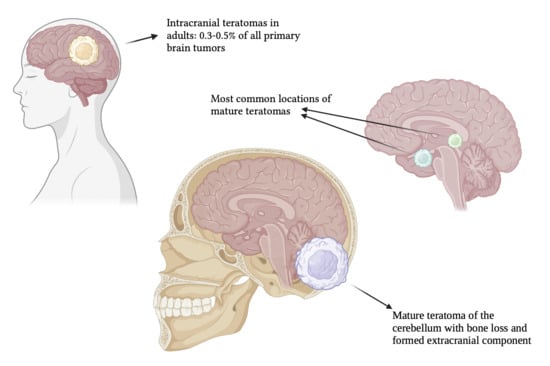
Mature Teratoma of the Cerebellum with Formed Extracranial Component
Abstract
1. Introduction
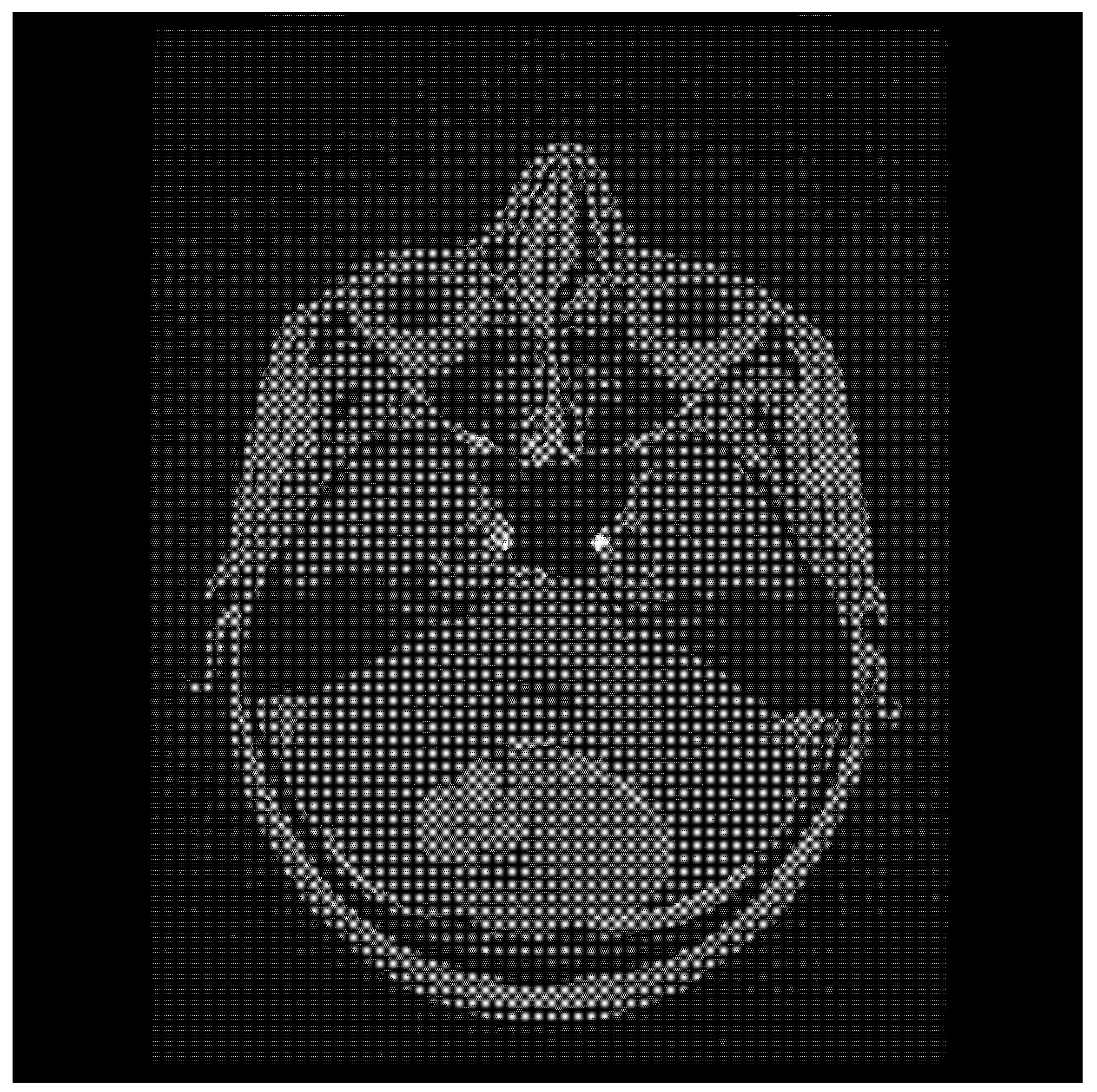
2. Case Report
3. Discussion
- Mature teratomas are typically benign tumors composed of well-differentiated tissues from all three germ layers (ectoderm, mesoderm, and endoderm), with low mitotic activity, without necrosis [2,20,21]. While they can occur in various locations within the central nervous system, they are often found in midline structures, such as the pineal and suprasellar regions [2]. Complete surgical resection, as the preferred treatment for mature teratomas, is usually associated with a favorable prognosis [22] [2,20].
- Immature teratomas are characterized by the presence of undifferentiated or embryonic tissues, often with neuroectodermal components [21,23,24,25]. This presence of immature tissue signifies incomplete differentiation, and is associated with a higher risk of malignant transformation and, subsequently, poorer outcomes compared to mature teratomas [23,24,25]. Immature teratomas exhibit rapid growth and can lead to substantial complications, especially in cases involving infants [26].
- Teratomas with somatic-type malignancy exhibit aggressive characteristics, including rapid growth and the potential for metastasis [27]. They can arise de novo or from pre-existing immature teratomas that undergo malignant transformation, which most commonly manifests as rhabdomyosarcoma or an undifferentiated sarcoma, and, less frequently, as squamous cell carcinoma or adenocarcinoma [21,27,28]. The presence of yolk sac tumor elements can also give rise to enteric-type adenocarcinoma [21]. Immunohistochemistry plays a crucial role in diagnosing malignant teratomas, differentiating the malignant components from the benign tissues within the teratoma [21]. Markers such as vimentin, desmin, smooth muscle actin, S-100, CD99, and glial fibrillary acidic protein are used to identify sarcomatous transformation, while cytokeratins (CK20, CK7) and p53 are helpful in cases of carcinomatous transformation [21]. The presence of these markers, along with the histologic appearance, confirms the diagnosis and guides a multimodal treatment approach involving surgery, chemotherapy, and radiation therapy [21,27,29].
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gondowardojo, Y.R.B.; Argie, D. AB056. Management of a Rare Case of Adult Intracranial Large Temporal Mature Teratoma in Limited Neurosurgical Care Settings: A Case Report. Chin. Clin. Oncol. 2024, 13, AB056. [Google Scholar] [CrossRef]
- Nery, B.; Felix Fernandes, R.D.; do Rego Nobrega, E.A.; da Silva, A.C.T.; Liebig, M.S.; Eloy Nóbrega, C.C.; Braga, J.L.; Souto Fernandes, T.D.; Quaggio, E.; de Sousa Segundo, J.A. Mature Congenital Intraventricular Intracranial Teratoma: A Case Report and Literature Review. Surg. Neurol. Int. 2024, 15, 259. [Google Scholar] [CrossRef]
- Jafri, S.K.K.; Hussain, N.; Bari, M.E. Mature Teratoma of the Cisterna Magna in an Adult Patient. Surg. Neurol. Int. 2022, 13, 432. [Google Scholar] [CrossRef]
- Sánchez Medina, Y.; Robles Hidalgo, E.; Domínguez Baez, J.; Gómez Perals, L.F. Mature Teratoma in the Third Ventricle: A Case Report and Review of the Literature. Surg. Case Rep. 2021, 2021, 1–4. [Google Scholar] [CrossRef]
- Romić, D.; Raguž, M.; Marčinković, P.; Sesar, P.; Spero, M.; Čolak Romić, Z.; Dlaka, D.; Chudy, D. Intracranial Mature Teratoma in an Adult Patient: A Case Report. J. Neurol. Surg. Rep. 2019, 80, e14–e17. [Google Scholar] [CrossRef] [PubMed]
- Zavanone, M.; Alimehmeti, R.; Campanella, R.; Rampini, P.; Locatelli, M.; Egidi, M.; Righini, A.; Bauer, D. Cerebellar Mature Teratoma in Adulthood. J. Neurosurg. Sci. 2002, 46, 35–38. [Google Scholar] [PubMed]
- Park, K.-B.; Park, H.-S.; Lee, J.-I.; Suh, Y.-L. Mature Teratoma in the Cerebellar Hemisphere of an Adult. J. Korean Neurosurg. Soc. 2007, 41, 180–181. [Google Scholar] [CrossRef]
- Beschorner, R.; Schittenhelm, J.; Bueltmann, E.; Ritz, R.; Meyermann, R.; Mittelbronn, M. Mature cerebellar teratoma in adulthood. Neuropathology 2009, 29, 176–180. [Google Scholar] [CrossRef]
- Coulibaly, O.; El Kacemi, I.; Fatemi, N.; Gana, R.; Saïdi, A.; El Maaqili, R.; Jiddane, M.; Bellakhdar, F. Mature Posterior Fossa Teratoma Mimicking Infratentorial Meningioma: A Case Report. Neurochirurgie 2012, 58, 40–43. [Google Scholar] [CrossRef]
- Bohara, M.; Yonezawa, H.; Karki, P.; Bakhtiar, Y.; Hirano, H.; Kitazono, I.; Matsuyama, N.; Arita, K. Mature Posterior Fossa Teratoma Mimicking Dermoid Cyst. Brain Tumor Pathol. 2013, 30, 262–265. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, X.; Liu, X.; Hui, X. Mature Teratoma in Cerebellopontine Angle in a 70-Year-Old Female: A Rare Tumor with Exceptional Location, Age, and Presentation. Neurol. India 2012, 60, 660–661. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, P.; Barui, S.; Mathur, S.; Basak, U. A Case of Mature Cystic Teratoma Arising from the Fourth Ventricle. Case Rep. Pathol. 2013, 2013, 702424. [Google Scholar] [CrossRef]
- Saura, H.; Beppu, T.; Matsuura, H.; Asahi, S.; Uesugi, N.; Sasaki, M.; Ogasawara, K. Intractable Yawning Associated with Mature Teratoma of the Supramedial Cerebellum. J. Neurosurg. 2014, 121, 387–389. [Google Scholar] [CrossRef] [PubMed]
- Pöschl, J.; Berger, F.; Kretzschmar, H.A.; Schüller, U. A 59-Year-Old Man with Two Cerebellar Lesions and Disturbed Cerebellar Morphology. Brain Pathol. 2015, 25, 790–791. [Google Scholar] [CrossRef] [PubMed]
- Balasa, D.; Carp, O.; Mocanu, L.; Butoi, G.; Taran, V. Giant Posterior Fossa Mature Teratoma with Adjacent Subacute Haematoma, Compressive on the Brainstem, with Acute Hydrocephalus. Case Report. Rom. Neurosurg. 2016, 30, 330–335. [Google Scholar] [CrossRef]
- Sattar, R.; Ratha, V.; Kandallu, S.B.R.; Kapilavayi, S.; Sampath, N.; Sankaran, V.; Balasundaram, P. Mature Cystic Teratoma of the Right Cerebellopontine Angle: A Rare Case Report. Br. J. Neurosurg. 2021, 38, 722–725. [Google Scholar] [CrossRef]
- Shin, D.-W.; Kim, J.H.; Song, S.W.; Kim, Y.H.; Cho, Y.H.; Hong, S.H.; Nam, S.J. Posterior Fossa Teratomas in Adults: A Systematic Review. J. Korean Neurosurg. Soc. 2021, 64, 975–982. [Google Scholar] [CrossRef]
- Xi, Z.; Lu, S. Spontaneous Rupture of Mature Teratoma at the Cerebellar Vermis Comorbid with Dermal Sinus Tract and Subcutaneous Lipoma. Cureus 2024, 16, e67634. [Google Scholar] [CrossRef]
- Mahajan, S.; Suri, V.; Sahu, S.; Sharma, M.C.; Sarkar, C. World Health Organization Classification of Tumors of the Central Nervous System 5th Edition (WHO CNS5): What’s New? Indian J. Pathol. Microbiol. 2022, 65, 5–13. [Google Scholar] [CrossRef]
- Wu, A.; Jin, M.C.; Vogel, H.; Hiniker, S.; Campen, C.; Prolo, L.M.; Grant, G.A. Treatment Course and Outcomes of Intracranial Teratomas in Pediatric Patients: A Retrospective 15-Year Case Series Study. Pediatr. Neurosurg. 2023, 58, 429–438. [Google Scholar] [CrossRef]
- Diyora, B.; Devani, K.; Epari, S.; Deshpande, G.; Purandare, A.; Wankhade, R. Mature Teratoma with Somatic-Type Malignancy: An Entity of Unacquaintance—A Case Report. Asian J. Neurosurg. 2023, 18, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Kürner, K.; Greuter, L.; Roethlisberger, M.; Brand, Y.; Frank, S.; Guzman, R.; Soleman, J. Pediatric Sellar Teratoma—Case Report and Review of the Literature. Childs Nerv. Syst. 2024, 40, 1259–1270. [Google Scholar] [CrossRef]
- Nariai, H.; Price, D.E.; Jada, A.; Weintraub, L.; Weidenheim, K.M.; Gomes, W.A.; Levy, A.S.; Abbott, R.; Malbari, F. Prenatally Diagnosed Aggressive Intracranial Immature Teratoma-Clinicopathological Correlation. Fetal Pediatr. Pathol. 2016, 35, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Dubinski, D.; Mittelbronn, M.; Marquardt, G.; Tews, D.S.; Noack, A.; Behmanesh, B.; Seifert, V.; Freiman, T.M. Immature Teratoma of the Tectum Mesencephali with Histopathological Detection of Rudimentary Eye Anlage in a 3-Year-Old Boy: Report of a Rare Case. Neuropathology 2016, 36, 556–560. [Google Scholar] [CrossRef]
- Da Silva, A.; Lessa Barreto, C.M.; Kummer, L.L.M.; Correia, B.P.B.; Udihara, R.A.T. Large Immature Intracranial Teratoma in an Infant: A Case Report. Cureus 2024, 16, e51891. [Google Scholar] [CrossRef] [PubMed]
- Sarva, S.; Venkataraman, M. Congenital Intracranial Teratoma: Management Challenges! J. Fetal Med. 2023, 10, 128–132. [Google Scholar] [CrossRef]
- Satake, D.; Natsumeda, M.; Satomi, K.; Tada, M.; Sato, T.; Okubo, N.; Kawabe, K.; Takahashi, H.; Tsukamoto, Y.; Okada, M.; et al. Successful Multimodal Treatment of Intracranial Growing Teratoma Syndrome with Malignant Features. Curr. Oncol. 2024, 31, 1831–1838. [Google Scholar] [CrossRef]
- Georgiu, C.; Opincariu, I.; Cebotaru, C.L.; Mirescu, Ş.C.; Stănoiu, B.P.; Domşa, T.A.M.; Şovrea, A.S. Intracranial Immature Teratoma with a Primitive Neuroectodermal Malignant Transformation—Case Report and Review of the Literature. Rom. J. Morphol. Embryol. 2016, 57, 1389–1395. [Google Scholar]
- Akchurina, Y.R.; Gorozhanin, V.A.; Shatokhin, T.A.; Lukyanchikov, V.A. Third Ventricle Teratoma with Malignant Transformation. Heйрoхирургия 2024, 26, 79–88. [Google Scholar] [CrossRef]
- Marques, N.K.; Haag, K.; Buffara, L.T.; Steclan, C.A.; Della, A.P.; Nones, D.P.; Junior, O.N.R.; Peres, G.H.; de Oliveira, A.A.; de Oliveira Júnior, A.B.; et al. Intracranial Teratoma in Young Adult Female: Case Report. Arq. Bras. Neurocir. 2022, 42, e68–e72. [Google Scholar] [CrossRef]
- Dimov, I.; Tasić, D.; Stojanovic, I.; Stojanović, S.; Stojanović, N.; Stefanovic, I.; Dimov, D. Mature Intracranial Teratoma. Acta Fac. Medicae Naissensis 2013, 30, 97–102. [Google Scholar] [CrossRef]
- Romero, L.R.; Chen, B.; Guzman, M.A.; Zhou, Y.; Lai, J.; Chen, F.X. Ruptured Intracranial Teratoma: A Case Report and Literature Review. Clin. Med. Rev. Case Rep. 2015, 2, 29. [Google Scholar] [CrossRef]
- Osborne-Grinter, M.; Lopez, A.M.C.; Solth, A.; Hughes, M.A.; Woodfield, J. Ruptured Posterior Fossa Teratoma Mimicking Intracranial Haemorrhage: A Case Report. Neurosurg. Cases Rev. 2022, 5, 98. [Google Scholar] [CrossRef]
- Chiloiro, S.; Giampietro, A.; Bianchi, A.; De Marinis, L. Clinical Management of Teratoma, a Rare Hypothalamic-Pituitary Neoplasia. Endocrine 2016, 53, 636–642. [Google Scholar] [CrossRef]
- Guk, M.O.; Danevych, O.; Chukov, A.A.; Zemskova, O.; Chuvashova, O.; Iegorova, K.S.; Ukrainets, O.V.; Kulichenko, A.Y.; Tsiurupa, D.M.; Shmelyova, A.A. Suprasellar Mature Teratoma: Case Report. Ukr. Neurosurg. J. 2021, 27, 44–50. [Google Scholar] [CrossRef]
- Thoe, J.; Ducis, K.; Eldomery, M.K.; Marshall, M.S.; Ferguson, M.F.; Vortmeyer, A.O.; Raskin, J.S.; Coven, S.L. Pineal Teratoma with Nephroblastic Component in a Newborn Male: Case Report and Review of the Literature. J. Clin. Neurosci. 2020, 80, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Lv, X.; Wang, W.; An, J.; Duan, F.; Feng, X.; Chen, X.; Ouyang, B.; Li, S.; Singh, S.; et al. Imaging Characteristics of Primary Intracranial Teratoma. Acta Radiol. 2014, 55, 874–881. [Google Scholar] [CrossRef] [PubMed]
- Amshavalli, R.S.; Ramkumar, P.G.; Reshath, M.; Sheena, B.G.; Kumar, V. Efficient Diagnosis of Intracranial Tumor on MRI Scans. In Proceedings of the 2024 10th International Conference on Communication and Signal Processing (ICCSP), Melmaruvathur, India, 12–14 April 2024. [Google Scholar] [CrossRef]
- Vigo, V.; Prolo, L.M.; Nunez, M.A.; Nayak, J.V.; Fernandez-Miranda, J.C. Endoscopic Endonasal Approach for Suprasellar Mature Teratoma in Growing Teratoma Syndrome: 2-Dimensional Operative Video. Oper. Neurosurg. 2022, 22, e268. [Google Scholar] [CrossRef]
- Kong, Z.; Wang, Y.; Dai, C.; Yao, Y.; Ma, W.; Wang, Y. Central Nervous System Germ Cell Tumors: A Review of the Literature. J. Child Neurol. 2018, 33, 610–620. [Google Scholar] [CrossRef]
- Takeuchi, S.; Arakawa, Y.; Takeuchi, Y.; Minamiguchi, S.; Tanji, M.; Mineharu, Y.; Miyamoto, S. Central Nervous System Mature Teratoma Producing Carbohydrate Antigen 19-9: Illustrative Case. J. Neurosurg. 2022, 4. [Google Scholar] [CrossRef]
- Zhou, J.; Wu, C.; Li, S. CNS Germ Cell Tumors: Molecular Advances, Significance in Risk Stratification and Future Directions. Brain Sci. 2024, 14, 445. [Google Scholar] [CrossRef] [PubMed]
- Gwak, Y.G.; Yang, S.H.; Yoo, Y.J.; Kim, H.H.; Lee, Y.; Kim, Y.I. A Rare Case of Intracranial Growing Teratoma Syndrome in a Young Adult. Brain Tumor Res. Treat. 2024, 12, 200–203. [Google Scholar] [CrossRef] [PubMed]
- Shatara, M.; Cantor, E.; Ramos, K.N.; Yu, J.; Hassan, A.; Shimony, J.S.; Han, P.; Meyer, A.; Ogle, A.; McHugh, M.; et al. GCT-06. Management of a Congenital Intracranial Teratoma: A Case Report and Review of Literature. Neuro-Oncol. 2022, 24, i55. [Google Scholar] [CrossRef]
- Ogawa, K.; Toita, T.; Nakamura, K.; Uno, T.; Onishi, H.; Itami, J.; Shikama, N.; Saeki, N.; Yoshii, Y.; Murayama, S. Treatment and Prognosis of Patients with Intracranial Nongerminomatous Malignant Germ Cell Tumors: A Multiinstitutional Retrospective Analysis of 41 Patients. Cancer 2003, 98, 369–376. [Google Scholar] [CrossRef]
- Matsutani, M.; Sano, K.; Takakura, K.; Fujimaki, T.; Nakamura, O.; Funata, N.; Seto, T. Primary Intracranial Germ Cell Tumors: A Clinical Analysis of 153 Histologically Verified Cases. J. Neurosurg. 1997, 86, 446–455. [Google Scholar] [CrossRef]

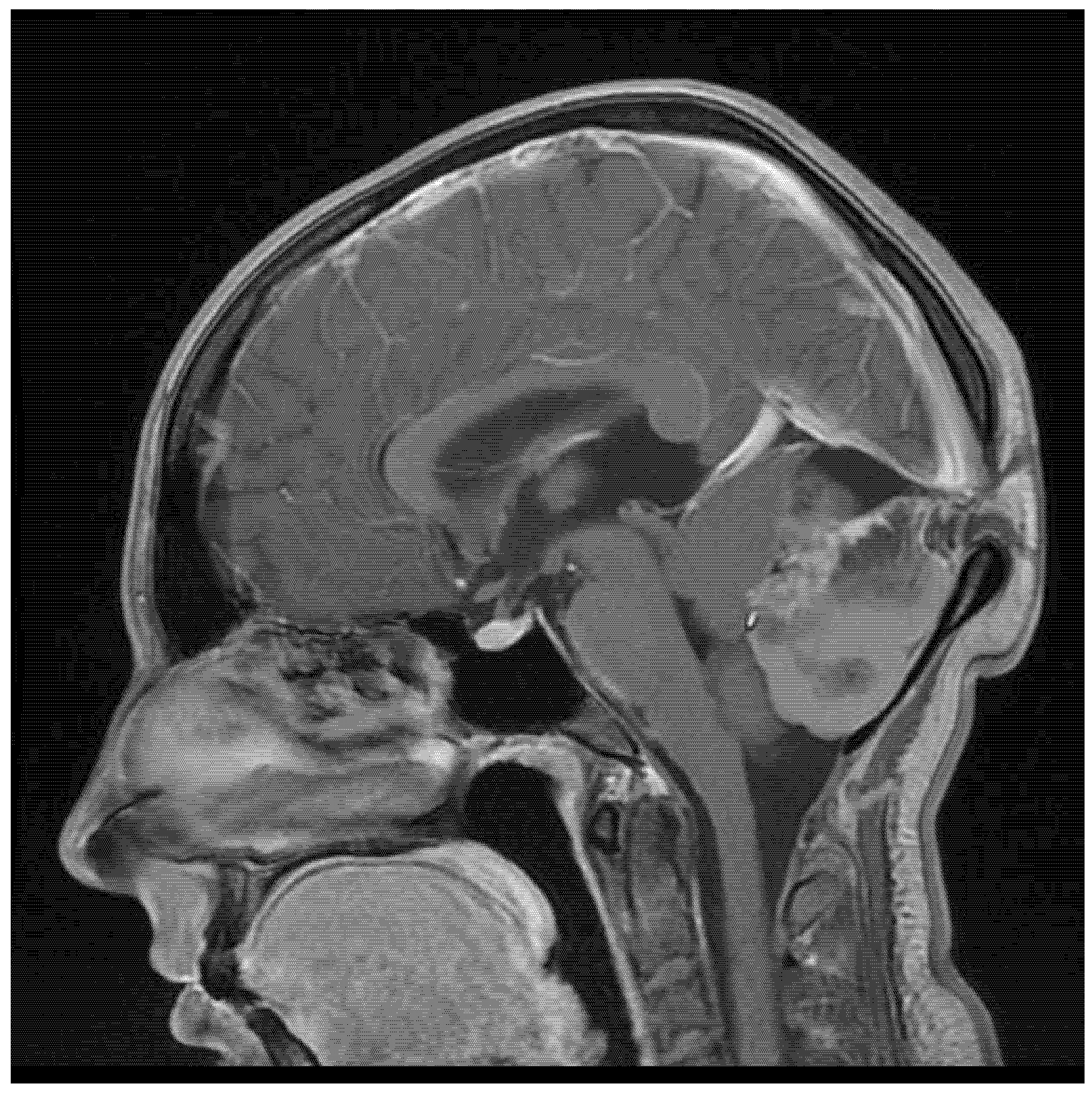
| Age/Sex | Location | Histology | Symptoms | References |
|---|---|---|---|---|
| 50/male | vermis and left hemisphere | mature teratoma | nausea, vomiting, headache, dizziness | [6] |
| 47/female | left cerebellar hemisphere, apart from midline | mature teratoma | headache, dizziness, and nausea | [7] |
| 66/male | vermis | mature teratoma | chronic occipital headache, episodes of severe vertigo | [8] |
| 42/female | vermis and left hemisphere | mature teratoma | vomiting, progressive headache | [9] |
| 41/female | cisterna magna | mature teratoma | right-sided sudden hearing loss, vertigo, dizziness | [10] |
| 70/female | cerebellopontine angle | mature teratoma | headache, vomiting, gait disturbance, right-sided ataxia | [11] |
| 28/male | whole posterior fossa, arising from roof of fourth ventricle | mature teratoma | occipital headache | [12] |
| 19/female | supramedial cerebellum (dorsal side of midbrain and upper pons) | mature teratoma | intractable yawning | [13] |
| 59/male | cerebellum—midline | mature teratoma | evaluation for metastases | [14] |
| 65/male | vermis, paravermis, and in fourth ventricle | mature teratoma | intense headache, nausea, vomiting, gait ataxia, orizontal nistagmus, dismetria, disdiadocokinezia—predominant on left side, long tract signs—predominant on left side | [15] |
| 24/female | right cerebellopontine angle | mature teratoma | cerebellar ataxia, sensorineural hearing loss, decreased palatal movements and facial sensations, nystagmus | [16] |
| 60/male | vermis | mature teraroma | dysarthria, left hemiparesis | [17] |
| 26/female | cisterna magna | mature teratoma | vomiting, headache, vertigo | [3] |
| 41/male | vermis | mature teratoma | headache, coma | [18] |
| 22/female | cerebellum—midline | mature teratoma | severe headache | current case |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowacka, A.; Ziółkowska, E.; Smuczyński, W.; Bożiłow, D.; Śniegocki, M. Mature Teratoma of the Cerebellum with Formed Extracranial Component. J. Clin. Med. 2025, 14, 1994. https://doi.org/10.3390/jcm14061994
Nowacka A, Ziółkowska E, Smuczyński W, Bożiłow D, Śniegocki M. Mature Teratoma of the Cerebellum with Formed Extracranial Component. Journal of Clinical Medicine. 2025; 14(6):1994. https://doi.org/10.3390/jcm14061994
Chicago/Turabian StyleNowacka, Agnieszka, Ewa Ziółkowska, Wojciech Smuczyński, Dominika Bożiłow, and Maciej Śniegocki. 2025. "Mature Teratoma of the Cerebellum with Formed Extracranial Component" Journal of Clinical Medicine 14, no. 6: 1994. https://doi.org/10.3390/jcm14061994
APA StyleNowacka, A., Ziółkowska, E., Smuczyński, W., Bożiłow, D., & Śniegocki, M. (2025). Mature Teratoma of the Cerebellum with Formed Extracranial Component. Journal of Clinical Medicine, 14(6), 1994. https://doi.org/10.3390/jcm14061994