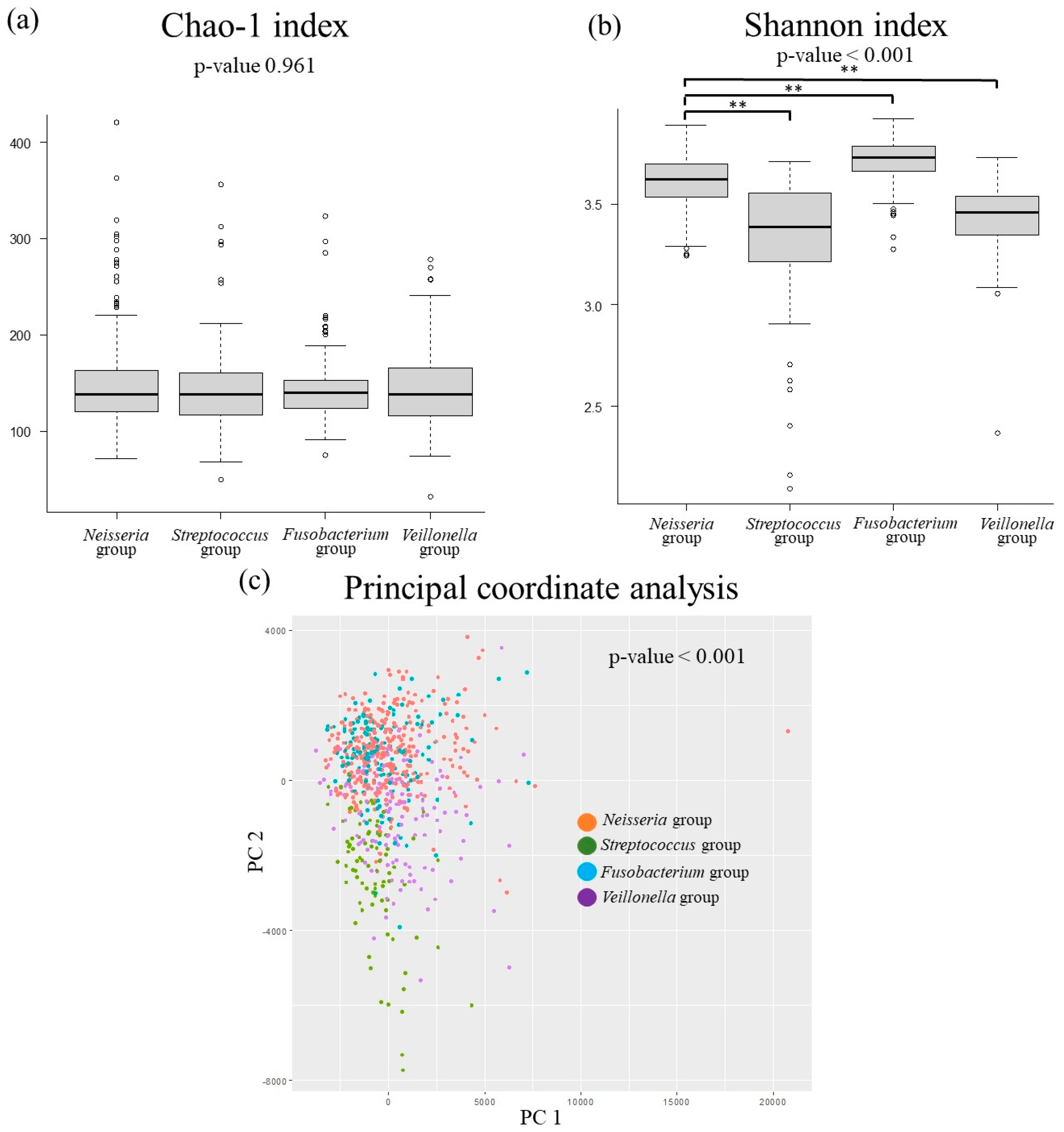
3.3. The Relationship Between MASLD-Related Items and Oral Microbiota
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MASLD | metabolic dysfunction-associated steatotic liver disease |
| IL | interleukin |
| TNF | tumor necrosis factor-alpha |
| NAFLD | non-alcoholic fatty liver disease |
| NASH | non-alcoholic steatohepatitis |
| CAP | controlled attenuation parameter |
| SLD | steatotic liver disease |
| LDL | low-density lipoprotein |
| HDL | high-density lipoprotein |
References
- Castillo-Núñez, Y.; Almeda-Valdes, P.; González-Gálvez, G.; Arechavaleta-Granell, M.D.R. Metabolic dysfunction-associated steatotic liver disease and atherosclerosis. Curr. Diab Rep. 2024, 24, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Leite, N.C.; Villela-Nogueira, C.A.; Cardoso, C.R.; Salles, G.F. Non-alcoholic fatty liver disease and diabetes: From physiopathological interplay to diagnosis and treatment. World J. Gastroenterol. 2014, 20, 8377–8392. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Gramlich, T.; Matteoni, C.A.; Boparai, N.; McCullough, A.J. Nonalcoholic fatty liver disease in patients with type 2 diabetes. Clin. Gastroenterol. Hepatol. 2004, 2, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology 2023, 78, 1966–1986. [Google Scholar] [CrossRef]
- Riazi, K.; Azhari, H.; Charette, J.H.; Underwood, F.E.; King, J.A.; Afshar, E.E.; Swain, M.G.; Congly, S.E.; Kaplan, G.G.; Shaheen, A.A. The prevalence and incidence of NAFLD worldwide: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2022, 7, 851–861. [Google Scholar] [CrossRef]
- Nazir, M.A. Prevalence of periodontal disease, its association with systemic diseases and prevention. Int. J. Health Sci. 2017, 11, 72–80. [Google Scholar]
- Genco, R.J.; Borgnakke, W.S. Diabetes as a potential risk for periodontitis: Association studies. Periodontology 2000 2020, 83, 40–45. [Google Scholar] [CrossRef]
- Nocini, R.; Favaloro, E.J.; Sanchis-Gomar, F.; Lippi, G. Periodontitis, coronary heart disease and myocardial infarction: Treat one, benefit all. Blood Coagul. Fibrinolysis 2020, 31, 339–345. [Google Scholar] [CrossRef]
- Pardo, A.; Barilli, A.; Signoriello, A.; Gualtieri, M.; Brancato, G.; Colapinto, G.; Lombardo, G.; Albanese, M. Oral health conditions and hygiene procedures in patients with Parkinson’s disease: A systematic review. Explor. Med. 2024, 5, 852–869. [Google Scholar] [CrossRef]
- Shine, B.K.; Son, M.; Moon, S.Y.; Han, S.H. Metabolic Dysfunction-Associated Steatotic Liver Disease and the Risk of Chronic Periodontitis: A Nationwide Cohort Study. Nutrients 2024, 17, 125. [Google Scholar] [CrossRef]
- Chen, T.P.; Yu, H.C.; Lin, W.Y.; Chang, Y.C. The role of microbiome in the pathogenesis of oral-gut-liver axis between periodontitis and nonalcoholic fatty liver disease. J. Dent. Sci. 2023, 18, 972–975. [Google Scholar] [CrossRef] [PubMed]
- Konkel, J.E.; O’Boyle, C.; Krishnan, S. Distal Consequences of Oral Inflammation. Front. Immunol. 2019, 10, 1403. [Google Scholar] [CrossRef]
- Kuraji, R.; Sekino, S.; Kapila, Y.; Numabe, Y. Periodontal disease-related nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: An emerging concept of oral-liver axis. Periodontol. 2000 2021, 87, 204–240. [Google Scholar] [CrossRef]
- Cekici, A.; Kantarci, A.; Hasturk, H.; Van Dyke, T.E. Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontol. 2000 2014, 64, 57–80. [Google Scholar] [CrossRef]
- Nakaji, S.; Ihara, K.; Sawada, K.; Parodi, S.; Umeda, T.; Takahashi, I.; Murashita, K.; Kurauchi, S.; Tokuda, I. Social innovation for life expectancy extension utilizing a platform-centered system used in the Iwaki health promotion project: A protocol paper. SAGE Open Med. 2021, 9, 20503121211002606. [Google Scholar] [CrossRef] [PubMed]
- Masaki, K.; Takaki, S.; Hyogo, H.; Kobayashi, T.; Fukuhara, T.; Naeshiro, N.; Honda, Y.; Nakahara, T.; Ohno, A.; Miyaki, D.; et al. Utility of controlled attenuation parameter measurement for assessing liver steatosis in Japanese patients with chronic liver diseases. Hepatol. Res. 2013, 43, 1182–1189. [Google Scholar] [CrossRef]
- Takahashi, S.; Tomita, J.; Nishioka, K.; Hisada, T.; Nishijima, M. Development of a prokaryotic universal primer for simultaneous analysis of Bacteria and Archaea using next-generation sequencing. PLoS ONE 2014, 9, e105592. [Google Scholar] [CrossRef]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar]
- Schenkein, H.A.; Loos, B.G. Inflammatory mechanisms linking periodontal diseases to cardiovascular diseases. J. Clin. Periodontol. 2013, 40 (Suppl. S14), S51–S69. [Google Scholar] [CrossRef]
- Madureira, D.F.; Lucas De Abreu Lima, I.; Costa, G.C.; Lages, E.M.B.; Martins, C.C.; Aparecida Da Silva, T. Tumor Necrosis Factor-alpha in Gingival Crevicular Fluid as a Diagnostic Marker for Periodontal Diseases: A Systematic Review. J. Evid. Based Dent. Pract. 2018, 18, 315–331. [Google Scholar] [CrossRef] [PubMed]
- Bansal, M.; Khatri, M.; Taneja, V. Potential role of periodontal infection in respiratory diseases—A review. J. Med. Life 2013, 6, 244–248. [Google Scholar]
- Mojon, P.; Bourbeau, J. Respiratory infection: How important is oral health? Curr. Opin. Pulm. Med. 2003, 9, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Mojon, P. Oral health and respiratory infection. J. Can. Dent. Assoc. 2002, 68, 340–345. [Google Scholar] [PubMed]
- Yu, B.; Wang, C.Y. Osteoporosis and periodontal diseases—An update on their association and mechanistic links. Periodontology 2000 2022, 89, 99–113. [Google Scholar] [CrossRef]
- Elghannam, M.T.; Hassanien, M.H.; Ameen, Y.A.; Turky, E.A.; Elattar, G.M.; ElRay, A.A.; Eltalkawy, M.D. Oral microbiota and liver diseases. Clin. Nutr. ESPEN 2023, 54, 68–72. [Google Scholar] [CrossRef]
- Lei, Y.; Li, S.; He, M.; Ao, Z.; Wang, J.; Wu, Q.; Wang, Q. Oral Pathogenic Bacteria and the Oral-Gut-Liver Axis: A New Understanding of Chronic Liver Diseases. Diagnostics 2023, 13, 3324. [Google Scholar] [CrossRef]
- Yamashita, Y.; Takeshita, T. The oral microbiome and human health. J. Oral Sci. 2017, 59, 201–206. [Google Scholar] [CrossRef]
- Takeshita, T.; Nakano, Y.; Kumagai, T.; Yasui, M.; Kamio, N.; Shibata, Y.; Shiota, S.; Yamashita, Y. The ecological proportion of indigenous bacterial populations in saliva is correlated with oral health status. Isme J. 2009, 3, 65–78. [Google Scholar] [CrossRef]
- Kelly, T.N.; Bazzano, L.A.; Ajami, N.J.; He, H.; Zhao, J.; Petrosino, J.F.; Correa, A.; He, J. Gut Microbiome Associates With Lifetime Cardiovascular Disease Risk Profile Among Bogalusa Heart Study Participants. Circ. Res. 2016, 119, 956–964. [Google Scholar] [CrossRef]
- Takeshita, T.; Kageyama, S.; Furuta, M.; Tsuboi, H.; Takeuchi, K.; Shibata, Y.; Shimazaki, Y.; Akifusa, S.; Ninomiya, T.; Kiyohara, Y.; et al. Bacterial diversity in saliva and oral health-related conditions: The Hisayama Study. Sci. Rep. 2016, 6, 22164. [Google Scholar] [CrossRef]
- Wang, M.; Yan, L.-Y.; Qiao, C.-Y.; Zheng, C.-C.; Niu, C.-G.; Huang, Z.-W.; Pan, Y.-H. Ecological shifts of salivary microbiota associated with metabolic-associated fatty liver disease. Front. Cell. Infect. Microbiol. 2023, 13, 1131255. [Google Scholar] [CrossRef]
- Zhao, F.; Dong, T.; Yuan, K.Y.; Wang, N.J.; Xia, F.Z.; Liu, D.; Wang, Z.M.; Ma, R.; Lu, Y.L.; Huang, Z.W. Shifts in the Bacterial Community of Supragingival Plaque Associated With Metabolic-Associated Fatty Liver Disease. Front. Cell Infect. Microbiol. 2020, 10, 581888. [Google Scholar] [CrossRef]
- Dong, L.; Yin, J.; Zhao, J.; Ma, S.R.; Wang, H.R.; Wang, M.; Chen, W.; Wei, W.Q. Microbial Similarity and Preference for Specific Sites in Healthy Oral Cavity and Esophagus. Front. Microbiol. 2018, 9, 1603. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Chaparro, P.J.; McCulloch, J.A.; Mamizuka, E.M.; Moraes, A.; Faveri, M.; Figueiredo, L.C.; Duarte, P.M.; Feres, M. Do different probing depths exhibit striking differences in microbial profiles? J. Clin. Periodontol. 2018, 45, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Zaura, E.; Keijser, B.J.; Huse, S.M.; Crielaard, W. Defining the healthy “core microbiome” of oral microbial communities. BMC Microbiol. 2009, 9, 259. [Google Scholar] [CrossRef] [PubMed]
- Bahadoran, Z.; Mirmiran, P.; Carlström, M.; Ghasemi, A. Inorganic nitrate: A potential prebiotic for oral microbiota dysbiosis associated with type 2 diabetes. Nitric Oxide 2021, 116, 38–46. [Google Scholar] [CrossRef]
- Chen, B.; Wang, Z.; Wang, J.; Su, X.; Yang, J.; Zhang, Q.; Zhang, L. The oral microbiome profile and biomarker in Chinese type 2 diabetes mellitus patients. Endocrine 2020, 68, 564–572. [Google Scholar] [CrossRef]
- Li, B.Z.; Zhou, H.Y.; Guo, B.; Chen, W.J.; Tao, J.H.; Cao, N.W.; Chu, X.J.; Meng, X. Dysbiosis of oral microbiota is associated with systemic lupus erythematosus. Arch. Oral. Biol. 2020, 113, 104708. [Google Scholar] [CrossRef]
- Bui, F.Q.; Almeida-da-Silva, C.L.C.; Huynh, B.; Trinh, A.; Liu, J.; Woodward, J.; Asadi, H.; Ojcius, D.M. Association between periodontal pathogens and systemic disease. Biomed. J. 2019, 42, 27–35. [Google Scholar] [CrossRef]
- Zhai, Q.; Wu, H.; Zheng, S.; Zhong, T.; Du, C.; Yuan, J.; Peng, J.; Cai, C.; Li, J. Association between gut microbiota and NAFLD/NASH: A bidirectional two-sample Mendelian randomization study. Front. Cell Infect. Microbiol. 2023, 13, 1294826. [Google Scholar] [CrossRef]
- Qin, N.; Yang, F.; Li, A.; Prifti, E.; Chen, Y.; Shao, L.; Guo, J.; Le Chatelier, E.; Yao, J.; Wu, L.; et al. Alterations of the human gut microbiome in liver cirrhosis. Nature 2014, 513, 59–64. [Google Scholar] [CrossRef]
- Palmas, V.; Pisanu, S.; Madau, V.; Casula, E.; Deledda, A.; Cusano, R.; Uva, P.; Vascellari, S.; Loviselli, A.; Manzin, A.; et al. Gut microbiota markers associated with obesity and overweight in Italian adults. Sci. Rep. 2021, 11, 5532. [Google Scholar] [CrossRef]
- Aranaz, P.; Ramos-Lopez, O.; Cuevas-Sierra, A.; Martinez, J.A.; Milagro, F.I.; Riezu-Boj, J.I. A predictive regression model of the obesity-related inflammatory status based on gut microbiota composition. Int. J. Obes. 2021, 45, 2261–2268. [Google Scholar] [CrossRef]
- Chen, B.Y.; Lin, W.Z.; Li, Y.L.; Bi, C.; Du, L.J.; Liu, Y.; Zhou, L.J.; Liu, T.; Xu, S.; Shi, C.J.; et al. Roles of oral microbiota and oral-gut microbial transmission in hypertension. J. Adv. Res. 2023, 43, 147–161. [Google Scholar] [CrossRef]
- Wang, L.; Xu, J.; You, N.; Shao, L.; Zhuang, Z.; Zhuo, L.; Liu, J.; Shi, J. Characteristics of intestinal flora in nonobese nonalcoholic fatty liver disease patients and the impact of ursodeoxycholic acid treatment on these features. Lipids 2024, 59, 193–207. [Google Scholar] [CrossRef]
- Huang, F.; Lyu, B.; Xie, F.; Li, F.; Xing, Y.; Han, Z.; Lai, J.; Ma, J.; Zou, Y.; Zeng, H.; et al. From gut to liver: Unveiling the differences of intestinal microbiota in NAFL and NASH patients. Front. Microbiol. 2024, 15, 1366744. [Google Scholar] [CrossRef]
- Lei, W.Y.; Chang, W.H.; Shih, S.C.; Liu, C.J.; Shih, C.H. Pyogenic liver abscess with Prevotella species and Fusobacterium necrophorum as causative pathogens in an immunocompetent patient. J. Formos. Med. Assoc. 2009, 108, 253–257. [Google Scholar] [CrossRef]
- Jiang, W.; Wu, N.; Wang, X.; Chi, Y.; Zhang, Y.; Qiu, X.; Hu, Y.; Li, J.; Liu, Y. Dysbiosis gut microbiota associated with inflammation and impaired mucosal immune function in intestine of humans with non-alcoholic fatty liver disease. Sci. Rep. 2015, 5, 8096. [Google Scholar] [CrossRef]
- Tonomura, S.; Naka, S.; Tabata, K.; Hara, T.; Mori, K.; Tanaka, S.; Sumida, Y.; Kanemasa, K.; Nomura, R.; Matsumoto-Nakano, M. Relationship between Streptococcus mutans expressing Cnm in the oral cavity and non-alcoholic steatohepatitis: A pilot study. BMJ Open Gastroenterol. 2019, 6, e000329. [Google Scholar] [CrossRef]
| Classification | Species | Factor 1 | Factor 2 | Factor 3 | Factor 4 |
|---|---|---|---|---|---|
| Phylum | Actinobacteria | 0.01 | 0.129 | −0.092 | 0.029 |
| Bacteroidetes | 0.005 | −0.111 | −0.002 | −0.028 | |
| Candidatus Saccharibacteria | −0.044 | −0.001 | −0.106 | −0.004 | |
| Firmicutes | 0.079 | 0.017 | 0.083 | −0.015 | |
| Fusobacteria | 0.035 | 0.039 | 0.03 | 0.164 | |
| Proteobacteria | −0.064 | −0.021 | 0.037 | −0.017 | |
| Class | Actinobacteria | 0.01 | 0.129 | −0.092 | 0.029 |
| Bacilli | 0.035 | 0.028 | 0.066 | −0.04 | |
| Bacteroidia | 0.008 | −0.111 | −0.004 | −0.033 | |
| Clostridia | 0.029 | 0.023 | −0.029 | 0.116 | |
| Fusobacteriia | 0.035 | 0.039 | 0.03 | 0.164 | |
| Gammaproteobacteria | 0.028 | −0.009 | 0.124 | 0.036 | |
| Negativicutes | 0.104 | −0.034 | 0.062 | 0.016 | |
| Order | Actinomycetales | −0.005 | 0.143 | −0.089 | 0.035 |
| Bacteroidales | 0.008 | −0.111 | −0.004 | −0.033 | |
| Betaproteobacteria | −0.102 | −0.021 | −0.027 | −0.047 | |
| Clostridiales | 0.03 | 0.023 | −0.029 | 0.117 | |
| Coriobacteriales | 0.043 | −0.013 | −0.031 | −0.011 | |
| Fusobacteriales | 0.035 | 0.039 | 0.03 | 0.164 | |
| Lactobacillales | 0.036 | 0.025 | 0.065 | −0.043 | |
| Neisseriales | −0.102 | −0.021 | −0.028 | −0.047 | |
| Pasteurellales | 0.029 | −0.01 | 0.124 | 0.036 | |
| Selenomonadales | 0.104 | −0.034 | 0.062 | 0.016 | |
| Family | Actinomycetaceae | 0.013 | 0.066 | −0.098 | 0.051 |
| Carnobacteriaceae | −0.018 | 0.032 | 0.026 | −0.007 | |
| Coriobacteriaceae | 0.043 | −0.013 | −0.031 | −0.011 | |
| Fusobacteriaceae | 0.026 | 0.027 | 0.053 | 0.143 | |
| Lachnospiraceae | 0.04 | 0.023 | −0.036 | 0.094 | |
| Micrococcaceae | −0.012 | 0.111 | −0.033 | 0.006 | |
| Neisseriaceae | −0.102 | −0.021 | −0.028 | −0.047 | |
| Pasteurellaceae | 0.029 | −0.01 | 0.124 | 0.036 | |
| Porphyromonadaceae | −0.044 | −0.008 | 0.016 | 0.026 | |
| Prevotellaceae | 0.021 | −0.104 | −0.009 | −0.039 | |
| Streptococcaceae | 0.041 | 0.022 | 0.066 | −0.043 | |
| Veillonellaceae | 0.104 | −0.034 | 0.062 | 0.016 | |
| Genus | Actinomyces | 0.012 | 0.066 | −0.098 | 0.051 |
| Atopobium | 0.042 | −0.015 | −0.032 | −0.013 | |
| Fusobacterium | 0.026 | 0.027 | 0.053 | 0.143 | |
| Granulicatella | −0.018 | 0.032 | 0.026 | −0.007 | |
| Haemophilus | 0.029 | −0.01 | 0.124 | 0.037 | |
| Neisseria | −0.102 | −0.021 | −0.028 | −0.047 | |
| Porphyromonas | −0.045 | −0.01 | 0.016 | 0.023 | |
| Prevotella | 0.027 | −0.098 | −0.009 | −0.046 | |
| Rothia | −0.012 | 0.111 | −0.033 | 0.006 | |
| Saccharibacteria_genera_incertae_sedis | −0.044 | −0.001 | −0.106 | −0.004 | |
| Streptococcus | 0.041 | 0.022 | 0.066 | −0.043 | |
| Veillonella | 0.105 | −0.035 | 0.069 | 0.013 |
| Genera | First Group n = 334 | Second Group n = 86 | Third Group n = 149 | Fourth Group n = 143 |
|---|---|---|---|---|
| Actinomyces | 6.4 (4.5–8.9) | 7.1 (3.3–11.2) | 6.9 (4.6–10.3) | 6.1 (4.5–7.3) |
| Atopobium | 1.5 (0.7–2.8) | 1.4 (0.5–2.5) | 1.5 (0.6–2.7) | 2.5 (0.9–4.0) |
| Fusobacterium | 2.0 (1.2–2.7) | 0.9 (0.4–1.8) | 4.1 (2.8–5.6) | 1.3 (0.5–2.2) |
| Granulicatella | 1.2 (0.8–1.8) | 1.9 (1.2–2.5) | 1.2 (0.7–1.7) | 1.2 (0.7–1.9) |
| Haemophilus | 4.2 (2.1–6.1) | 4.3 (1.2–7.6) | 4.4 (2.3–7.6) | 4.3 (1.9–8.0) |
| Neisseria | 10.6 (4.2–17.9) | 2.3 (0.4–5.4) | 6.9 (2.6–11.1) | 1.6 (0.5–4.8) |
| Porphyromonas | 1.8 (0.6–4.4) | 0.3 (0.1–1.1) | 2.8 (1.3–5.1) | 0.5 (0.2–1.5) |
| Prevotella | 16.8 (10.6–21.9) | 7.0 (3.3–11.1) | 14.5 (8.7–20.6) | 18.6 (11.8–23.8) |
| Rothia | 3.1 (1.8–5.3) | 13.7 (9.2–20.3) | 2.8 (1.5–5.2) | 4.8 (2.9–6.3) |
| Saccharibacteria_genera_incertae_sedis | 9.0 (4.8–14.5) | 2.3 (0.5–4.5) | 6.6 (3.4–11.2) | 2.6 (0.9–5.6) |
| Streptococcus | 17.3 (13.8–21.1) | 32.7 (25.5–38.9) | 15.5 (11.9–19.6) | 26.2 (22.0–32.1) |
| Veillonella | 7.4 (5.3–9.6) | 8.3 (5.7–20.3) | 8.3 (6.4–10.6) | 12.7 (9.8–15.7) |
| Neisseria Group n = 334 | Streptococcus Group n = 86 | Fusobacterium Group n = 149 | Veillonella Group n = 143 | Neisseria vs. Streptococcus | Neisseria vs. Fusobacterium | Neisseria vs. Veillonella | |
|---|---|---|---|---|---|---|---|
| sex, male | 94 (28.1%) | 41 (47.7%) | 62 (41.6%) | 40 (28.0%) | 0.005 | 0.029 | 0.999 |
| Age (year) | 50.0 (37.0–64.0) | 57.0 (38.0–68.0) | 57.0 (42.0–66.0) | 56.0 (39.0–66.0) | 0.225 | 0.114 | 0.183 |
| BMI (kg/m2) | 22.2 (19.6–24.6) | 21.8 (20.2–23.6) | 23.2 (20.7–25.8) | 22.3 (20.0–25.1) | 0.964 | 0.009 | 0.639 |
| Waist circumference (cm) | 74.0 (67.2–82.5) | 74.2 (68.9–82.8) | 79.0 (71.0–86.4) | 74.4 (68.0–83.8) | 0.762 | <0.001 | 0.667 |
| Fasting blood sugar (mmHg) | 90.0 (85.0–98.0) | 92.5 (86.8–100.3) | 92.0 (87.5–100.5) | 92.0 (85.0–98.0) | 0.263 | 0.041 | 0.794 |
| HbA1c (%) | 5.7 (5.5–5.9) | 5.7 (5.5–6.0) | 5.7 (5.5–5.9) | 5.7 (5.5–5.9) | 0.633 | 0.890 | 0.895 |
| Systolic blood pressure (mmHg) | 120.0 (109.0–131.3) | 121.5 (110.5–133.5) | 127.0 (114.5–139.0) | 123.0 (111.0–134.0) | 0.997 | 0.004 | 0.498 |
| Diastolic blood pressure (mmHg) | 76.0 (69.0–83.0) | 76.0 (69.0–82.8) | 78.0 (69.5–87.0) | 77.0 (71.0–86.0) | 0.987 | 0.188 | 0.196 |
| Triglycerides (mg/dL) | 72.0 (50.0–105.0) | 77.0 (53.8–97.3) | 82.0 (57.0–123.5) | 75.0 (54.0–110.0) | 0.962 | 0.036 | 0.663 |
| HDL cholesterol (mg/dL) | 63.0 (53.0–74.3) | 62.0 (54.8–78.3) | 62.0 (50.0–75.0) | 64.0 (55.0–78.0) | 0.907 | 0.999 | 0.475 |
| LDL cholesterol (mg/dL) | 116.0 (96.8–135.3) | 112.0 (98.5–136.5) | 119.0 (99.5–142.0) | 118.0 (99.0–138.0) | 0.999 | 0.299 | 0.535 |
| Aspartate aminotransferase (IU/L) | 20.0 (17.0–24.0) | 22.0 (17.0–25.0) | 21.0 (17.5–26.0) | 20.0 (17.0–25.0) | 0.447 | 0.099 | 0.955 |
| Alanine aminotransferase (IU/L) | 17.0 (12.0–23.0) | 18.0 (13.0–23.0) | 20.0 (14.0–29.0) | 17.0 (13.0–24.0) | 0.762 | 0.006 | 0.646 |
| γ-Glutamyl TransPeptidase (IU/L) | 19.0 (14.0–31.0) | 21.0 (15.0–35.0) | 21.0 (17.0–35.0) | 20.0 (15.0–30.0) | 0.355 | 0.017 | 0.739 |
| CAP (dB/m) | 208.0 (168.8–251.3) | 211.5 (174.5–261.3) | 223.0 (195.0–274.0) | 228.0 (185.0–267.0) | 0.943 | 0.002 | 0.084 |
| LSM (kPa) | 4.3 (3.5–5.4) | 4.1 (3.5–5.6) | 4.4 (3.6–5.3) | 4.3 (3.6–5.4) | 0.982 | 0.986 | 0.999 |
| Fatty liver index | 10.7 (4.1–28.4) | 11.8 (4.7–23.0) | 20.2 (6.8–45.8) | 13.4 (5.7–31.7) | 0.868 | <0.001 | 0.338 |
| Smoking habit | 35 (10.5%) | 17 (19.8%) | 19 (12.8%) | 22 (15.4%) | 0.190 | 0.999 | 0.999 |
| Exercise habit | 57 (17.1%) | 18 (20.9%) | 30 (20.1%) | 18 (12.6%) | 0.999 | 0.999 | 0.999 |
| MASLD | 115 (34.4%) | 31 (36.0%) | 66 (44.3%) | 65 (45.5%) | 0.999 | 0.300 | 0.180 |
| Cardiometabolic risk factors | |||||||
| High blood pressure | 144 (43.1%) | 44 (51.2%) | 91 (61.1%) | 71 (49.7%) | 0.999 | 0.002 | 0.999 |
| Obesity/central obesity | 85 (25.4%) | 19 (22.1%) | 58 (38.9%) | 40 (28.0%) | 0.999 | 0.023 | 0.999 |
| Hyperglycemia or diabetes | 182 (54.5%) | 56 (65.1%) | 86 (57.7%) | 84 (58.7%) | 0.590 | 0.999 | 0.999 |
| Resuce HDL-cholesterol | 12.3% | 8 (9.3%) | 19 (12.8%) | 11 (7.7%) | 0.999 | 0.999 | 0.999 |
| High triglycerides | 68 (20.4%) | 17 (19.8%) | 41 (27.5%) | 30 (21.0%) | 0.999 | 0.630 | 0.999 |
| Cardiometabolic crieria ≥ 3 | 76 (22.8%) | 17 (19.8%) | 53 (35.6%) | 37 (25.9%) | 0.999 | 0.028 | 0.999 |
| Univariable | Multivariable | |||||||
|---|---|---|---|---|---|---|---|---|
| OR | 95%CI | p-Value | OR | 95%CI | p-Value | |||
| Male | 1.64 | 1.19 | 2.25 | 0.002 | 1.41 | 0.95 | 2.09 | 0.084 |
| Age | 1.02 | 1.01 | 1.03 | <0.001 | 1.01 | 0.99 | 1.02 | 0.405 |
| smoking habit | 1.56 | 1.01 | 2.42 | 0.045 | 1.51 | 0.87 | 2.61 | 0.140 |
| exercise habit | 1.14 | 0.77 | 1.69 | 0.522 | 0.93 | 0.58 | 1.49 | 0.773 |
| Obesity/central obesity | 7.28 | 5.06 | 10.50 | <0.001 | 4.91 | 3.27 | 7.35 | <0.001 |
| Hyperglycemia or diabetes | 4.22 | 3.01 | 5.92 | <0.001 | 2.90 | 1.92 | 4.36 | <0.001 |
| High blood pressure | 2.55 | 1.87 | 3.48 | <0.001 | 1.42 | 0.95 | 2.14 | 0.092 |
| High triglycerides | 3.69 | 2.54 | 5.35 | <0.001 | 2.29 | 1.48 | 2.55 | <0.001 |
| Reduce HDL-cholesterol | 4.54 | 2.72 | 7.58 | <0.001 | 2.70 | 1.47 | 4.95 | <0.001 |
| Oral microbiota pattern | ||||||||
| Neisseria group | 1.00 | 1.00 | ||||||
| Streptococcus group | 1.07 | 0.65 | 1.76 | 0.779 | 0.96 | 0.54 | 1.72 | 0.894 |
| Fusobacterium group | 1.51 | 1.02 | 2.25 | 0.039 | 1.08 | 0.67 | 1.73 | 0.767 |
| Veillonella group | 1.59 | 1.06 | 2.37 | 0.023 | 1.68 | 1.05 | 2.70 | 0.031 |
| Neisseria | Streptococcus | Fusobacterium | Veillonella | |||||
|---|---|---|---|---|---|---|---|---|
| r | p-Value | r | p-Value | r | p-Value | r | p-Value | |
| CAP | −0.132 | <0.001 | −0.094 | 0.012 | 0.035 | 0.345 | 0.158 | <0.001 |
| BMI | −0.121 | 0.001 | −0.054 | 0.153 | 0.014 | 0.712 | 0.138 | <0.001 |
| Waist circumference | −0.124 | 0.001 | −0.048 | 0.203 | 0.045 | 0.236 | 0.118 | 0.002 |
| Systolic blood pressure | −0.065 | 0.084 | −0.049 | 0.192 | 0.001 | 0.988 | 0.112 | 0.003 |
| Diastolic blood pressure | −0.067 | 0.072 | 0.007 | 0.846 | −0.028 | 0.462 | 0.097 | 0.010 |
| Blood glucose | −0.092 | 0.014 | 0.013 | 0.725 | −0.033 | 0.382 | 0.095 | 0.012 |
| HbA1c | −0.109 | 0.004 | 0.027 | 0.465 | −0.075 | 0.046 | 0.104 | 0.006 |
| Triglycerides | −0.124 | 0.001 | −0.05 | 0.186 | −0.001 | 0.970 | 0.148 | <0.001 |
| HDL cholesterol | 0.076 | 0.041 | 0.023 | 0.533 | 0.015 | 0.689 | −0.079 | 0.034 |
| Aspartate aminotransferase | −0.06 | 0.107 | −0.053 | 0.155 | 0.072 | 0.054 | 0.092 | 0.014 |
| Alanine aminotransferase | −0.107 | 0.004 | −0.078 | 0.037 | 0.115 | 0.002 | 0.089 | 0.018 |
| Gamma-glutamyl transpeptidase | −0.14 | <0.001 | −0.075 | 0.046 | 0.042 | 0.267 | 0.160 | <0.001 |
| Fatty liver index | −0.083 | 0.026 | −0.063 | 0.092 | 0.095 | 0.011 | 0.070 | 0.062 |
| LSM | −0.04 | 0.283 | 0.018 | 0.637 | 0.030 | 0.425 | 0.020 | 0.602 |
| Neisseria | Streptococcus | Fusobacterium | Veillonella | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | p | R2 | β | p | R2 | β | p | R2 | β | p | R2 | |
| CAP | −0.092 | 0.017 | 0.065 | −0.125 | 0.001 | 0.030 | 0.053 | 0.178 | 0.028 | 0.137 | <0.001 | 0.038 |
| Body mass index | −0.099 | 0.010 | 0.067 | −0.071 | 0.070 | 0.021 | 0.001 | 0.972 | 0.025 | 0.137 | <0.001 | 0.038 |
| Waist circumference | −0.097 | 0.023 | 0.065 | −0.090 | 0.040 | 0.022 | 0.030 | 0.495 | 0.026 | 0.133 | 0.002 | 0.034 |
| Systolic blood pressure | −0.058 | 0.150 | 0.061 | −0.059 | 0.155 | 0.019 | −0.002 | 0.969 | 0.025 | 0.101 | 0.015 | 0.029 |
| Diastolic blood pressure | −0.057 | 0.136 | 0.061 | 0.007 | 0.855 | 0.016 | −0.040 | 0.306 | 0.027 | 0.088 | 0.023 | 0.028 |
| Blood glucose | −0.059 | 0.135 | 0.061 | 0.001 | 0.981 | 0.016 | −0.029 | 0.465 | 0.026 | 0.069 | 0.087 | 0.025 |
| HbA1c | −0.077 | 0.046 | 0.063 | 0.016 | 0.678 | 0.016 | −0.060 | 0.129 | 0.028 | 0.073 | 0.062 | 0.026 |
| Triglycerides | −0.073 | 0.065 | 0.062 | −0.087 | 0.031 | 0.023 | 0.002 | 0.963 | 0.025 | 0.136 | <0.001 | 0.036 |
| HDL cholesterol | 0.050 | 0.207 | 0.060 | 0.046 | 0.256 | 0.018 | 0.047 | 0.250 | 0.027 | −0.098 | 0.016 | 0.029 |
| Aspartate aminotransferase | −0.066 | 0.100 | 0.062 | −0.058 | 0.157 | 0.019 | 0.060 | 0.137 | 0.028 | 0.094 | 0.021 | 0.028 |
| Alanine aminotransferase | −0.103 | 0.011 | 0.067 | −0.101 | 0.014 | 0.025 | 0.100 | 0.014 | 0.033 | 0.104 | 0.011 | 0.030 |
| Gamma-glutamyl transpeptidase | −0.052 | 0.199 | 0.060 | −0.095 | 0.021 | 0.024 | 0.087 | 0.034 | 0.031 | 0.074 | 0.074 | 0.025 |
| Fatty liver index | −0.105 | 0.012 | 0.066 | −0.129 | 0.003 | 0.029 | 0.040 | 0.347 | 0.026 | 0.172 | <0.001 | 0.043 |
| LSM | −0.038 | 0.302 | 0.059 | 0.017 | 0.651 | 0.016 | 0.025 | 0.497 | 0.026 | 0.022 | 0.560 | 0.021 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sato, S.; Iino, C.; Furusawa, K.; Yoshida, K.; Chinda, D.; Sawada, K.; Mikami, T.; Nakaji, S.; Fukuda, S.; Sakuraba, H. Effect of Oral Microbiota Composition on Metabolic Dysfunction-Associated Steatotic Liver Disease in the General Population. J. Clin. Med. 2025, 14, 2013. https://doi.org/10.3390/jcm14062013
Sato S, Iino C, Furusawa K, Yoshida K, Chinda D, Sawada K, Mikami T, Nakaji S, Fukuda S, Sakuraba H. Effect of Oral Microbiota Composition on Metabolic Dysfunction-Associated Steatotic Liver Disease in the General Population. Journal of Clinical Medicine. 2025; 14(6):2013. https://doi.org/10.3390/jcm14062013
Chicago/Turabian StyleSato, Satoshi, Chikara Iino, Keisuke Furusawa, Kenta Yoshida, Daisuke Chinda, Kaori Sawada, Tatsuya Mikami, Shigeyuki Nakaji, Shinsaku Fukuda, and Hirotake Sakuraba. 2025. "Effect of Oral Microbiota Composition on Metabolic Dysfunction-Associated Steatotic Liver Disease in the General Population" Journal of Clinical Medicine 14, no. 6: 2013. https://doi.org/10.3390/jcm14062013
APA StyleSato, S., Iino, C., Furusawa, K., Yoshida, K., Chinda, D., Sawada, K., Mikami, T., Nakaji, S., Fukuda, S., & Sakuraba, H. (2025). Effect of Oral Microbiota Composition on Metabolic Dysfunction-Associated Steatotic Liver Disease in the General Population. Journal of Clinical Medicine, 14(6), 2013. https://doi.org/10.3390/jcm14062013






